Abstract
The R10 and R11 late blight differentials of Black (tetraploid clones 3681ad1 and 5008ab6) were crossed with the susceptible potato (Solanum tuberosum) cultivar Maris Piper and the progeny were assessed for blight resistance in a whole plant glasshouse test using race 1,2,3,4,6,7 of Phytophthora infestans. The disease scores for the R10 population displayed a continuous distribution whereas the progeny in the R11 population could be categorised as resistant or susceptible. A bulk segregant analysis using amplified fragment length polymorphism assays was done on the ten most resistant and ten most susceptible progeny in each population and two closely linked markers were found to be associated with resistance. R11 mapped to 8.5 cM from marker PAG/MAAG_172.3 and R10 mapped as a quantitative trait locus in which marker PAC/MATC_264.1 explained 56.9% of the variation in disease scores. The results were consistent with R10 and R11 being allelic versions of genes at the R3 locus on chromosome 11. The implications are discussed for mapping R-genes which fail to give complete immunity to a pathogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Late blight disease of potato (Solanum tuberosum), caused by the oomycete pathogen Phytophthora infestans (Mont.) de Bary, first made its impact outside of Mexico in the mid-1840s when severe epidemics swept through North America and Europe and resulted in the Irish potato famine (Large 1940). The need for resistant cultivars was clear and an apparent breakthrough came in 1909 when Salaman recognised the Mexican wild species S. demissum as a source of extreme resistance that could be backcrossed into S. tuberosum (Muller and Black 1951). Breeding for late blight resistance therefore concentrated on using S. demissum’s major dominant R-genes, of which 11 were identified (Muller and Black 1951; Malcolmson and Black 1966; Malcolmson 1969). For example, at the Scottish Plant Breeding Station, cultivar Pentland Ace (with resistance gene R3) resulted from a cross made in 1937 between S. phureja and S. demissum, and was released in 1951 after just three backcrosses to S. tuberosum. The commercially more successful cultivar Pentland Dell (having resistances R1, R2 and R3) required two further backcrosses, and entered ware production in 1963 at a time when race 4 was the prevalent race of P. infestans, but succumbed to blight in 1967. In 1967 and 1968 races overcoming the more recently discovered R-genes (R5–R11) were widely distributed in Great Britain although these genes were not present in common commercial varieties, and it was clear that they would not provide durable resistance either singly or in combination due to the evolution of new races of P. infestans (Malcolmson 1969). As a consequence, many breeders switched to selecting for quantitative field resistance either by using races of P. infestans compatible with the R-genes present in their material or by creating R-gene free germplasm so that screening could be done with any race (Toxopeus 1964; Black 1970; Wastie 1991; Ortiz 2001).
Today there is renewed interest in the use of R-genes for resistance to late blight. Since 1984, new populations of P. infestans have been spreading from Mexico to the rest of the world (Goodwin and Drenth 1997). These new populations are seen as an emerging threat to food security because they comprise both the A1 and A2 mating types, some strains have increased aggressiveness and there is insensitivity to the widely used fungicide metalaxyl (GILB 1999). Breeding for resistance has therefore intensified and new sources of major R-gene resistance have been found in Solanum species other than S. demissum. These include: S. berthaultii (Ewing et al. 2000), S. bulbocastanum (Naess et al. 2000; Park et al. 2003; van der Vossen et al. 2004), S. mochiquense (Smilde et al. 2005) and S. pinnatisectum (Kuhl et al. 2001). Some authors advocate the independent cloning and transfer of these new R-genes into a single cultivar by genetic transformation to produce a multi-line cultivar as a way of achieving durable resistance (Niederhauser et al. 1996; Huang 2005; Smilde et al. 2005). There is also some evidence that defeated R-genes may contribute to late blight resistance and that combining R-genes with high levels of field resistance is therefore a desirable goal (Stewart et al. 2003). Hence there is now great interest in mapping R-genes and quantitative trait loci (QTLs) for field resistance, including those from S. demissum, to see if new R-genes are alleles at a known genetic locus or a new locus and to see if QTLs are also alleles at known R-gene loci or closely linked to them. Of the 11 S. demissum R-genes, R1 has been mapped to chromosome 5 of the 12 potato chromosomes (Leonards-Schippers et al. 1992), R2 to chromosome 4 (Li et al. 1998), R3, R6 and R7 to a complex locus on chromosome 11 (El-Kharbotly et al. 1994; Huang et al. 2004) and R5, R8, R9, R10 and R11 have been shown to be allelic versions of the R3 complex locus by marker analysis and mapping results (Huang 2005). However, R10 and R11 have not been mapped in the original R-gene differentials of Black, namely tetraploid clones 3681ad1 and 5008ab6, which are maintained at SCRI. This paper therefore presents the results of mapping them in these clones, both as quantitative and qualitative traits through their linkage to molecular markers, and demonstrates that R10, unlike R11, behaves more like a QTL than a major dominant R-gene.
Materials and methods
Crosses and plant material
In the year 2000, the R10 and R11 differentials (tetraploid clones 3681ad1 and 5008ab6, respectively) were crossed as female parents with the cultivar Maris Piper which does not carry any R-genes. In 2001, 200 seeds of each cross were sown in a glasshouse and 150 seedlings raised and their tubers harvested to provide up to 150 clones of each cross. These clones were maintained by planting three tubers of each in the glasshouse in 2002 and 2003, and the blight resistance tests were done in 2003 on the 146 clones from each cross with enough tubers. Leaf samples for molecular marker analysis were taken from the maintenance plants during the week beginning 12 May 2003. Between 5 and 10 g of young, fully expanded leaves were harvested from each clone, “flash-frozen” in liquid nitrogen, and stored at −80°C.
Blight isolates
Two isolates were used in the blight tests. Isolate 99/23, race 1,2,3,4,6,7 (isolated in 1999), originated in Scotland, and isolate CP, race 1,3,4,7,10,11 (isolated in 1994) came from Wales. Both isolates were passaged through potato tubers to select for increased aggressiveness before use in the resistance tests. The virulence characteristics of each isolate were verified in a whole plant test using Black’s differential series of R-gene-bearing clones (Black et al. 1953; Malcolmson and Black 1966; Malcolmson 1969).
Blight resistance tests
A whole-plant glasshouse test, similar to that described by Stewart et al. (1983), was used to determine blight resistance. Blight inoculum was prepared as described by Malcolmson (1976), using the zoospore suspension resulting from a sporangial suspension at an initial concentration of 14×103 sporangia/ml. A hand sprayer was used to apply the inoculum and the spray was allowed to fall evenly on the plants from above. For each progeny, two plants of each of the 146 clones, and six of each parent, in flower bud, were inoculated with race 1,2,3,4,6,7 (avirulent against the genes R10 and R11). The plants were dampened, inoculated and incubated for 24 h in either a misted controlled environment cabinet or a misted cooled glasshouse maintained at 15°C and 95–100% humidity, then kept at 15°C in a cooled glasshouse. Seven days after inoculation each plant was scored using Malcolmson’s 1–9 disease scoring scale where 9 is resistant (Cruickshank et al. 1982). Disease on lower leaves was ignored. The apparent presence or absence of the R-gene was noted: plants that were symptomless or had only limited necrotic lesions, or spreading lesions in the oldest leaves only, were assumed to carry the R-gene, whilst plants with spreading lesions on the other leaves were not. In 29 cases where the R-gene status was unclear, and two where it was clear, (31 clones of the R10 progeny), two further plants per clone (and six per parent) were inoculated with the avirulent race, and two plants per clone (six per parent) were inoculated with the virulent race 1,3,4,7,10,11 for comparison. Clones that showed R-gene phenotype (described above) with the avirulent race but spreading lesions with the virulent race were considered to carry the R-gene, whilst clones that developed spreading lesions similarly with both isolates were not. The differences in disease scores between the two races were also taken into account (see Results).
DNA isolation from plants
DNA was extracted from frozen plant tissue using the DNeasy Plant DNA Extraction kit (Qiagen cat.no 69106) according to the manufacturer’s instructions.
Amplified fragment length polymorphism (AFLP) assays
Amplified fragment length polymorphism assays were performed using a modification of the protocol of Vos et al. (1995) as described in Bryan et al. (2002). AFLP fragment nomenclature has been described elsewhere (Bryan et al. 2002). The products of selective AFLP amplification were run on acrylamide gels and the products detected by autoradiography.
Bulked segregant analysis (BSA)
Two bulks (resistant and susceptible) were constructed from the offspring of each of the populations using the blight score data. The resistant bulks consisted of the ten clones with the least disease symptoms (highest scores on 1–9 scale) and the susceptible bulks consisted of the ten clones with the most disease (lowest scores on 1–9). DNA samples, in the form of AFLP secondary template taken from each of the selected progeny clones, were pooled in equimolar amounts to form the bulk samples.
Each of the bulk and parental DNA samples were subjected to AFLP analysis using 65 PstI/MseI AFLP primer combinations. Primer combinations showing at least one AFLP band present in the resistant parent and the resistant pool, and absent from the susceptible parent and the susceptible pool, were selected for application to all of the individuals in the bulk. Primer combinations, which produced a band that was present in all individuals in the resistant bulks but absent from all individuals in the susceptible bulks, were then applied to all individuals within both populations. Parents of a Ultra High Density mapping population, SH and RH, were included in the AFLP analyses to enable putative identification of markers which co-segregated with previously mapped markers (Isidore et al. 2003).
Analyses of the data
Analyses of variance of the disease scores and regressions of clone means on the presence and absence of the molecular markers were done using GENSTAT 5 Release 3 (GENSTAT 5 Committee 1993).
Segregation ratios of the markers and R11 (R10 could not be analysed as a qualitative trait) and the association of the markers with R11 were tested using Pearson’s chi-square statistic. As the markers and R11 segregated in 1:1 ratios, simplex inheritance was inferred and the recombination frequency (r) for a marker and R11 was calculated as: r=(b+c)/n with standard error the square root of r(1−r)/n where b+c is the number of recombinant clones and n is the total number of clones in the R11 population.
Results
Late blight resistance
The analyses of variance for the two populations (R10 and R11) are shown in Tables 1 and 2. For both populations there were statistically significant (P<0.001) differences between clones and most of the variation between clone means (mean of two replicates) was genetical rather than environmental (79.3% for R10 and 89.0% for R11). The histograms of the clone means for the two populations are shown in Figs. 1 and 2. The distribution for R10 is continuous and skewed with its mean (5.90) closer to the maximum value (8.50) than to the minimum value (1.5). In contrast, the distribution for R11 is clearly bimodal with the two peaks coinciding with the blight scores of Maris Piper (the susceptible parent) and the R11 differential (the resistant parent).
Bulked segregant analysis using AFLP markers
Bulks containing the most late blight resistant (R), and susceptible (S), clones from each of the crosses were screened using BSA. The ten resistant clones from the R10 population all had scores of 8.0 or 8.5 and the ten susceptible clones all had scores of 1.5, 2.0 or 2.5. Likewise for the R11 population, the disease scores were 7.5 or 8.0 for the resistant bulk and 1.0 or 1.5 for the susceptible bulk.
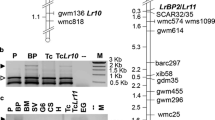
Each of the bulks and parents were subjected to AFLP analysis using 65 PstI/MseI AFLP primer combinations. Nineteen primer combinations showing at least one AFLP band present in the R10 resistant parent and the R10 resistant pool, and absent from the corresponding susceptible parent and the susceptible pool, were selected for application to all of the individuals in the bulks. Likewise, six primer combinations were found and applied to all individuals within the bulks of the R11 population. Two primer combinations, PAC/MATC and PAG/MAAG, produced a fragment (PAC/MATC_264.1 and PAG/MAAG_172.3) which was present in all individuals within the resistant bulks but absent from all individuals within the susceptible bulks of both populations. The results for PAC/MATC_264.1 in the R11 population are shown in Fig. 3 (those for R10 are identical). These bands were also present in SH and absent in RH, the parents of the UHD mapping population, and had been mapped in adjacent bins, SH11B066 (Bin 66) and SH11B065 (Bin 65) (http://www.dpw.wageningen-ur.nl/uhd). These bins are located on the distal end of chromosome 11 and according to Huang et al. (2004) the R3 locus also maps to this location.
Association of bands PAC/MATC_264.1 and PAG/MAAG_172.3 with blight scores
The results of the regressions of blight scores on the presence and absence of the markers are shown in Tables 3 and 4. The regressions were statistically significant (P<0.001) and explained between 48 and 64% of the variation in blight scores. With the R10 population, the average differences in blight scores between the presence and absence of the markers were 2.75 and 2.49 for PAC/MATC_264.1 and PAG/MAAG_172.3, respectively, compared with 5.66 for the difference between the R10 differential and Maris Piper. With the R11 population, the corresponding differences were 3.41 and 3.73 compared with 4.67 for the difference between the R11 differential and Maris Piper. Hence the markers explained more of the variation in the R11 population than in the R10 population.
Classification of clones with respect to R10
The blight scores for the R10 population displayed a continuous distribution, and hence could not be simply classified into resistant and susceptible. Although there was a big difference between the scores of the R10 parent (7.83) and Maris Piper (2.17), many clones had intermediate values with both replicates in agreement. The parents and 31 clones including 29 with uncertain R-gene status were tested with an avirulent and a virulent race to see if their R-gene status could be determined. As expected, Maris Piper was overcome by both the avirulent and virulent races of P. infestans resulting in mean disease scores of 3.67 and 3.50, respectively, with a maximum difference in disease scores of 1.5. The disease scores recorded on the R10 parent were 7.17 when the clone was challenged with the avirulent isolate and 4.14 when challenged with the virulent isolate. As a result of these further tests, 11 clones were categorised as having R10 based on a reduction in blight score of 3.0 or more when tested with the virulent as opposed to the avirulent isolate and seven of these clones had the expected type of lesion. Eleven clones were thought to lack R10 based on a reduction in blight score of 1.5 or less and nine of these clones had the expected lesion type. The presence of R10 in nine clones remained undetermined based on a reduction in blight score of 2.0 or 2.5. Two of these clones had a resistant lesion type, five had a susceptible type and two were intermediate. The mean disease scores of the three groups (R10 present, R10 absent and undetermined) with the avirulent race in the original test were almost identical, namely 6.95, 6.77 and 6.83. It was concluded that the R-gene status of the clones with intermediate disease scores could not be determined with sufficient certainty to test the association of the presence and absence of the markers with the presence and absence of R10. We have therefore treated R10 only as a QTL.
Association of bands PAC/MATC_264.1 and PAG/MAAG_172.3 with R11
In contrast, in the R11 population, the clones scoring 6 and above were clearly resistant and contained R11, and those scoring 3.5 and below were clearly susceptible and lacked R11 (Fig. 2). When the raw data were examined for the 16 clones scoring 4.0, 4.5, 5.0 and 5.5, it was found that for 13 of them one replicate was resistant and the other one was susceptible so that it was not possible to tell whether the clone contained the R-gene or not. Hence these clones were omitted from the analysis of the association of the presence and absence of the markers with the presence and absence of R11. The two clones scoring 5.5 were classed as resistant and the one scoring 4.0 as susceptible. The results of the classification are presented in Table 5. A few clones (7 and 4, respectively) are missing because of lack of marker data. Both the markers and R11 displayed a 1:1 segregation ratio (chi-square test statistic, P>0.05) from which simplex inheritance can be inferred. The tests for linkage were statistically significant (P<0.001) and the estimates of the recombination frequency were r=0.143±0.031 and r=0.085±0.025 for R11 with PAC/MATC_264.1 and PAG/MAAG_172.3, respectively. As the markers were linked with a recombination frequency of r=0.081±0.024, the gene order on the linkage map is PAC/MATC_264.1–PAG/MAAG_172.3–R11.
Discussion
Mapping confirmed that the R10 and R11 genes in Black’s R10 and R11 differentials map to the distal end of chromosome 11 (as defined by the UHD map), as found by Huang (2005) using the Dutch differential set of Mastenbroek which is equivalent to Black’s for R5–R11. Huang (2005) was able to conclude from his marker analysis and mapping results that the R5–R11 differentials all contain allelic versions of genes at the R3 locus. The mapping in this paper was not quite so clear. Two molecular markers on the distal end of chromosome 11 were 8.1 cM apart compared with being in adjacent bins, separated by a single recombination event, in the UHD mapping population (http://www.dpw.wageningen-ur.nl/uhd). R11 mapped to 8.5 cM from PAG/MAAG_172.3 and R10 mapped as a QTL in which PAC/MATC_264.1 explained 56.9% of the variation in disease scores, and 2.75 of the 5.66 difference in blight scores between the R10 differential and cultivar Maris Piper. The apparent lack of precision in chromosomal locations could be due to increased recombination in our tetraploid mapping populations or errors in the blight scores or marker data. Considerable additional resources would be required to eliminate all possible errors in this kind of experiment, and precision in mapping can only be increased by increasing the population size (not the number of replicates) as it depends upon the number of recombinants produced during meiosis. Nevertheless, there was good agreement from mapping R11 as both a qualitative and quantitative trait. The estimated effect of R11 when treated as a QTL (the difference in marker means divided by 1−2r, where r is the recombination frequency between marker and QTL) was 4.49 for marker PAG/MAAG_172.3 compared with 4.67 for the difference in blight scores between the R11 differential and cultivar Maris Piper.
In this work, R10 could only be mapped as a quantitative trait because of the continuous distribution of blight scores in the progeny resulting from the cross between the R10 containing parent and the susceptible cultivar Maris Piper. There was also a lack of clear differences in disease score and lesion appearance when clones with intermediate values were inoculated with compatible (virulent) and incompatible (avirulent) isolates. This result was unexpected but can be related to previous results from SCRI with the R10 and R11 differentials where neither gene gave complete immunity to P. infestans. Stewart (1990) found that the resistances of R10 and R11 to avirulent isolates were not always fully expressed in detached potato leaflets and depended on plant age and inoculum concentration. In her results, resistance was best expressed in R5 in two experiments and R8 in a third experiment. In a further experiment, Stewart and Gourlay (1995) compared the results from glasshouse detached leaflet tests with glasshouse whole plant tests and field tests and found that R5, R8 and R10 were all highly resistant in all of the tests whereas R11 was highly resistant only in the glasshouse whole plant test. Compared with R5 and R8, R10 had a slightly lower field score (7 compared with 8 on a 1 to 9 scale of increasing resistance) and a slightly larger lesion size in the detached leaflet test. Again, Stewart and Bradshaw (2001) found that both R10 and R11 were highly resistant but not immune in whole plant glasshouse tests with avirulent isolates. The results of field experiments using virulent isolates (Stewart and Gourlay 1995; Stewart and Bradshaw 2001) have shown that Black’s R10 differential possesses more background field resistance to P. infestans than R11 and the other differentials tested (R1, R3, R4 and R7). However, in the whole plant glasshouse experiments of Stewart and Bradshaw (2001), the field resistance of R10 was underestimated and that of R11 was overestimated, although the former was still higher than the latter. Interestingly, in whole seedling tests, Malcolmson and Killick (1980) found that out of nine clones and cultivars, the offspring of the R10 differential had the highest average score (general combining ability) for overall blight resistance and small lesion size in the leaf, petiole and stem. The higher field resistance of R10 compared with R11 was also apparent in the experiments of Stewart et al. (2003) which provided evidence of a small contribution to field resistance from defeated R-genes, including similar sized effects from R10 and R11. Where comparisons could be made, the differences in blight scores between virulent and avirulent isolates were smallest for the R10 differential (Stewart and Gourlay 1995; Stewart and Bradshaw 2001). In conclusion, it seems reasonable to attribute the continuous distribution of blight scores in the cross between the R10 differential and cultivar Maris Piper to the background field resistance of the R10 differential.
The contrasting results for R10 and R11 suggest that the differences between major dominant R-genes and QTLs of large effect are not always clear cut. Both R10 and R11 are accepted as major dominant R-genes for blight resistance although their expression is variable. When mapped as QTLs, associated markers explained similar amounts of the phenotypic variation, namely 56.9 and 63.6%, although the distributions of blight scores were continuous and bimodal, respectively. Interestingly, a major QTL for field resistance to late blight has been found on chromosome 4 in cultivar Stirling which explains 37.2% of the variation in blight scores in the progeny of a cross to a susceptible clone (Bradshaw et al. 2004). Stirling also possesses a major dominant R-gene and the molecular mechanisms of R-gene mediated and field resistance are being compared in appropriate crosses, but the genes which are specifically upregulated in the two types of resistance are still to be identified (Avrova et al. 2004).
Isolates of P. infestans capable of overcoming the resistance of R10 and R11 are common throughout the world and hence these resistances are of little value in breeding for durable resistance (Swiezynski et al. 2000). However, the results in this paper, and the previous work done at SCRI and discussed above highlight some of the difficulties in working with these resistance genes which should be avoided in seeking new R-genes for use in breeding. Ideally, R-genes should be sought which give immunity or consistently high levels of resistance in detached leaflets (for speed of screening), whole plants and the field. It is easier to study the genetics of R-gene mediated resistance in a background of low levels of field resistance. However, in breeding for resistance, one wants to combine R-genes with high levels of field resistance in anticipation of breakdown of the R-gene resistance. Molecular markers that are always associated with the R-genes would be extremely useful in achieving this aim. Finally, one needs to remember that the durability of the R-gene resistance will be determined by the relative fitnesses of strains of P. infestans with and without the corresponding virulence genes, so that trying to determine these fitnesses would be worthwhile.
References
Avrova AO, Taleb N, Rokka V-M, Heilbronn J, Campbell E, Hein I, Gilroy EM, Cardle L, Bradshaw JE, Stewart HE, Fakim YJ, Loake G, Birch PRJ (2004) Potato oxysterol binding protein and cathepsin B are rapidly up-regulated in independent defence pathways that distinguish R gene-mediated and field resistances to Phytophthora infestans. Mol Plant Pathol 5:45–56
Black W (1970) The nature and inheritance of field resistance to late blight (Phytophthora infestans) in potatoes. Am Potato J 47:279–288
Black W, Mastenbroek C, Mills WR, Peterson LC (1953) A proposal for an international nomenclature of races of Phytophthora infestans and of genes controlling immunity in Solanum demissum derivatives. Euphytica 2:173–179
Bradshaw JE, Pande B, Bryan GJ, Hackett CA, McLean K, Stewart HE, Waugh R (2004) Interval mapping of quantitative trait loci for resistance to late blight [Phytophthora infestans (Mont.) de Bary], height and maturity in a tetraploid population of potato (Solanum tuberosum subsp. tuberosum). Genetics 168:983–995
Bryan GJ, McLean K, Bradshaw JE, De Jong WS, Phillips M, Castelli L, Waugh R (2002) Mapping QTLs for resistance to the cyst nematode Globodera pallida derived from the wild potato species Solanum vernei. Theor Appl Genet 105:68–77
Cruickshank G, Stewart HE, Wastie RL (1982) An illustrated assessment key for foliage blight of potatoes. Potato Res 25:213–214
El-Kharbotly A, Leonards-Schippers C, Huigen DJ, Jacobsen E, Pereira A, Stiekema WJ, Salamini F, Gebhardt C (1994) Segregation analysis and RFLP mapping of the R1 and R3 alleles conferring race-specific resistance to Phytophthora infestans in progeny of dihaploid potato parents. Mol Gen Genet 242:749–754
Ewing EE, Simko I, Smart CD, Bonierbale MW, Izubuti ESG, May GD, Fry WE (2000) Genetic mapping from field tests of qualitative and quantitative resistance to Phytophthora infestans in a population derived from Solanum tuberosum and Solanum berthaultii. Mol Breed 6:25–36
GENSTAT 5 Committee (1993) GENSTAT 5 Release 3 reference manual. Clarendon Press, Oxford
GILB (1999) Late blight: a threat to global food security. In: Proceedings of the global initiative on late blight conference, March 16–19, 1999, Quito, Ecuador
Goodwin SB, Drenth A (1997) Origin of the A2 mating type of Phytophthora infestans outside Mexico. Phytopathology 87:992–999
Huang S (2005) Discovery and characterization of the major late blight resistance complex in potato. Thesis, Wageningen University
Huang S, Vleeshouwers VGAA, Werij JS, Hutten RCB, Van Eck HJ, Visser RGF, Jacobsen E (2004) The R3 resistance to Phytophthora infestans in potato is conferred by two closely linked R genes with distinct specificities. Mol Plant Microbe Interact 17:428–435
Isidore E, Van Os H, Andrzejewski S, Bakker J, Barrena I et al (2003) Toward a marker-dense meiotic map of the potato genome: lessons from linkage group 1. Genetics 165:2107–2116
Kuhl JC, Hanneman RE, Havey MJ (2001) Characterization and mapping of Rpi1, a late blight resistant locus from diploid (1EBN) Mexican Solanum pinnatisectum. Mol Genet Genomics 265:977–985
Large EC (1940) Advance of the fungi. Jonathan Cape, London
Leonards-Schippers C, Gieffers W, Salamini F, Gebhardt C (1992) The R1 gene conferring race-specific resistance to Phytophthora infestans in potato is located on potato chromosome V. Mol Gen Genet 233:278–283
Li X, van Eck HJ, Rouppe van der Voort JNAM, Huigen DJ, Stam P, Jacobsen E (1998) Autotetraploids and genetic mapping using common AFLP markers: the R2 allele conferring resistance to Phytophthora infestans mapped on potato chromosome 4. Theor Appl Genet 96:1121–1128
Malcolmson JF (1969) Races of Phytophthora infestans occurring in Great Britain. Trans Br Mycol Soc 53:417–423
Malcolmson JF (1976) Assessment of field resistance to blight (Phytophthora infestans) in potatoes. Trans Br Mycol Soc 67:321–325
Malcolmson JF, Black W (1966) New R genes in Solanum demissum Lindl. and their complementary races of Phytophthora infestans (Mont.) de Bary. Euphytica 15:199–203
Malcolmson JF, Killick RJ (1980) The breeding values of potato parents for field resistance to late blight measured by whole seedlings. Euphytica 29:489–495
Muller KO, Black W (1951) Potato breeding for resistance to blight and virus diseases during the last hundred years. Z Pflanzenzuchtg 31:305–318
Naess SK, Bradeen JM, Wielgus SM, Haberlach GT, McGrath JM, Helgeson JP (2000) Resistance to late blight in Solanum bulbocastanum is mapped to chromosome 8. Theor Appl Genet 101:697–704
Niederhauser JS, Alvarez-Luna E, Mackenzie DR (1996) RETONA, a new strategy in the control of potato late blight. Am Potato J 73:225–229
Ortiz R (2001) The state of the use of potato genetic diversity. In: Cooper HD, Spillane C, Hodgkin T (eds) Broadening the genetic base of crop production. CABI Publishing, Wallingford, pp 181–200
Park T-H, Vleeshouwers VGAA, van der Vossen E, Visser RF, van Eck HJ (2003) A novel resistance gene against Phytophthora infestans derived from Solanum bulbocastanum. Poster S10-S30 In: 7th International congress on plant molecular biology, ISPMP, Barcelona
Smilde WD, Brigneti G, Jagger L, Perkins S, Jones JDG (2005) Solanum mochiquense chromosome IX carries a novel late blight resistance gene Rpi-moc1. Theor Appl Genet 110:252–258
Stewart HE (1990) Effect of plant age and inoculum concentration on expression of major gene resistance to Phytophthora infestans in detached potato leaflets. Mycol Res 94:823–826
Stewart HE, Bradshaw JE (2001) Assessment of the field resistance of potato genotypes with major gene resistance to late blight (Phytophthora infestans (Mont.) de Bary) using inoculum comprised of two complementary races of the fungus. Potato Res 44:41–52
Stewart HE, Gourlay F (1995) Recognising race-specific resistance to Phytophthora infestans. In: Dowley LJ, Bannon E, Cooke LR, Keane T, O’Sullivan E (eds) Phytophthora infestans 150. Boole Press, Dublin, pp 255–260
Stewart HE, Flavelle PH, McCalmont DC, Wastie RL (1983) Correlation between glasshouse and field tests for resistance to foliage blight caused by Phytophthora infestans. Potato Res 26:41–48
Stewart HE, Bradshaw JE, Pande B (2003) The effect of the presence of R-genes for resistance to late blight (Phytophthora infestans) of potato (Solanum tuberosum) on the underlying level of field resistance. Plant Pathol 52:193–198
Swiezynski KM, Domanski L, Zarzycka H, Zimnoch-Guzowska E (2000) The reaction of potato differentials to Phytophthora infestans isolates collected in nature. Plant Breed 119:119–126
Toxopeus HJ (1964) Treasure-digging for blight resistance in potatoes. Euphytica 13:206–222
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Vossen E van der, Muskens M, Sikkema A, Gros J, Wouters D, Wolters P, Pereira A, Allefs S (2004) The Rpi-blb2 gene from Solanum bulbocastanum is allelic to the Mi-1 gene from tomato and confers broad-spectrum resistance to Phytophthora infestans both in cultivated potato and tomato. Lecture abstract L28. In: 1st Solanaceae genome workshop 2004, 19–21 September 2004, Wageningen
Wastie RL (1991) Breeding for resistance. Adv Plant Pathol 7:193–224
Acknowledgements
Thanks are due to Hayley Mathews, Moira Myles, Louise Sullivan and Geoff Swan for technical assistance, Helen Stewart for helpful discussions and the Scottish Executive Environment and Rural Affairs Department for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Wenzel
Rights and permissions
About this article
Cite this article
Bradshaw, J.E., Bryan, G.J., Lees, A.K. et al. Mapping the R10 and R11 genes for resistance to late blight (Phytophthora infestans) present in the potato (Solanum tuberosum) R-gene differentials of Black. Theor Appl Genet 112, 744–751 (2006). https://doi.org/10.1007/s00122-005-0179-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-0179-9