Abstract
The non-structural dry matter content of onion bulbs consists principally of fructose, glucose, sucrose and fructans. The objective of this study was to understand the genetic basis for the wide variation observed in the relative amounts of these carbohydrates. Bulb carbohydrate composition was evaluated in progeny from crosses between high dry matter storage onion varieties and sweet, low dry matter varieties. When samples were analysed on a dry weight basis, reducing sugar and fructan content exhibited high negative correlations and bimodal segregation suggestive of the action of a major gene. A polymorphic SSR marker, ACM235, was identified which exhibited strong disequilibrium with bulb fructan content in F2:3 families from the ‘W202A’ × ‘Texas Grano 438’ mapping population evaluated in two environments. This marker was mapped to chromosome 8 in the interspecific population ‘Allium cepa × A. roylei’. Mapping in the ‘Colossal Grano PVP’ × ‘Early Longkeeper P12’ F2 population showed that a dominant major gene conditioning high-fructan content lay in the same genomic region. QTL analysis of total bulb fructan content in the intraspecific mapping population ‘BYG15-23’ × ‘AC43’ using a complete molecular marker map revealed only one significant QTL in the same chromosomal region. This locus, provisionally named Frc, may account for the major phenotypic differences in bulb carbohydrate content between storage and sweet onion varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
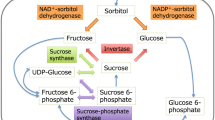
Onions have been valued over millennia not only for their culinary qualities but also for their medicinal value, which has been confirmed by modern medical science (Griffiths et al. 2002). Selection by growers and breeders has produced cultivars with widely varying adaptation, bulb size, sweetness, storability and processing quality, with correspondingly large variation in bulb carbohydrate composition. Bulbs of onion and other Allium species store fructans, fructose polysaccharides formed by the cumulative addition of a fructosyl group to a sucrose molecule, which also function as protectants against cold and drought stress (Ritsema and Smeekens 2003). Onions are a major source of dietary fructans, contributing 25% of the average American intake (Moshfegh et al. 1999). Fructans enhance the value of onion as a functional food by conferring prebiotic properties and lowering blood lipid and insulin levels (Ritsema and Smeekens 2003).
Fructans, glucose, fructose and sucrose comprise the majority (65–80%) of onion bulb dry matter content (Darbyshire and Henry 1978). Dry matter content (DM) exhibits wide genetic variation, from around 6% in sweet onions up to over 25% in dehydration varieties (Jones and Mann 1963) and is commonly estimated indirectly as soluble solids content (SSC) by refractometry (Mann and Hoyle 1944). Although higher DM is associated with higher total fructan content, higher degree of polymerisation (DP) and lower content of fructose, glucose and sucrose (Darbyshire and Henry 1979), the relationship between carbohydrate content and bulb water content is poorly understood (Sinclair et al. 1995a, b).
Onion contains fructans with DP 3–15 of the inulin neo-series, in which β(1–2)-linked fructose chains can be attached to the fructose C1 or the glucose C6 of the sucrose starter unit (Darbyshire and Henry 1978; Ernst et al. 1998; Vijn et al. 1997). Comparative chromatographic analyses of bulb fructo-oligosaccharides from sweet, storage and dehydrator-type cultivars have shown that C6-linked (neokestose) derivatives predominate over C1-linked (1-kestose) types and that cultivar differences are primarily quantitative (Shiomi et al. 1997). Fructan biosynthesis is initiated by the enzyme sucrose-sucrose 1-fructosyltransferase (SS1FT), which catalyses the formation of 1-kestose from sucrose (Vijn et al. 1998). Chain elongation is catalysed by fructan:fructan 6G-fructosyltransferase (6G-FFT) and fructan:fructan 1-fructosyltransferase (1-FFT) activities. An onion fructan:fructan fructosyltransferase cloned by Vijn et al. (1998) was subsequently shown to be a bi-functional enzyme possessing both 6G-FFT and 1-FFT activities capable of producing the entire range of fructans observed in onion (Fujishima et al. 2005; Ritsema et al. 2003). In vitro (Kahane et al. 2001a) and field-based (Kahane et al. 2001b) studies of bulb fructan accumulation during development have shown that lines with high DM continue to accumulate fructan throughout bulbing but low DM lines plateau early, suggesting stronger sink strength in high DM lines.
Onion bulb carbohydrate content is highly heritable. Several studies have obtained broad-sense heritability estimates of 0.6–0.83 for bulb SSC and also reported significant correlation with other traits such as bulb size and pungency (Galmarini et al. 2001; Kadams and Nwasike 1986; Lin et al. 1995; McCollum 1968; Simon 1995). Many dehydration onion breeding programmes have also made substantial progress in raising DM through selection (Wall and Corgan 1999).
Onion is an outcrossing, biennial diploid (2n=2x=16) with a very large genome (32 pg/2n; Jones 1990). Genetic analyses in onion have previously been limited by laborious breeding systems, population heterozygosity, limited genetic and DNA sequence resources, and poor knowledge of population structure. The only extensive genetic dissection of bulb traits to date has been conducted in the cross ‘BYG15–23’ × ‘AC43’ made between the storage onion ‘Brigham Yellow Globe’ and the low DM onion ‘Alisa Craig’ (King et al. 1998). An initial QTL analysis identified several genomic regions affecting DM, soluble solids and other correlated traits (Galmarini et al. 2001). A subsequent study based on analysis of fructan content from replicated field trials confirmed that a genome region containing a functionally characterised acid invertase gene (Vijn et al. 1998) was associated with bulb sugar content (Havey et al. 2004).
The objective of this research was therefore to identify genome regions affecting onion bulb carbohydrate composition in crosses between commercially relevant storage and sweet onion varieties using PCR-based markers. Analysis of phenotypic variation in several independent pedigrees showed common patterns of variation, suggesting segregation of a major gene affecting fructan content. Following identification of a linked marker, the onion linkage map (Martin et al. 2005) was used to confirm that a major gene in the same genome region was the main determinant of fructan content in all these populations.
Materials and methods
Plant material
Pair- and self-pollinations were carried out by enclosing umbels in perforated plastic bags and introducing blowfly pupae. Mass pollinations were performed in 1.5 m3 cages with honeybees. General plant husbandry and post-harvest practices were as described in McCallum et al. (2001a). Field evaluations of F2 populations were conducted by transplanting three plots of 40 plants each of the F2, maternal and paternal populations in a 3×3 Latin Square design.
The ‘Colossal Grano’ × ‘Early Longkeeper P12’ (C×P12) population was derived by self-pollinating an F1 bulb from the pair cross ‘Colossal PVP’ (lot 37018 Sunseeds) × ‘Early Longkeeper P12’ (Crop & Food Research). Parental and F2 plants were grown hydroponically without supplementary lighting in the glasshouse at Lincoln, New Zealand (lat 43°39′), in a high sulphur medium (4 Meq l−1 S) as described previously (McCallum et al. 2002; Randle et al. 1995). Six 18 l tubs each containing nine F2 plants and two tubs of nine plants of each parental line were transplanted in October 1999 and 2000, harvested in late January and cured for 2 weeks in the glasshouse.
The ‘W429A’ × ‘Houston Grano’ F2 population was derived by self-pollinating a single F1 bulb from the pair cross between ‘W429A’ (Goldman et al. 2000) and ‘Houston’ (Texas Grano 1025Y; Asgrow Vegetable Seeds; Wehner 1999).
The ‘W202A’ × ‘Texas Grano 438’ (W×T) population was established by mass-pollinating four bulbs of the male sterile line ‘W202A’ (Goldman et al. 2000) with a selection of 20 bulbs from ‘Texas Grano 438’ (Asgrow Vegetable Seeds, lot VGM 2112; Wehner 1999). Selected F1 bulbs were self-pollinated to generate multiple F1:2 families. Eighty cured F2 bulbs from one F1:2 family (‘47’) were vernalised and self-pollinated to generate 59 F2: 3 families for replicated field trials (one quarter of the F2 were sterile due to segregation for fertility restoration at the Ms locus). In spring 2002 these F2:3 families were transplanted at Pukekohe, New Zealand (lat 37°12′; 58 families) and Lincoln (45 families) in plots of 40 plants in a randomised block design with three blocks per site. Replication of lines depended on seed availability and averaged 2.5 at both sites. Parental plots were allocated in a pseudo-Latinised arrangement to provide estimates of spatial variation within sites (Timmerman-Vaughan et al. 2005). Single F2 bulbs from another F1:2 family (‘29F’) were evaluated in the field at Lincoln in 2002 as described above.
Carbohydrate analysis
Cured bulbs were stored under ambient conditions and bulb sampling was performed between 8 and 10 weeks post-harvest, as described previously (McCallum et al. 2005). Freeze-dried bulb tissues were stored at room temperature in a tightly sealed container over silica gel prior to carbohydrate analyses.
For detailed qualitative analysis of fructans in ‘W202A’ and ‘Texas Grano 438’, water-soluble carbohydrates were separated by reverse-phase HPLC on an Aqua C18 column (250×4.0 mm; Phenomenex, NZ). Samples (~0.5 mg in 20 ml H2O) were eluted with water at a flow rate of 1.0 ml min−1 and the eluant monitored by differential refractometry. Isomeric oligosaccharides were identified as described by Sims et al. (1992).
Quantitative analysis of total fructan and free reducing sugar content in freeze-dried samples was conducted according to AOAC Method 999.03 (McCleary et al. 2000) using a Megazyme Fructan Assay Kit (Megazyme International, Bray, Ireland) and control fructan flour. For quantitative HPLC analysis of glucose, fructose, sucrose and DP 3–4 fructo-oligosaccharides, ground, freeze-dried tissue (20 mg) was extracted in 1 ml of 80% v/v ethanol by agitation for 15 min at room temperature, centrifuged (13,000×g for 5 min) and filtered through a 0.2 μm Minisart filter. For preliminary HPLC analyses of the C×P12 population, juice extracts were prepared from bulb tissue as described by McCallum et al. (2005), diluted by addition of four volumes of ethanol and clarified as above. Carbohydrates in these extracts were fractionated by HPLC on a 220×4.6 mm Applied Biosystems (Foster City, CA, USA) Brownlee amino column and 15×3.2 mm NewGuard amino guard column, on a Waters (Milford, MA, USA) liquid chromatograph (model 626 pump and controller, model 717-plus autosampler). The mobile phase was degassed HPLC-grade acetonitrile:water (80:20 v/v for simple sugars and 60:40 v/v for fructans) at 1.5 ml min-1. Separations were performed at 30°C and sugars were detected using a refractive index detector (Waters model 410). Analytical standards were obtained from Fluka (Buchs, Switzerland) and identification of each sugar was based on retention times. Detector response to all sugars was linear over the concentration 0–10 mg ml-1 and standards exhibited less than 2% variability in individual concentrations between triplicate injections of the same sample.
DNA marker development and analysis
DNA was isolated from leaf of individual F2 plants or at least 25 germinated seedlings of parental and F2:3 lines (1–2 g fresh weight) by the protocol of Aljanabi et al. (1999) with minor modifications. Chloroform:sec octanol (24:1) was used instead of phenol/chloroform/isoamyl alcohol and tissue homogenisation and DNA pellet washes were performed as described earlier (McCallum et al. 2001b). EST-SSR and SSCP markers were developed and evaluated as described previously (Kuhl et al. 2004; Martin et al. 2005; McCallum et al. 2001b). Primer sets not reported in these previous publications are shown in Table 1. AFLP analysis was carried out according to Vos et al. (1995) with minor modifications. DNA templates (0.15–1 μg) were digested with Pst1+Mse1 and ligated with oligonucleotide adaptors. Pre-amplification was carried out with Pst1+A and Mse1+C primers and selective amplification was carried out with three base selective primers PstI (±33P) +ACW and MseI+CNN. Denatured radiolabelled PCR products were resolved by electrophoresis on 6% denaturing gels and detected by autoradiography. Template DNAs were arranged by fructan phenotype class to facilitate visual screening of banding patterns associated with these phenotypes.
DNA templates and genetic map data from the interspecific Allium cross A. cepa × A. roylei were used as described by van Heusden et al. (2000a, b). Allium fistulosum-shallot (A. cepa L. aggregatum group) alien addition line stocks (Shigyo et al. 1996) were used for chromosomal assignment of markers as described by Martin et al. (2005).
Statistical analysis
Analysis of phenotypic data was carried out using GenStat 7 (Payne et al. 2003). Tests for departure from normality were performed as described in Pearson and Hartley (1970). Principal components analysis was based on trait correlation matrices. Linkage analysis was performed using Joinmap 3.0 (van Ooijen and Voorips 2002) using a minimum of LOD 4 to form linkage groups. QTL mapping by single marker Kruskal–Wallis tests and standard interval mapping was performed using MapQTL (van Ooijen and Maliepaard 2002). Significance thresholds (P<0.05) for QTL mapping were obtained by permutation testing with 1,000 permutations (Churchill and Doerge 1994). Standard errors of recombination distances were calculated as described by Weir (1990). Analyses of phenotypic data from the W202A × Texas Grano QTL mapping trial were conducted in Genstat (Payne et al. 2003) using a mixed model analysis fitted with restricted maximum likelihood (REML). Check plot genotype and genotype for the linked marker ACM235 were included as fixed effects. Line genotype, block, row and bed were included as random effects. Using variance components estimated with the REML analysis, narrow-sense heritability was calculated as described by Timmerman-Vaughan et al. (2005). QTL analysis of total fructan content in the population ‘BYG15-23’ × ‘AC43’ was carried out with marker data used to construct the latest onion linkage map (Martin et al. 2005) and phenotypic data from replicated field trials reported previously (Havey et al. 2004).
Results
Phenotypic evaluations of F2 onion populations provide evidence of major gene action
Phenotyping of F2 bulb carbohydrate content in the C×P12 population was initially conducted by direct HPLC of juice samples prepared by homogenising bulb tissues with an equal volume of water. This is the sampling procedure most widely used for phenotyping onion bulbs for breeder selections and in previous studies of pungency and SSC. HPLC revealed that fructose and glucose concentrations were strongly skewed (coefficient of skewness √b 1=1.29, P<0.01 and √b 1=0.82, P<0.01, respectively). Exploration of the correlation structure of the sugar concentrations by principal components analysis (PCA) indicated that the first principal component (PC) accounted for 58% of total trait variation (Table 2) and that weightings of monosaccharides in PC1 were in opposite sign to those of oligosaccharides. When extracts from freeze-dried bulb tissue were analysed from a further sample of F2 bulbs in the following season a non-overlapping, bimodal segregation was observed for fructose and fructan content (Fig. 1a). The frequency of high-fructan and low-fructan individuals fitted a 3:1 ratio (33/13; χ 2=0.26, 1 df, P=0.61), suggesting action of a dominant single gene. Because of this clearer resolution into non-overlapping phenotypic classes, all subsequent phenotyping of bulbs was conducted on freeze-dried samples.
Subsequent analyses revealed similar bimodal trait distributions and correlation structures in other onion pedigrees (Tables 2 and 3). Total carbohydrate content, on a dry weight basis, across these populations was in the range 550–700 mg g−1 dry weight, as reported in previous studies (Darbyshire and Henry 1979; Jaime et al. 2001). Evaluations of field-grown single F2 bulbs from W×T (family 29F), and ‘W429A × Houston Grano’ revealed trait distributions that were bimodal (but overlapping) and skewed toward high fructan and low reducing sugars (Fig. 1). Inspection of trait correlations (Table 2) revealed consistently high (r~0.9) negative correlations between total fructan and fructose content in these populations but no consistently high correlation of sucrose concentrations with other components. PCA of carbohydrate concentrations in these populations and also the F3M families evaluated by Havey et al. (2004) showed that the first PC accounted for 60–70% of phenotypic variation (Table 3). Inspection of the weightings for individual components in the first PC showed that in all cases fructose and glucose were of opposite sign to fructans, suggesting that this PC can be interpreted as a measure of the balance between reducing sugars and fructans. These phenotypic observations suggested that in all the populations examined, the balance between fructan and reducing sugar levels is conditioned by a major gene and that there is dominance for high fructan.
Phenotypic evaluation of ‘W202A × Texas Grano 438’ mapping population
Reverse-phase HPLC of bulb carbohydrate extracts from samples of the ‘W202A’ and ‘Texas Grano 438’ parental populations showed a pattern of oligosaccharides typical of other onion extracts previously analysed on this system (O’Donoghue et al. 2004). Neokestose and its related tetrasaccharides were more abundant than 1-kestose and nystose, as reported in previous comparisons of onion cultivars (Shiomi et al. 1997) and relative proportions of these fructo-oligosaccharides were similar in both parents (Fig. 2). Since these observations confirmed that variation in fructan content was a quantitative trait, subsequent analysis of the mapping population was conducted by enzymatic analysis of total fructan.
F2:3 families derived from ‘W202A × Texas Grano 438’ (from F2 family ‘47’) were evaluated at Pukekohe (n=57 F2:3 families) and Lincoln (n=46 F2:3 families) sites in 2002/2003. Total fructan content was bimodal and strongly skewed (coefficient of skewness √b 1=−1.09; P<0.01) toward the higher levels of the ‘W202A’ parent (Fig. 1). Estimates of narrow-sense heritability (H2) of fructan content from the two sites were 0.95 (SE=0.010; Pukekohe site) and 0.94 (SE=0.015; Lincoln site).
Genetic mapping of the Frc locus
Following the observation of bimodal segregation in fructan content in the C×P12 population, attempts were made to map the putative locus using EST and Pst1-Mse1 AFLP markers. Following the screening of 15 combinations of Pst1/Mse1 selective primers, we scored 53 polymorphic AFLP markers from ten of these primer combinations. Two AFLP bands revealed by the Pst1-ACT/Mse1-CTA primer combination (pACTmCTA_7 and pACTmCTA_2), which were absent in the P12 (high fructan) parent, were associated (in repulsion) with fructan content. This was confirmed by formal linkage analysis which showed that these were linked (6.7% recombination; LOD 15.4) and both were significantly associated with carbohydrate content measured both on a fresh weight basis (P<0.02) and on a dry weight basis (P<0.00001) by one-way ANOVA.
Although 14 EST markers were scored in this family, heterozygosity of many markers in the parent populations and poor Pst1-AFLP reproducibility prevented effective mapping and few marker linkages were obtained. However, a polymorphic, co-dominant SSCP marker was developed for 6GFFT, the fructosyltransferase involved in fructan chain elongation. This marker revealed a minimum of four single-stranded DNA bands, consistent with two tightly linked loci (Fig. 3a). Single-marker analyses showed that it was not associated with fructan content (χ 2=2.6, 2 df, P>0.1). The marker was unambiguously assigned to Allium cepa chromosome 6 using monosomic addition lines (Fig. 3b). No other markers evaluated in this preliminary analysis exhibited association with fructan phenotype by single marker analyses.
a Segregation of a co-dominant SSCP in the 6GFFT gene in ‘Colossal × ELK P12’ F2 population. b Assignment of the 6-GFFT marker to chromosome 6 using a panel of independently isolated A. fistulosum-shallot alien addition lines. Control lanes on left of gel contained amplicons from Alliumfistulosum (Af) and shallot donor lines
During evaluation of EST-SSR markers in the W×T F2 family ‘47’ a single-copy (TTTG)4 tetranucleotide simple sequence repeat (SSR) marker (ACM235) was identified that was significantly associated with bulb fructan, fructose and glucose content, but not DM, in both sites (Table 4). Comparison of models fitted by REML, with and without ACM235, indicated that the marker could account for 35% of the genotypic variance. No polymorphic markers linked to ACM235 have yet been identified in this family.
We also identified polymorphic markers in W×T from two chromosomal regions previously shown to affect bulb composition by Galmarini et al. (2001). The EST-SSR marker ACM171, which is closely linked to the sucrose transporter RFLP locus API66C-E5-6.7/9.5 on chromosome 5 (Martin et al. 2005), showed significant association with bulb DM (Table 4), as reported previously (Galmarini et al. 2001). Only weak association of the chromosome 3 acid invertase locus API89 with bulb composition was observed (Table 4), in contrast to observations in ‘BYG15-23 × AC43’(Galmarini et al. 2001; Havey et al. 2004).
The chromosomal location of ACM235 was first determined by mapping in the interspecific population A. cepa × A. roylei, in which linkage groups have been assigned to chromosomes using monosomic addition lines (van Heusden et al. 2000a). ACM235 exhibited linkage to AFLP markers assigned to chromosome 8 (Fig. 4). Additional co-dominant SSR and EST markers from the onion linkage map (Martin et al. 2005) were scored to permit an unambiguous assignment of this marker to the distal region of the chromosome. Although ACM235 was monomorphic in ‘BYG15-123 × AC43’ it exhibited close linkage in C×P12 (6 cM) and A. cepa × A. roylei (1 cM) to the single-copy (CAT)5 SSR marker ACM033. Both SSR markers showed highly significant associations with fructan, fructose and sucrose content in C×P12 by single marker analyses (χ2>13, P<0.005). Interval mapping revealed that this genome region contained a major QTL (LOD>6; Fig. 4) explaining 93% of phenotypic variation in fructan content. Fructan content was evaluated as a Mendelian trait in the 46 C×P12 F2 individuals in which this exhibited non-overlapping bimodal segregation. This provided estimates of 10.5±5 cM recombination for ACM235 and 16.5±5 cM for ACM033 with the putative locus, which we denote Frc (Fig. 4).
Assignment of the Frc locus to chromosome 8 of onion by QTL and linkage analysis in inter- and intraspecific Allium gene mapping populations. Scale denotes recombination distance in Kosambi units. Names of AFLP loci in the interspecific map are omitted for clarity. Location of Frc is denoted by (1) QTL location bounds for bulb fructan and sucrose content as 1- and 2-LOD intervals from simple interval mapping and (2) 1 and 2-LOD confidence intervals distance of Frc, evaluated as a Mendelian character, from SSR loci ACM033 and ACM235. The cross-hatched interval in BYG15-23 × AC43 was reported by Galmarini et al. (2001) to affect bulb dry matter
The location of Frc was confirmed by QTL mapping in the population ‘BYG15-123’ × ‘AC43’ using least-square means estimates of total bulb fructo-oligosaccharide components obtained in replicated field trials (Havey et al. 2004) and the more detailed genetic map recently reported for this population (Martin et al. 2005), which spans >2 M and all eight chromosomes of the onion genome. The complete genome scan revealed a single QTL (LOD 6) affecting bulb total fructan content in the interval on chromosome 8 flanked by ACM033 and the SNP marker ACABE58, which explained 50.3% of total phenotypic variation. Galmarini et al. (2001) previously reported a QTL affecting bulb DM in an interval (AJB72-E1-4Y-API54C-E1-15/9) proximal to this position but at that time the map lacked co-dominant markers distal to AJB72-E1-4Y. A QTL previously reported on chromosome 4 in the interval API18-AOB77 (Havey et al. 2004) did not exceed the genome-wide significance threshold (LOD 2.6) using the new map data.
No segregation distortion was evident in the markers linked to Frc in any of the populations studied. Single locus QTL models fitted by interval mapping in C×P12 and ‘BYG15-123’ × ‘AC43’ populations showed partial dominance of high fructan content over low, with d/a ratios of 0.52 and 0.80, respectively.
Conclusion
It is likely that Frc conditions the principal differences in bulb carbohydrate phenotype between sweet and storage onion types, since fructose quantitatively dominates sweetness in onion. However, the observation that fructan content and DM in the ‘W202A × Texas Grano 438’ population are affected by QTL in unlinked genomic regions suggests that interactions between loci may be important in determining onion bulb carbohydrate phenotype. The expense of developing and trialling large replicated onion families does not permit population sizes sufficiently large for detecting epistasis. Because bulb carbohydrate content is highly heritable, more rapid and economical progress in genetic analysis of onion carbohydrate content should be achievable by analysis of larger numbers of individual bulbs from F1, F2, BC or test-cross populations.
Since none of markers reported in this paper show tight linkage to Frc, it is desirable to tag it more closely to enable marker-assisted selection and more detailed studies of the role and evolution of the locus. Because genomics resources are very limited in Allium and the pathway of fructan metabolism is well understood, the most efficient means to achieve this may be through molecular analysis of carbohydrate physiology in high- and low-fructan lines. Studies by Shiomi et al. (1997) showed that higher fructan accumulation is associated with higher levels of fructan:fructan fructosyltransferase activity, suggesting that this enzyme is a candidate for higher sink strength in genotypes with high fructan accumulation. Studies of sucrose-metabolising enzymes in developing onion have reported decreased acid invertase (Lercari 1982) and increased sucrose synthase (Thomas et al. 1997) following induction of bulbing but no comparisons of different genotypes were included.
The assignment of a major gene conditioning bulb non-reducing sugar content to onion chromosome 8 is supported by the recent findings of Hang et al. (2004), who reported increased winter content of non-reducing sugars in A. fistulosum monosomic addition lines carrying shallot (A. cepa aggregatum group) chromosome 8. This suggests that the inbred lines developed in the present study and the addition lines developed by Shigyo et al. (1997) will provide complementary genetic stocks for elucidating the biochemical phenotype and molecular nature of the Frc locus.
Abbreviations
- DM:
-
Dry matter content
- SSC:
-
Soluble solids content
- PCA:
-
Principal components analysis
- REML:
-
Restricted maximum likelihood
- SSCP:
-
Single-stranded conformation polymorphism
References
Aljanabi SM, Forget L, Dookun A (1999) An improved and rapid protocol for the isolation of polysaccharide- and polyphenol-free sugarcane DNA. Plant Mol Biol Rep 17:1–8
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Darbyshire B, Henry RJ (1978) The distribution of fructans in onions. New Phytol 81:29–34
Darbyshire B, Henry RJ (1979) The association of fructans with high percentage dry weight in onion cultivars suitable for dehydration. J Sci Food Agric 30:1035–1038
Ernst MK, Chatterton NJ, Harrison PA, Matitschka G (1998) Characterization of fructan oligomers from species of the genus Allium L. J Plant Physiol 153:53–60
Fujishima M, Sakai H, Ueno K, Takahashi N, Onodera S, Benkeblia N, Shiomi N (2005) Purification and characterization of a fructosyltransferase from onion bulbs and its key role in the synthesis of fructo-oligosaccharides in vivo. New Phytol 165:513–524
Galmarini CR, Goldman IL, Havey MJ (2001) Genetic analyses of correlated solids, flavor, and health-enhancing traits in onion (Allium cepa L.). Mol Genet Genomics 265:543–551
Goldman IL, Schroek G, Havey MJ (2000) History of public onion breeding programs in the United States. Plant Breed Rev 20:68–103
Griffiths G, Trueman L, Crowther T, Thomas B, Smith B (2002) Onions-a global benefit to health. Phytother Res 16:603–615
Hang TT, Shigyo M, Yaguchi S, Yamauchi N, Tashiro Y (2004) Effect of single alien chromosome from shallot (Allium cepa L. Aggregatum group) on carbohydrate production in leaf blade of bunching onion (A. fistulosum L.). Genes Genet Syst 79:345–350
Havey MJ, Galmarini CR, Gokce AF, Henson C (2004) QTL affecting soluble carbohydrate concentrations in stored onion bulbs and their association with flavor and health-enhancing attributes. Genome 47:463–468
van Heusden AW, Shigyo M, Tashiro Y, Vrielink-van Ginkel R, Kik C (2000a) AFLP linkage group assignment to the chromosomes of Allium cepa L. via monosomic addition lines. Theor Appl Genet 100:480–486
van Heusden AW, van Ooijen JW, Vrielink-van Ginkel R, Verbeek WHJ, Wietsma WA, Kik C (2000b) A genetic map of an interspecific cross in Allium based on amplified fragment length polymorphism (AFLPTM) markers. Theor Appl Genet 100:118–126
Jaime L, Martin-Cabrejas M, Molla E, Lopez-Andreu F, Esteban R (2001) Effect of storage on fructan and frucyooligosaccharide of onion (Allium cepa L.). J Agric Food Chem 49:982–988
Jones RN (1990) Cytogenetics. In: Brewster HRRJL (ed) Onions and allied crops. CRC Press, Boca Raton, pp 199–214
Jones HA, Mann LK (1963) Onions and their allies, 1st edn. Interscience Publishers, London, New York
Kadams AM, Nwasike CC (1986) Heritability and correlation studies of some vegetative traits in Nigerian local white onion. Plant Breed 97:232–236
Kahane R, Pitiot C, Chamaux C, Vialle-Guerin E, Schweisguth B (2001a) Non-structural carbohydrate composition of long-day onion in vitro. J Hortic Sci Biotechnol 76:320–326
Kahane R, Vialle-Guerin E, Boukema I, Tzanoudakis D, Bellamy C, Chamaux C, Kik C (2001b) Changes in non-structural carbohydrate composition during bulbing in sweet and high-solid onions in field experiments. Environ Expt Bot 45:73–83
King JJ, Bradeen JM, Bark O, McCallum JA, Havey MJ (1998) A low-density genetic map of onion reveals a role for tandem duplication in the evolution of an extremely large diploid genome. Theor Appl Genet 96:52–62
Kuhl JC, Cheung F, Yuan Q, Martin W, Zewdie Y, McCallum J, Catanach A, Rutherford P, Sink KC, Jenderek M, Prince JP, Town CD, Havey MJ (2004) A unique set of 11,008 onion expressed sequence tags reveals expressed sequence and genomic differences between the monocot orders Asparagales and Poales. Plant Cell 16:114–125
Lercari B (1982) Changes in invertase activities during the photoperiodically induced bulb formation of onion (Allium cepa L.). Physiol Plant 54:480–484
Lin M, Watson JF, Baggett JR (1995) Inheritance of soluble solids and pyruvic acid content of bulb onions. J Am Soc Hort Sci 120:119–122
Mann L, Hoyle BJ (1944) Use of the refractometer for selecting onion bulbs high in dry matter for breeding. Proc Am Soc Hort Sci 46:285–292
Martin W, McCallum J, Shigyo M, Jakse J, Kuhl J, Yamane N, Pither-Joyce M, Gokce A, Sink K, Town C, Havey M (2005) Genetic mapping of expressed sequences in onion and in silico comparisons with rice show scant colinearity. Mol Genet Genomics 274:197–204
McCallum JA, Grant DG, McCartney EP, Scheffer J, Shaw ML, Butler RC (2001a) Genotypic and environmental variation in bulb composition of New Zealand adapted onion (Allium cepa) germplasm. N Z J Crop Hortic Sci 29:149–158
McCallum JA, Leite D, Pither-Joyce MD, Havey MJ (2001b) Expressed sequence markers for genetic analysis of bulb onion (Allium cepa. L.). Theor Appl Genet 103:979–991
McCallum JA, Pither-Joyce MD, Shaw ML (2002) Sulfur deprivation and genotype affect gene expression and metabolism of onion roots. J Am Soc Hort Sci 127:583–589
McCallum J, Porter N, Searle B, Shaw M, Bettjeman B, McManus M (2005) Sulfur and nitrogen fertility affects flavour of field-grown onions. Plant Soil 274:151–158
McCleary BV, Murphy A, Mugford DC (2000) Measurement of total fructan in foods by enzymatic/spectrophotometric method: collaborative study. J AOAC Int 83:356–364
McCollum G (1968) Heritability and genetic correlation of soluble solids, bulb size and shape in white sweet spanish onion. Can J Genet Cytol 10:508–514
Moshfegh AJ, Friday JE, Goldman JP, Ahuja JKC (1999) Presence of inulin and oligofructose in the diets of Americans. J Nutr 129:1407S–1411S
O’Donoghue EM, Somerfield SD, Shaw M, Bendall M, Hedderly D, Eason J, Sims I (2004) Evaluation of carbohydrates in Pukekohe Longkeeper and Grano cultivars of Allium cepa. J Agric Food Chem 52:5383–5390
van Ooijen JW, Maliepaard C (2002) MapQTL Version 5.0: Software for the calculation of QTL positions on genetic maps 5.0. Plant Research International BV, Wageningen
van Ooijen JW, Voorips RE (2002) JoinMap 3.0: software for the calculation of genetic linkage maps. Plant Research International BV, Wageningen
Payne RW, Baird DB, Cherry M, Gilmour AR, Harding SA, Kane AF, Lane PW, Murray DA, Soutar DM, Thompson R, Todd AD, Tunnicliffe Wilson G, Webster R, Welham SJ (2003) GenStat 7.1. Lawes Agricultural Trust
Pearson ES, Hartley HO, (1970) Biometrika tables for statisticians, 3rd ed. Cambridge University Press, Cambridge
Randle WM, Lancaster JE, Shaw ML, Sutton KH, Hay RL, Bussard ML (1995) Quantifying onion flavor compounds responding to sulfur fertility—sulfur increases levels of alk(en)yl cysteine sulfoxides and biosynthetic intermediates. J Am Soc Hort Sci 120:1075–1081
Ritsema T, Smeekens S (2003) Fructans: beneficial for plants and humans. Curr Opin Plant Biol 6:223–230
Ritsema T, Joling J, Smeekens S (2003) Patterns of fructan synthesized by onion fructan:fructan 6G-fructosyltransferase expressed in tobacco BY2 cells—is fructan:fructan 1-fructosyltransferase needed in onion? New Phytol 160:61–67
Shigyo M, Tashiro Y, Isshiki S, Miyazaki S (1996) Establishment of a series of alien monosomic addition lines of Japanese bunching onion (Allium fistulosum L) with extra chromosomes from shallot (A. cepa L. aggregatum group). Genes and Genet Syst 71:363–371
Shigyo M, Iino M, Isshiki S, Tashiro Y (1997) Morphological characteristics of a series of alien monosomic addition lines of Japanese bunching onion (Allium Fistulosum L.) with extra chromosomes from shallot (A.cepa L. aggregatum group). Genes Genet Syst 72:181–186
Shiomi N, Onodera S, Sakai H (1997) Fructo-oligosaccharide content and fructosyltransferase activity during growth of onion bulbs. New Phytol 136:105–113
Simon PW (1995) Genetic analysis of pungency and soluble solids in long-storage onions. Euphytica 82:1–8
Sims IM, Pollock CJ, Horgan R (1992) Structural analysis of oligomeric fructans from excised leaves of Lolium temulentum. Phytochemistry 31:2989–2992
Sinclair PJ, Blakeney AB, Barlow EWR (1995a) Relationships between bulb dry matter content, soluble solids concentration and non-structural carbohydrate composition in the onion (Allium cepa). J Sci Food Agric 69:203–209
Sinclair PJ, Neeson RJ, Barlow EWR (1995b) Osmotic potential and soluble solids concentration in onion (Allium cepa) bulbs. J Sci Food Agric 69:211–214
Thomas B, P. H, Partis M (1997) Gene regulation in onion. In: JL Burba CG (eds) First international symposium on edible Alliiaceae, Mendoza, Argentina, 1994. Acta Horticulturae 433:375–380
Timmerman-Vaughan GM, Mills A, Whitfield C, Frew T, Butler R, Murray S, Lakeman M, McCallum J, Russell A, Wilson D (2005) Linkage mapping of QTL for seed yield, yield components, and developmental traits in pea. Crop Sci 45:1336–1344
Vijn I, Vandijken A, Sprenger N, Vandun K, Weisbeek P, Wiemken A, Smeekens S (1997) Fructan of the inulin neoseries is synthesized in transgenic chicory plants (Cichorium Intybus L) harbouring onion (Allium Cepa L) fructan-fructan 6-fructosyltransferase. Plant J 11:387–398
Vijn I, Vandijken A, Luscher M, Bos A, Smeets E, Weisbeek P, Wiemken A, Smeekens S (1998) Cloning of sucrose—sucrose 1—fructosyltransferase from onion and synthesis of structurally defined fructan molecules from sucrose. Plant Physiol 117:1507–1513
Vos P, Hogers R, Bleeker M, Reijans M, Vandelee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP—a new technique for DNA fingerprinting. Nucleic Acids Res 22:4407–4414
Wall AD, Corgan JN (1999) Heritability estimates and progeny testing of phenotypic selections for soluble solids content in dehydrator onion. Euphytica 106:7–13
Wehner TC (1999) Vegetable cultivar descriptions for North America; List 25; 1999. Hortscience 34:957–1012
Weir BS (1990) Genetic data analysis: methods for discrete population data, 1st edn. Sinauer Associates, Sunderland
Acknowledgements
This research was funded by the New Zealand Foundation for Research, Science and Technology contracts C02X0203 and C02X0202. We acknowledge the assistance of Martyn Callaghan, Enza Zaden NZ Ltd, for technical advice, Paul McCartney and Winston Fahey for bulb and seed production and Doug Grant (Hybrid Seed Company) for population development.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Langridge
Rights and permissions
About this article
Cite this article
McCallum, J., Clarke, A., Pither-Joyce, M. et al. Genetic mapping of a major gene affecting onion bulb fructan content . Theor Appl Genet 112, 958–967 (2006). https://doi.org/10.1007/s00122-005-0199-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-0199-5