Abstract
Interspecific crossing of the African indigenous rice Oryza glaberrima with Oryza sativa cultivars is hindered by crossing barriers causing 100% spikelet sterility in F1 hybrids. Since hybrids are partially female fertile, fertility can be restored by back crossing (BC) to a recurrent male parent. Distinct genetic models on spikelet sterility have been developed predicting, e.g., the existence of a gamete eliminator and/or a pollen killer. Linkage of sterility to the waxy starch synthase gene and the chromogen gene C, both located on chromosome 6, have been demonstrated. We selected a segregating BC2F3 population of semi-sterile O. glaberrima × O. sativa indica hybrid progenies for analyses with PCR markers located at the respective chromosome-6 region. These analyses revealed that semi-sterile plants were heterozygous for a marker (OSR25) located in the waxy promoter, whereas fertile progenies were homozygous for the O. glaberrima allele. Adjacent markers showed no linkage to spikelet sterility. Semi-sterility of hybrid progenies was maintained at least until the F4 progeny generation, suggesting the existence of a pollen killer in this plant material. Monitoring of reproductive plant development showed that spikelet sterility was at least partially due to an arrest of pollen development at the microspore stage. In order to address the question whether genes responsible for F1 sterility in intraspecific hybrids (O. sativa indica × japonica) also cause spikelet sterility in interspecific hybrids, crossings with wide compatibility varieties (WCV) were performed. WCV accessions possess "neutral" S-loci (S n) improving fertility in intraspecific hybrids. This experiment showed that the tested S n-loci had no fertility restoring effect in F1 interspecific hybrids. Pollen development was completely arrested at the microspore stage and grains were never obtained after selfing. This suggests that distinct or additional S-loci are responsible for sterility of O. glaberrima × O. sativa hybrids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The African rice Oryza glaberrima (Steud.) is one out of two cultivated rice species and is traditionally found in diverse West African agro-ecosystems, e.g. in rainfed environments and deep water floating systems. O. glaberrima is today largely abandoned in favor of high yielding Oryza sativa cultivars due to its poor agronomic performance. However, rice cultivation in West Africa faces constraints specific to the region and O. sativa cultivars are often not sufficiently adapted. In contrast, O. glaberrima is resistant/tolerant to relevant diseases (e.g. African rice gall midge, rice yellow mottle virus) and stresses (e.g. drought, salinity, acidity). It is therefore a valuable source of useful genes (Jones et al. 1997a).
The introduction of the O. glaberrima desirable traits into O. sativa cultivars is hindered by reproductive barriers causing spikelet sterility of F1 hybrids (Bougerol and Pham 1989; Sano 1990; Jones et al. 1997b). Since some embryo sacs are fertile, fertility can be restored by back crossing (BC) to the recurrent parent and subsequent selection of fertile progenies in successive selfing generations (Jones et al. 1997b; Heuer et al. 2003).
Hybrid sterility is a common phenomenon and has also been described for intraspecific crosses of O. sativa ssp. indica × ssp. japonica (e.g. Yanagihara et al. 1995; Xu et al. 1997; Ikehashi and Wan 1998). Intraspecific F1 hybrids are, in contrast to O. glaberrima × O. sativa hybrids, only partially sterile (e.g. Ikehashi and Akari 1986). Numerous genomic sterility (S) loci and segregation distortion loci scattered over most of the rice chromosomes have been identified (for an overview see Xu et al. 1997). The discovery of wide compatibility varieties (WCV) possessing "neutral" sterility loci (S n) recently showed that individual S n-loci (e.g. S5 n) are sufficient to restore fertility in indica × japonica hybrids (Ikehashi and Akari 1986). This facilitated a breakthrough in hybrid rice production in Asia (for a review see Ikehashi and Wan 1998). S5 n is located on chromosome 6, where it is linked to chromogen gene C and the waxy (wx) starch synthase gene (Yanagihara et al. 1995).
Crossing experiments performed with advanced backcrosses of O. glaberrima × O. sativa hybrid progenies led to the identification of tentative S-loci also in O. glaberrima (Sano et al. 1979; Sano 1986). The observed elimination of female and male gametes was best explained by the "one-locus sporo-gametophytic interaction" model. This model predicts that female and male gametes of genotype Sa abort in heterozygous S/Sa plants whereas S-gametes remain viable (Oka 1974; Sano et al. 1979). It is presumed that O. glaberrima is of genotype S and O. sativa of genotype Sa (Sano 1990). According to the model, Sa gametes abort in heterozygous F1 plants and only S-gametes are represented in the F2, which would accordingly be fully fertile. This was indeed shown for a BC8F1 and the corresponding F2 generation of O. glaberrima × O. sativa ssp. indica hybrid progenies (Sano et al. 1979). An identical experiment with an O. glaberrima × O. sativa ssp. japonica back-cross population subsequently showed that female gamete abortion was absent or only partial in this plant material (Sano 1986, 1990). This led to the hypothesis that the gamete eliminator S1 is essentially a pollen killer but can become a gamete eliminator due to the activity of modifiers and depending on the genetic background (indica or japonica; Sano 1990). The respective locus was shown to be linked to C and wx located on chromosome 6, as is the case for S5 n (see above). An O. sativa introgression line suggests that S1 modifiers are located distally adjacent to the wx locus (Sano 1990).
Recent mapping analyses based on an interspecific O. glaberrima × O. sativa ssp. indica hybrid-progeny population showed a strong segregation distortion around the wx locus giving further evidence that a sterility locus (denoted S10) is located at this chromosomal region (Lorieux et al. 2000). Since S10 was first described in intraspecific indica/japonica hybrids (Sano 1994) it remains to be determined whether S10 is allelic to the S1-locus described by Sano et al. (1979).
Despite these crossing barriers, O. glaberrima × O. sativa hybrid progeny lines of agronomic value have been developed in Africa and demonstrated the potential of wide crossing for the improvement of local indica and japonica acccessions (Jones et al. 1997a, b; Dingkuhn et al. 1998; Heuer et al. 2003). However, access to O. glaberrima is still limited to few parental accessions and desirable traits are often lost in the fertility restoring process. In order to gain broader access to O. glaberrima it is therefore necessary to develop strategies to overcome or circumvent crossing barriers.
With the objective to better understand hybrid sterility, semi-sterile O. glaberrima × O. sativa indica hybrid progenies were selected, and analyzed at the molecular level and during plant development. Results obtained suggest that an arrest in early pollen development is at least partially responsible for spikelet sterility and showed that sterility was linked to a heterozygous wx promoter locus. Crossing experiments with wide compatibility varieties suggest that spikelet sterility of interspecific hybrids is caused by distinct or additional S-loci than those causing sterility in intraspecific hybrids.
Materials and methods
Plant material
Crosses were performed with O. glaberrima (Tog5681) as female parent and O. sativa ssp. indica (IR64) as male parent as described by Heuer et al. (2003). F1 hybrid plants were completely self-sterile and backcrossing (BC) to IR64 as the male parent was performed to restore fertility. Three BC1F2 families and 17 BC2F2 families of 20–100 plants each were subsequently screened for spikelet fertility under field conditions in Northern Senegal (Ndiaye) during the dry season (DS) 2000. Semi-sterile plants (seed set <50%) and fertile control plants (seed set >50%) were selected, and F3 progenies were cultivated under field conditions in the following wet season (WS) 2000. Only semi-sterile plants of similar plant type as fertile plants were selected to ensure that spikelet sterility was not due to a general weakness of the plant or environmental factors.
One BC2F3 family (#3–16) showing the most extreme range of spikelet fertility (12.4%–93%) was selected for more detailed analyses. For these analyses a different set of 33 BC2F3 plants (derived from the same semi-sterile BC2F2 progenitor as the BC2F3 field population) was cultivated in a greenhouse during the DS2001. A cooling system facilitated partial temperature control ensuring that temperatures stayed below 37 °C in order to prevent pollen sterility due to heat. Parental plants and F3 progenies of a fertile #3–16 BC2F2 plant were cultivated as controls. Plants were monitored for various vegetative and reproductive characters (height, leaf characteristics, ligule length, straw weight, anthocyanin coloration, tiller and panicle number, panicle structure, duration, spikelet and pollen fertility, and grain weight) throughout development. BC2F4 progenies were cultivated in parallel under field conditions and monitored for spikelet fertility.
Crossing with wide-compatibility varieties
The WCV accessions Aus373, Dular, Ketan Nangka, Kinandang Patong, N22 and Peta were kindly provided by the Genebank of the International Rice Research Institute (IRRI, Manila, the Philippines). They possess one or combinations of three to six neutral S-loci (AUS373: S5 n; Dular: S5 n, S7 n, S8 n, S9 n, S16 n, S17(t)n; N22: S5 n, S7 n, S8 n, S15 n, S16 n; Ketan Nangka: S5 n, S15 n, S17(t)n; Kinandang Patong: S5 n; Peta: S5 n; Ikehashi and Wan 1998; IRRI, personal communication). The O. glaberrima accessions Tog5681, Tog5674, Tog5672 and CG17, as well as the O. sativa accessions IR64 and IR67013-58 are available at WARDA. Crosses were performed in November/December 2000 using O. glaberrima as female and WCV accessions as male parents as described by Heuer et al. (2003). Reciprocal (N22 × CG17; CG17 × N22) and WCV × O. sativa crosses (Aus373 × IR64; Kinandang Patong × IR67013-58), as well as Dular × Dular crosses, were performed as controls. Representative F1 plants were cultivated in the greenhouse during DS2001 as described above.
Sampling of spikelets and pollen analyses
Spikelets 1–2 days before anthesis were dehydrated in an ethanol series (30%–70%) and stored in 70% ethanol at 4 °C for pollen analysis. Spikelets were washed in H2O before anthers were squeezed on a slide and pollen-stained with J2KJ solution. About 300 pollen grains from three spikelets were counted in independent experiments for an estimation of the percentage of microspores, bi-cellular and mature pollen. For monitoring of temporal endosperm and embryo development, spikelets were labeled at anthesis to facilitate documentation of grain development at defined stages (days after anthesis) until plant maturity.
DNA isolation and PCR analyses
Genomic DNA from young flag leaves frozen in liquid nitrogen was extracted with extraction buffer (1% Laurylsarcosine, 100 mM of Tris-HCl pH 8, 100 mM of NaCl and 10 mM of EDTA) and phenol:chloroform:isoamylalcohol (25:24:1) following the protocol of Pallotta et al. (2000). Amplification of DNA fragments by a hot-start PCR (5 min at 94 °C; 35 cycles: 30 s at 95 °C, 30 s at 55 °C, 30 s at 72 °C; and final elongation for 5 min at 72 °C) was performed as described by Heuer et al. (2000), except that 200 nM of primers, 200 μM of each dNTP and 1 U of Taq-DNA polymerase (GibcoBRL) were used. The primer pairs RM204 (Chen et al. 1997) and OSR19 (Akagi et al. 1996) were kindly provided by Dr. M. Lorieux (CIRAD, Montpellier, France). OSR25 primers (for: 5′-CAGAAACCACACACCACC-3′; rev: 5′-CTCCGCTCCGTTCTTTTC-3′) were designed according to the sequence published by Akagi et al. (1996). RG213 primers (for: 5′-CTGGATAAGAGTTTCATTGCA-3′; rev: 5′-GCACGTATTCTGAACTGCTA-3′) were derived from the sequence published under GenBank accession AQ074249 (McCouch et al. 1988). The primer pair MRG5263 (for: 5′-ATCTCCTGCCACTGCACA-3′; rev: 5′-AGGAGGAGGTGTCGATCT-3′) was designed from the sequence published by Monsanto (http://www.monsanto.com ) stating that the marker closest to MRG5263 is C1084 (Harushima et al. 1998).
Amplified DNA fragments were separated on 3.5–4% agarose gels in order to determine polymorphism. Representative samples were subsequently separated on 8% polyacrylamide gels and DNA fragments were silver-stained.
Data analyses
Data were analyzed with Microsoft Excel 97 software. Simple linear correlation (Pearson r) of plant traits was calculated with the STATISTICA 5 software package.
Results
Spikelet sterility is maintained in O. glaberrima × O. sativa F3 and F4 hybrid progenies
Spikelet fertility of Tog5681 × IR64 interspecific hybrid progenies (BC1/BC2F2–F4) was monitored during three seasons under field and greenhouse conditions. In the F2 generation, progeny families developed a variable number (11%–70%) of semi-sterile plants (<50% seed set), with the exception of two BC2F2 families developing only fertile plants (data not shown). F3 families derived from semi-sterile progenitors developed either a high (32%–64%) or very low percentage of semi-sterile plants (0%–20%). F3 families of fertile BC2F2 progenitors developed 4%–15% semi-sterile plants. Tog5681 and IR64 control plants were always fertile. Plants were monitored with the objective to identify progeny families segregating into semi-sterile and fertile plants of similar plant type. This criteria was applied in order to exclude the possibility that sterility was due to a general weakness of the plant or to environmental factors. One BC2F3 line (#3–16) was finally selected for more in-depth studies and a second set of #3–16 BC2F3 plants was cultivated in a greenhouse. Out of a total of 33 BC2F3 progenies, 15 plants showed >50% seed set (range: 56–88%; average 70.4%) and 14 plants were semi-sterile (range: 1%–36%; average 19.6%). Four plants were photosensitive and were excluded from further analyses. The number of semi-sterile plants was lower in the BC2F3 field population (13 semi-sterile: 26 fertile plants), which might be due to cross-pollination. A 1:1 segregation for spikelet fertility (24 semi-sterile:22 fertile plants) was again observed in BC2F4 progenies of a semi-sterile #3–16 F3 plant cultivated under field conditions. Spikelet fertility was on average 36.3% (range 15.9%–49%) in the semi-sterile F4 progenies and 71.5% (range: 51%–92%) among fertile F4 progenies.
Correlation of plants traits to spikelet fertility
Monitoring of the BC2F3 greenhouse population during vegetative and reproductive development revealed that spikelet sterility was significantly correlated to the number of secondary branches and spikelets per panicle, as well as to plant height and straw weight (Table 1). Semi-sterile and fertile plants differed in height (average: 1.18 m semi-sterile, 1.08 m fertile) and straw weight (average: 154 g semi-sterile, 123 g fertile). Semi-sterile plants developed on average 185 spikelets and 40 secondary branches per panicle, both numbers significantly higher than in fertile plants (average: 157 spikelets, 35 secondary branches). The same trend was also observed in BC1F2 and BC2F2 families, as well as in the #3–16 BC2F3 field population (data not shown). No correlation to spikelet sterility was observed for anthocyanin coloration (data not shown). Other plant characters monitored were very similar or identical in fertile and semi-sterile plants (number of primary branches: 10; ligule length: 27 mm; width of flag leaf: 18 mm; panicle number: 60), indicating a similar plant type (Table 1).
Analysis of pollen and grain development in hybrid progenies
Spikelets of BC2F3 hybrid progenies were morphologically normal and all reproductive organs were developed. Detailed microscopic analysis of anthers at anthesis revealed that semi-sterile plants developed a high number of immature pollen (Table 2). With the majority of immature pollen arrested at the one-cellular microspore stage, pollen appeared heterogeneous in terms of size and starch-filling status. The percentage of microspores (MS) was inversely correlated to the percentage of seed set. In semi-sterile plants up to 59% (average 44.9%) of the pollen was arrested at the microspore stage, whereas fertile plants showed on average 7.9% MS (Table 2). BC2F3 progenies of fertile progenitors and the O. sativa parent IR64 developed 90% and 97.5% mature pollen, respectively (Table 2). The Tog5681 parent is photosensitive and did not develop panicles under long-day conditions.
Monitoring of temporal grain development showed that at 3 days after pollination endosperm development was detectable in fertile spikelets, whereas sterile spikelets remained undistinguishable from unfertilized spikelets until plant maturity. This indicates that either no fertilization takes place or that reproductive development in sterile spikelets is arrested before 3 days after pollination.
PCR analysis of the chromosome-6 region adjacent to a tentative S-locus
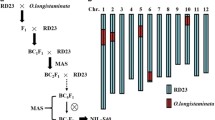
Genetic data give consistent evidence that genomic loci located on chromosome 6 are of importance for spikelet sterility in rice hybrids. A scheme of the respective chromosomal region, as was deduced from published molecular maps is given in Fig. 1 (Kinoshito 1993; Causse et al. 1994; Yanagihara et al. 1995; Chen et al. 1997; Harushima et al. 1998; Lorieux et al. 2000). The putative sterility locus S1 (or S10) is located on the short arm of chromosome 6 between the wx locus (at approximately 7.9 cM) and the marker RZ398 (at 25 cM). The markers OSR19 and OSR25 represent the wx gene and wx promoter, respectively. The S5 locus is located further downstream (at approximately 39 cM) and closely linked to C (at approximately 34 cM), Est-2 (at approximately 37.4) and RG213 (at approximately 43 cM). The marker MRG5263 is reported to be located close to C1084, the latter located at approximately 1.4 cM from wx. The microsatellite marker RM204 is located approximately 7.5 cM from the wx locus and flanks S1 downstream. The RFLP marker RG213 was shown to detect distinct alleles in O. sativa indica and japonica accessions, and in WCV accessions (Yanagihara et al. 1995). With the exception of the RG213 PCR-marker designed for this study, all markers were polymorphic in the parental accessions and were subsequently tested in the BC2F3 greenhouse population.
Genetic map of the region of the short arm of rice chromosome 6. The position of molecular makers, major genes (in italics), and sterility loci (in circles) was estimated from maps published by Kinoshito (1993), Causse et al. (1994), Yanagihara et al. (1995), Chen et al. (1997), Harushima et al. (1998), and Lorieux et al. (2000). PCR markers tested in a BC2F3 O. glaberrima × O. sativa hybrid progeny population are indicated by gray boxes. (*) The S1 locus is linked to wx and C (Sano 1990), but may be upstream or downstream of RM204; cM, centi Morgan
An overview of the size and different combinations of amplified DNA fragments is given in Table 2 and representative samples analyzed by acrylamide-gel electrophoresis are illustrated in Fig. 2. Progeny plants classified as fertile were exclusively homozygous for the O. glaberrima allele of the wx gene (marker OSR19) and promoter (marker OSR25). In contrast, all semi-sterile plants were heterozygous for OSR25. Three out of 14 semi-sterile plants were also heterozygous for the wx gene, whereas all remaining plants carried the O. glaberrima allele (Table 2, Fig. 2). The pattern obtained with the primer pair RM204 was complex. A 150-bp and a 110-bp DNA fragment not represented in either parent appeared in the analysis, which might represent variable numbers of CT-repeats constituting this microsatellite. The majority of fertile plants showed a combination of the 150-bp DNA fragment with either the O. sativa (169 bp) or the O. glaberrima (112 bp) allele (Table 2, Fig. 2). Most semi-sterile plants carried either the O. sativa allele in combination with the 150-bp DNA fragment, or a combination of the 110-bp and the 150-bp DNA fragments. The former was predominantly represented in semi-sterile plants with less than 25% spikelet fertility. The O. glaberrima allele was not represented in any combination. Both, fertile and semi-sterile plants, were homozygous for the O. sativa allele of MGR5263 (Table 2, Fig. 2).
PCR marker analysis of the chromosome-6 region adjacent to a putative sterility locus. Analysis was performed with genomic DNA of fertile (F) and semi-sterile (S) interspecific progenies (BC2F3), and the two parents (O. sativa, IR64; O. glaberrima, Tog5681). Representative samples were separated by polyacrylamide-(OSR25, RM204) and agarose gel-electrophoresis (MGR5262, OSR19). The size of DNA fragments is indicated in base pairs (bp)
Testing neutral-sterility loci by interspecific crossing with wide compatibility varieties
A total of 112 grains were obtained from 165 individual crossings performed with six WCV and four O. glaberrima accessions. With few exceptions, grains germinated normally and representative F1 plants developed into mature, morphologically normal plants. Individual plants showed a strong positive heterosis effect with respect to growth vigor, early tillering ability and duration (data not shown). Monitoring of hybrid plants at flowering showed that spikelets appeared normal, but that pollen development was completely arrested at the microspore stage (Fig. 3a). Plants were accordingly 100% male-sterile and grains were never obtained after selfing in any of the cross-combinations tested. Likewise, F1 hybrid plants derived from a reciprocal WCV × O. glaberrima cross (N22 × CG17; CG17 × N22) were completely self-sterile giving evidence that cytoplasmic factors are not responsible for spikelet sterility in this material. Intraspecific WCV × O. sativa indica F1 hybrids (Patong × IR67013-58; Aus373 × IR64) developed about 40% mature pollen (Fig. 3b) and showed partial seed set (data not shown). Parental accessions were fully fertile and representative pollen is shown in Fig. 3c–e.
Pollen grains of F1 hybrids derived from crosses with wide compatibility varieties (WCV). Pollen of O. glaberrima × WCV and WCV × O. sativa F1 hybrids was stained with iodine solution about 2 days before anthesis. In interspecific hybrids (a) pollen development was completely arrested at the microspore stage. Intraspecific hybrids were semi-sterile, and pollen at all developmental stages was represented (b). Pollen of parental accessions was fertile (c–e)
Discussion
Segregation of spikelet sterility in hybrid progenies suggests the existence of a "pollen killer"
In the present study it was shown that semi-sterility was maintained until the F4 generation in interspecific hybrid progenies. This is in agreement with the "pollen killer" model on hybrid sterility since semi-sterility can only be maintained after selfing if female (Sa) gametes remain viable (Oka 1974; Sano 1990). The basic assumption for both the "pollen killer" and the "gamete eliminator" models is that parental plants are of genotype S/S and S a/S a, respectively, and that S a gametes abort in heterozygous plants. In the case of a gamete eliminator, all male and female Sa gametes abort. Subsequent selfing of a heterozygous plant would produce exclusively F2 progenies of genotype S/S, which would accordingly be fully fertile. In the case of a pollen killer, pollen of genotype Sa abort whereas female Sa gametes remain viable. Semi-sterile, heterozygous S/Sa plants can therefore be obtained after selfing by combining S pollen and Sa embryo sacs. The semi-sterility of BC1F2 and BC2F2 to BC2F4 hybrid progenies reported here is accordingly in agreement with a putative pollen killer, but not with a gamete eliminator. The observed 1:1 segregation into semi-sterile and fertile progenies in the analyzed F3 and F4 progeny families is additionally expected if female Sa gametes remain viable.
According to the literature, there are two putative chromosomal locations for a pollen killer locus. One locus (S3) was extracted from BC8F2 interspecific progenies and was shown to be tightly linked to lazy growth habit (la) on chromosome 11 (Sano 1986). In our experiments, semi-sterile and fertile control plants were of similar plant type and duration, and a linkage to la was therefore not evident.
A second putative pollen killer is represented by S1 located on chromosome 6 (see below). This locus is complex in its effect on spikelet sterility since it can behave as a gamete eliminator or a pollen killer depending on the activity of modifiers and genetic background (Sano 1990). A region distal to S1 encompassing the wx locus was critical for such a conversion, as was shown in an O. sativa indica introgression line carrying O. glaberrima chromosome-6 segments of different size (Sano 1990). Female sterility was significantly higher in plants carrying the O. glaberrima distal segment than in plants carrying the O. sativa segment, suggesting that O. glaberrima carries a S1 modifier that enhances the abortion of female Sa gametes.
Semi-sterile plants are heterozygous for the OSR25 marker
The molecular analysis presented here showed that semi-sterile plants were heterozygous at the wx promoter (OSR25), whereas fertile progenies were homozygous. This is in agreement with the basic assumption that hybrid sterility is correlated to a heterozygous S-locus and its linkage to wx (Sano 1990). Surprinsingly, such a correlation was not observed for the adjacent wx gene (OSR19), expected to be tightly linked to its promoter. Only three semi-sterile plants were heterozygous for the OSR19 marker, whereas the remaining semi-sterile and fertile plants were homozygous for the O. glaberrima allele. This reduced number of heterozygous plants and the absence of plants homozygous for the O. sativa allele, for both the wx promoter and the wx gene, suggest selection against the O. sativa allele. This would be in agreement with the assumption that O. glaberrima is carrying the S-allele inducing abortion of the O. sativa Sa allele in heterozygous plants (Sano et al. 1979). Analysis of a higher plant number is necessary in order to exclude that absence of the O. sativa allele is due to a low sample number. No selection against the O. sativa allele was observed for the microsatellite marker RM204 distantly adjacent to wx. Instead, neither the O. glaberrima allele nor the heterozygous O. sativa × O. glaberrima combination was represented. For the marker MGR5263 it can not be excluded that it is not located at the presumed position close to wx, and the exact chromosomal localization of MGR5263 should therefore be determined before interpreting this result. Despite the fact that the linkage of C and S 1 with a recombination value of less than 0.05 has been reported for some O. glaberrima × O. sativa hybrids (Sano et al. 1979; Sano 1990), no such linkage was observed in our plant material, as was determined by the monitoring of anthocyanin coloration.
Analysis of a larger plant population of segregating hybrid progenies is needed in order to determine linkage of the tested markers to spikelet sterility. With the sequencing of the rice genome, detailed information on the respective chromosome-6 region is now available facilitating highly saturated marker analysis.
Hybrid sterility is correlated to total spikelet number and plant height
Semi-sterile hybrid progenies developed about 8% more spikelets per panicle than fertile plants and therefore had a higher-sink organ size. This might cause a sub-optimal sink/source ratio of the plant, i.e. the spikelet number might exceed the capacity of the plant to provide sufficient metabolites for grain filling, thereby partially accounting for the observed spikelet sterility. However, the high straw-weight of semi-sterile plants suggests that sufficient metabolites are provided but remain in vegetative organs instead of being translocated into grains. This was also observed in another context in otherwise fertile rice accessions when spikelet sterility was exceptionally high due to, e.g., heat sterility (Haefele et al. 2002).
A correlation of plant height and spikelet sterility observed in our plant material has also been reported from O. sativa indica × japonica crosses, where it has been attributed to a linkage of the gametophytic lethal 2 locus and the semi-dwarf gene d60 located on chromosome 7 (Tomita 1996).
Pollen development in BC2F3 hybrids is arrested at the microspore stages
Pollen or male sterility of interspecific hybrids has been described as one of the major components of crossing barriers in rice (e.g. Sano et al. 1979; Bougerol and Pham 1989; Zhang and Lu 1996). Our observations showed that sterile pollen mainly consists of microspores (often referred to as "small, empty" pollen) and pollen at the bi-cellular stage (often referred to as intermediate pollen or small stainable pollen). This indicates that pollen sterility is not due to a degeneration of pollen but to an arrest of pollen development before and shortly after the first pollen mitosis.
Despite a high percentage of immature pollen grains, the number of mature pollen grains was theoretically sufficient for the fertilization of the spikelet. This was also reported from advanced backcrosses of O. glaberrima × O. sativa japonica and O. rufipogon × O. sativa interspecific hybrids (Sano 1986, 1992). As was shown for intraspecific hybrids, the low seed-set observed, despite of a 50% pollen stainability, was due to impaired in vitro pollen germination since only about 10% of the pollen grains germinated (Lin et al. 1992). Pollen-germination tests should now be performed in order to determine whether this is also the case for interspecific hybrids, or whether the low seed set is due, e.g., to anther indehiscent or female sterility. Some genes triggering pollen meiosis and mitosis are known (see Heuer et al. 2000, and references therein) and might be putative target genes for functional genomics on pollen sterility in hybrids.
O. glaberrima × WCV F1 hybrids are male-sterile
The complete spikelet sterility of O. glaberrima × WCV F1 hybrids showed that the S n-loci tested by this approach had no fertility restoring effect in interspecific hybrids. This suggests that sterility is caused by genes distinct from those causing sterility in O. sativa indica × japonica hybrids, or that an additional sterility gene(s) dominates in the F1.
Complete pollen sterility in the F1 can not be explained by the gamete eliminator or pollen-killer model, since S gametes are expected to be fertile in both models (see above). Therefore, at least one additional sterility gene or modifying factor causing an arrest of pollen development in the F1 must be predicted. This tentative S-gene would be eliminated by back-crossing thereby facilitating partial pollen fertility. Since female F1 gametes are at least partially fertile (about 30%, Chu et al. 1969) this gene might have no, or a less severe, effect on female gametophytes. Since reciprocal crosses indicate that cytoplasmic factors are not generally involved in F1 hybrid sterility (this study; Bougerol and Pham 1989; Sano et al. 1979) it is likely to be a nuclear gene/locus.
References
Akagi H, Yokozeki Y, Inagaki A, Fujimura T (1996) Microsatellite DNA markers for rice chromosomes. Theor Appl Genet 93:1071–1077
Bougerol B, Pham JL (1989) Influence of the Oryza sativa genotype on the fertility and quantitative traits of F1 hybrids between the two cultivated rice species O. sativa L. and O. glaberrima Steud. Genome 32:810–815
Causse MA, Fulton TM, Gu Cho Y, Nag Ahn S, Chunwongse J, Wu K, Xiao J, Yu Z, Ronald PC, Harrington SE, Second G, McCouch SR, Tanksley SD (1994) Saturated molecular map of the rice genome based on an interspecific backcross population. Genetics 138:1251–1274
Chen X, Temnykh S, Xu Y, Cho YG, McCouch SR (1997) Development of a microsatellite framework map providing genome-wide coverage in rice (Oryza sativa L.). Theor Appl Genet 95:553–567
Chu YE, Morishima H, Oka HI (1969) Reproductive barriers distributed in cultivated rice species and their wild relatives. Jpn J Genet 44:207–223
Dingkuhn M, Jones M, Johnson DE, Sow A (1998) Growth and yield potential of Oryza sativa and O. glaberrima upland rice cultivars and their interspecific progenies. Field Crops Res 57:57–69
Haefele SM, Wopereis MCS, Wiechmann H (2002) Long-term fertility experiments for irrigated rice in the West African Sahel: agronomic results. Field Crops Res 132:31–40
Harushima Y, Yano M, Shomura A, Sato M, Shimano T, Kuboki Y, Yamamoto T, Yang Lin S, Antonio BA, Parco A, Kajiya H, Huang N, Yamamoto K, Nagamura Y, Kurata N, Khush GS, Sasaki T (1998) A high-density rice genetic linkage map with 2,275 markers using a single F2 population. Genetics 148:479–494
Heuer S, Loerz H, Dresselhaus T (2000) The maize MADS box gene ZmMADS2 is specifically expressed in maize pollen and during maize pollen-tube growth. Sex Plant Reprod 13:21–27
Heuer S, Miézan KM, Sié M, Gaye G (2003) Increasing biodiversity of irrigated rice in Africa by interspecific crossing of Oryza glaberrima (Steud.) × O. sativa indica (L.). Euphytica 132:31–40
Ikehashi H, Akari H (1986) Genetics of F1 sterility in remote crosses of rice. In: Kush GS (ed) Rice genetics. Proc Int Rice Genetics Symposium. International Rice Research Institute, Manila, The Philippines, pp 119–130
Ikehashi H, Wan J (1998) The wide compatibility system: current knowledge of its genetics and use for enhanced yield heterosis. In: Virmani SS, Siddiq EA, Muralidharan K (eds) Advances in hybrid rice technology. International Rice Research Institute, Manila, The Philippines, pp 67–77
Jones MP, Mande S, Aluko K (1997a) Diversity and potential of Oryza glaberrima Steud. in upland rice breeding. Breed Sci 47:395–398
Jones MP, Dingkuhn M, Aluko GK, Semon M (1997b) Interspecific Oryza sativa L. × O. glaberrima Steud. progenies in upland rice improvement. Euphytica 92:237–246
Kinoshito T (1993) Report of the committee on gene symbolization, nomenclature and linkage groups. RGN 10:7–39
Lin SY, Ikehashi H, Yanagihara S, Kawashima A (1992) Segregation distortion via male gametes in hybrids between Indica and Japonica or wide-compatibility varieties of rice (Oryza sativa L.). Theor Appl Genet 84:812–818
Lorieux M, Ndjiondjop MN, Ghesquière A (2000) A first interspecific Oryza sativa × Oryza glaberrima microsatellite-based genetic linkage map. Theor Appl Genet 100:593–601
McCouch SR, Kochert G, Yu ZH, Wang ZY, Khush GS (1988) Molecular mapping of rice chromosomes. Theor Appl Genet 76:815–829
Oka HI (1974) Analysis of genes controlling F1 sterility in rice by the use of isogenic lines. Genetics 77:521–534
Pallotta MA, Graham RD, Langridge P, Sparrow DHB, Barker SJ (2000) RFLP mapping of manganese efficiency in barley. Theor Appl Genet 101:1100–1108
Sano Y (1986) Sterility barriers between Oryza sativa and O. glaberrima. In: Kush GS (ed) Rice genetics. Proc Int Rice Genetics Symposium International Rice Research Institute, Manila, The Philippines, pp 109–118
Sano Y (1990) The genetic nature of gamete eliminator in rice. Genetics 125:183–191
Sano Y (1992) Genetic comparison of chromosome 6 between wild and cultivated rice. Jpn J Breed 42:561–572
Sano Y (1994) Gamete eliminator adjacent to the wx locus as revealed by pollen analysis in rice. J Hered 85:310–312
Sano Y, Chu YE, Oka HI (1979) Genetic studies of speciation in cultivated rice. 1. Genetic analysis for the F1 sterility between O. sativa L. and O. glaberrima STEUD. Jpn J Genet 54:121–132
Tomita M (1996) The gametic lethal gene gal: activated only in the presence of the semidwarfing gene d60 in rice. In: Kush GS (ed) Rice genetics III. Proc 3rd Int Rice Genetics Symposium. International Rice Research Institute, Manila, The Philippines, pp 396–403
Xu Y, Zhu L, Xiao J, Huang N, McCouch SR (1997) Chromosomal regions associated with segregation distortion of molecular markers in F2, backcross, doubled-haploid, and recombinant inbred populations in rice (Oryza sativa L.). Mol Gen Genet 253:535–545
Yanagihara S, McCouch SR, Ishikawa K, Ogi Y, Maruyama K, Ikehashi H (1995) Molecular analysis of the inheritance of the S-5 locus, conferring wide compatibility in Indica/Japonica hybrids of rice (O. sativa L.). Theor Appl Genet 90:182–188
Zhang G, Lu Y (1996) Genetics of F1 pollen sterility in O. sativa. In: Kush GS (ed) Rice genetics III. Proc 3rd Int Rice Genetics Symposium. International Rice Research Institute, Manila, The Philippines, pp 418–422
Acknowledgements
We thank the Deutsche Akademischer Austauschdienst (DAAD) for granting a post-doc scholarship for Dr. S. Heuer (D/99/18141). We are grateful to Dr. S. Braconnier and colleagues at the CERAAS (CIRAD) institute in Thiès (Senegal), as well as to Prof. Dr. H. Loerz, Dr. T. Dresselhaus and the team at Hamburg University (Germany), for the provision of greenhouse and laboratory facilities. Many thanks to Dr. A. Ghesquière and Dr. M. Lorieux (IRD, Montpellier, France) for the PCR primers and stimulating discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.C. Becker
Rights and permissions
About this article
Cite this article
Heuer, S., Miézan, K.M. Assessing hybrid sterility in Oryza glaberrima × O. sativa hybrid progenies by PCR marker analysis and crossing with wide compatibility varieties. Theor Appl Genet 107, 902–909 (2003). https://doi.org/10.1007/s00122-003-1325-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-003-1325-x