Abstract
Seed and seedling traits related to germination and stand establishment are important in the production of cultivated lettuce (Lactuca sativa L.). Six seed and seedling traits segregating in a L. sativa cv. Salinas x L. serriola recombinant inbred line population consisting of 103 F8 families revealed a total of 17 significant quantitative trait loci (QTL) resulting from three seed production environments. Significant QTL were identified for germination in darkness, germination at 25 and 35°C, median maximum temperature of germination, hypocotyl length at 72 h post-imbibition, and plant (seedling) quality. Some QTL for germination and early seedling growth characteristics were co-located, suggestive of pleiotropic loci regulating these traits. A single QTL (Htg6.1) described 25 and 23% of the total phenotypic variation for high temperature germination in California- and Netherlands-grown populations, respectively, and was significant between 33 and 37°C. Additionally, Htg6.1 showed significant epistatic interactions with other Htg QTL and a consistent effect across all the three seed production environments. L. serriola alleles increased germination at these QTL. The estimate of narrow-sense heritability (h2) of Htg6.1 was 0.84, indicating potential for L. serriola as a source of germination thermotolerance for lettuce introgression programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of molecular maps using DNA markers has enabled quantitative trait loci (QTL) for morphological and physiological traits to be identified in cultivated plant species. The degree of dormancy in seed populations can be problematic; too little or too much can result in an economic loss or a delay in the use of the seed lot (Gale et al. 2002). Accordingly, a number of QTL studies have focused specifically on seed dormancy and germination, including those in Arabidopsis thaliana L. (van der Schaar et al. 1997; Alonso-Blanco et al. 2003; Clerkx et al. 2004), tomato (Lycopersicon esculentum Mill.) (Foolad et al. 1999), wheat (Triticum aestivum L.) (Anderson et al. 1993; Kato et al. 2001), wild oat (Avena fatua L.) (Fennimore et al. 1999), barley (Hordeum vulgare L.) and rice (Foley 2002). Alonso-Blanco et al. (2003) identified seven loci affecting the after-ripening requirement to alleviate seed dormancy in a cross between dormant and non-dormant accessions of Arabidopsis. A major and two minor QTL controlling approximately 80% of phenotypic variation in seed dormancy of wheat have also been identified (Kato et al. 2001).
Inhibition of lettuce (Lactuca sativa L.) seed (achene) germination by high temperature (termed thermoinhibition) can lead to poor field emergence and stand establishment (Valdes et al. 1985), and reduced yield (Cantliffe et al. 1981). The maximum germination temperature is affected by cultivar (Gray 1975; Thompson et al. 1979), production environment (Reynolds 1973; Sung et al. 1998), light (Fielding et al. 1992; Toyomasu et al. 1998; Roth-Bejerano et al. 1999), and hormones such as ethylene, gibberellic acid (GA) and abscisic acid (ABA) (Yoshioka et al. 1998; Nascimento et al. 2000; Gonai et al; 2004). In general, exposure of lettuce seeds to light, ethylene, and GA tends to increase the temperature at which thermoinhibition occurs, while darkness and ABA lower the inhibitory temperature (Cantliffe et al. 2000). Similar responses of germination to temperature, light, and hormones have also been documented in L. serriola, the progenitor species of cultivated lettuce (Marks and Prince 1982; Small and Gutterman 1992).
Because many crops are propagated by seeds, and seeds often represent a significant investment as the delivery system for improved germplasm, a number of QTL analyses have focused specifically on seed traits associated with their role as propagules. Doganlar et al. (2000) summarized several studies that describe 24 QTL responsible for seed weight variation in tomato. Seed weight and oil content QTL have also been reported in sunflower (Helianthus annuus L.) and soybean (Glycine max [L.] Merr.) (Csanadi et al. 2001; Bert et al. 2003; Gandhi et al. 2005). QTL associated with seed and seedling vigour were identified in cabbage (Brassica oleracea L.) (Bettey et al. 2000), tomato (Foolad et al. 1999) and rice (Oryza sativa L.) (Redoña and Mackill 1998; Cui et al. 2002).
The pronounced germination/dormancy phenotype at high temperatures as well as the availability of genotyped recombinant inbred lines (RILs) derived from an interspecific L. sativa x L. serriola cross (Johnson et al. 2000) make lettuce an attractive candidate for QTL analysis of seed and seedling traits. The present study identified 17 QTL associated with seed and seedling traits segregating in this population. This QTL analysis provides a new approach for elucidating the physiological factors controlling the imposition and release of lettuce seed thermoinhibition.
Materials and Methods
Seed production environments
Replicate samples of seed of an existing RIL mapping population comprised of 103 F8 families derived by single-seed descent from a L. sativa cv. Salinas x L. serriola (UC96US23) F2 population (Johnson et al. 2000) were produced at three separate locations. In the summer of 2002 at Davis, California, the RILs were transplanted to the field in rows of 10 to 15 plants per genotype. Plants were irrigated by sprinklers after transplanting and thereafter by furrow irrigation as no rain fell during growth and seed production. Average daily minimum and maximum temperatures from the first to last seed harvest (79 days) were 12 and 32°C, respectively. At 50% flowering, inflorescences from 5 to 10 plants of each family were covered with air-permeable plastic-mesh pollination bags (Vilutis and Co., Inc., Frankfort, IL) and allowed to mature for 14–21 days. Seed of the earliest flowering lines was harvested beginning 30 July and harvesting continued thereafter as RILs matured until 16 October. Following harvest, seeds were bulked together for each RIL, cleaned, and stored in canvas bags at 10°C and 50% relative humidity (RH) until moisture content (MC) equilibration at 6–8%, at which time they were transferred to plastic bottles and stored at 5°C.
The same RIL population was also grown at the University of Arizona Research Center in Yuma. Plants were transplanted to the field on 22 October 2001. Seeds were hand-harvested beginning 5 March 2002 and continuing until 20 July 2002. Average daily minimum and maximum temperatures from the first to last seed harvest (∼130 days) were 11 and 29°C, respectively. Some plants were harvested for seed up to three times; seeds from the final harvest, in which all RILs were represented, were used for phenotypic analysis. Following harvest, seeds were kept at room temperature in paper bags from 1–14 days, transferred to sealed plastic bottles, and held at 5°C until evaluated.
The RIL population was grown a third time in The Netherlands in 2002 by the seed company Rijk Zwaan Zaadteelt en Zaadhandel B.V. (De Lier, The Netherlands). Seed production was performed in a glasshouse with temperatures ranging between 15 and 35°C during the day and between 10 and 15°C at night. Seeds were sown in April, transplanted to a greenhouse in May, and harvested in August–September, according to maturity.
Accumulated degree days (DD) (single sine method and horizontal cutoff; http://www.ipm.ucdavis.edu) from flowering to harvest in the Davis and Yuma seed production environments were calculated to assess possible effects of temperature during seed development and maturation on germination characteristics. DD were used to integrate the heat units accumulated during the seed development period. DD data were not available for Netherlands-grown seed.
Map construction and genotyping
Plant material was prepared and genotyped as described previously (Kesseli et al. 1994; Johnson et al. 2000). A genetic linkage map based on the F7 RIL population comprising over 1,700 AFLP, SSR, and EST markers consisting of nine chromosomal linkage groups and spanning 1,254 cM was constructed using JOINMAP v2.0 (Stam 1993; http://cgpdb.ucdavis.edu/database/genome_viewer/viewer/; M. Truco, unpublished). A subset of 224 robust markers approximately 10–15 cM apart were chosen as the framework map for QTL analysis of the F7 generation (http://cgpdb.ucdavis.edu/database/supplemental_data).
Phenotypic analyses
Germination assays
Tests for germination at 25 (low-temperature germination, LTG) and 35°C (high-temperature germination, HTG) were performed. A temperature of 25°C is not “low” with respect to germination per se, as the base temperature for lettuce seed germination is near 0°C. However, it is at the lower end of the temperature range at which thermoinhibition occurs and therefore is termed LTG here. LTG and HTG tests were conducted on three replicates of 25 seeds each from all three production environments. Seeds were distributed on two absorbent blotter paper discs (VWR Scientific Products, West Chester, PA) saturated with 5 ml of de-ionized water in 5 cm-diameter Petri dishes (Gelman Sciences, Ann Arbor, MI). Seeds were placed into a germination cabinet (Hoffman Manufacturing, Albany, OR) under continuous fluorescent light and constant temperature conditions and evaluated for germination (radicle emergence) at 72 h.
Germination in light-requiring lettuce seeds is controlled by photoreversible phytochromes, the active form of which (Pfr) results in synthesis of gibberellins that stimulate germination (Toyomasu et al 1998). To test for light requirements for germination, assays for germination in darkness after exposure to far-red light (GFR) were performed on four replicates of 50 seeds produced in the Davis and Yuma environments. Exposing the seeds to far-red light should drive all phytochrome into the non-active Pr form. Therefore, germination after exposure to far-red light (GFR) assesses the extent to which germination is under the control of photoreversible (low-fluence response) phytochrome. The very low fluence response (VLFR) is controlled by phytochrome A, is irreversible, and can be triggered by far-red light (Botto et al. 1996). The VLFR could also contribute to germination following far-red light exposure. Seeds were exposed to far-red light (intensity 22.5 mW/nm) during imbibition for 24 h at 20°C, and then incubated in the dark at the same temperature and evaluated for radicle emergence beginning 24 h after transfer to the dark and every 24 h thereafter for a total of 7 days.
To further characterize the temperature response, two experiments were performed on a thermogradient table with subsets of the Davis RIL population to determine the median maximum temperature of germination (T50), or the temperature at which germination was reduced to 50% in a given RIL. The first subset was composed of 57 families tested at 2°C intervals from 27 to 33°C and the second subset was composed of 35 families tested from 33 to 39°C. The temperature range over which to test RIL families was based on their germination percentages in the 35°C test. Germination protocols were as described above, except with two replicates of 25 seeds per RIL.
Seedling growth
Lengths of radicles (RDL) and hypocotyls (HYL) of 3-day old seedlings were evaluated using OSUSVIS software as detailed in Sako et al. (2001). Two replicates of 50 seeds of each family were placed onto water-saturated blue blotter paper (Anchor Paper Co., St. Paul, MN) on Plexiglass slant boards. Slant boards were held at a 10° angle from vertically in sealed plastic bags at 100% RH and 20°C and exposed to 24 h of continuous fluorescent light followed by 48 h of darkness. Slant boards were then placed under an inverted flatbed scanner (UMax Data Systems, Inc., Dallas, Texas) and digital images were captured using VistaScan v3.77 software (Umax). Images were imported into OSUSVIS for measurements of hypocotyl and radicle lengths of individual seedlings.
Plant quality (PQ)
Plant quality assessments were performed by Rijk Zwaan on seeds produced in the Davis and Netherlands environments. Seeds (150) of each RIL family were sown on peat soil in trays. Trays were held in a climate room at 15°C for 72 h to ensure optimal germination. Subsequently, trays were placed in a greenhouse at 15°C under natural light (8 h/day). PQ was evaluated visually 7 days after sowing by counting the number of seedlings that had acceptable commercial quality, i.e., seedlings of adequate size and uniformity, lacking aberrations or deformed leaves, and which experience indicates will develop into marketable plants.
Statistical analyses
Mean percent germination was transformed by the probit method (PROC PROBIT, SAS Institute, Inc., Cary, NC) to normalize variances in germination percentage and data distribution. Data for seedling trait assays were not transformed. Transformed germination data were used to detect QTL with Windows QTL Cartographer V. 2.0 (Basten et al. 2001). QTL analyses were performed first using composite interval mapping (CIM) and subsequently using multilocus mixed model ANOVA (PROC MIXED). QTL and additive effects were identified by CIM utilizing standard model 6 with the following options: (1) five markers were chosen to control for genetic background at testing positions; (2) forward and backward regression method was selected with a window size of 5 cM flanking the test site; (3) a 2 cM walking speed along chromosomes was used. QTL were identified initially at a LOD threshold of 2.5+. Subsequently, QTL were verified by 1,000 permutations of phenotypic data and declared significant (p = 0.05) above the permutated LOD threshold value. Untransformed data were used to estimate additive effects of QTL for germination traits from CIM results and to estimate additive effects of QTL for all other seed and seedling traits.
Multilocus QTL analysis was performed to identify intralocus and interlocus effects of QTL for germination traits within and across environments, and to further define significant single effects and interactions of QTL found to be nonsignificant in CIM (below permutated threshold values, but LOD ≥ 2.5+). Homozygous RIL genotypes at the AFLP marker loci that maximized LOD scores for QTLs in CIM analysis were used as an independent variable in a mixed linear model analysis (SAS PROC MIXED) according to the method of Gandhi et al. (2005). Significant epistatic effects were identified for up to 23 factorial models where location, rep and genotype x location were random and genotype effects were fixed. The magnitude of Type III multilocus additive interactions were identified using the ESTIMATE statements in PROC MIXED. Least squares means, and Type III sums of squares and F-tests were estimated for genotypes. Linear contrasts among least square means were used to calculate intralocus and interlocus effects among loci as in Gandhi et al. (2005). An analysis of covariance (ANCOVA) was conducted for germination (HTG, LTG, and GFR) and production environment (DD from flowering to harvest) to determine whether temperature during seed development was a significant source of variation for germination. When DD was detected as a significant covariate, mean germination values were estimated by least squares means, and adjusted according to the mean of DD.
RIL-mean narrow sense heritabilities were estimated for germination at 25 and 35°C in light and in darkness at 20°C following far-red light exposure. Heritabilities were estimated by
where \(\sigma^{2} _{\rm g}\) is the between-RIL variance component, \(\sigma^{2} _{\rm p}\) is the phenotypic variance component, \(\sigma^{2} _{\rm e}\) is the residual variance component, \(\sigma^{2} _{\rm gl}\) is the between-RIL x location variance component, r is the number of replications, and l is the number of locations. The linear model was completely random and restricted maximum likelihood method (REML) estimates of the variance components were found using SAS PROC VARCOMP. The between-RIL variance component is equal to twice the additive genetic variance when epistasis is absent; hence, h 2 is a narrow-sense heritability estimator (Bernardo 2002). Phenotypic correlations for measured traits were calculated using SAS PROC CORR.
Results
Germination phenotypes
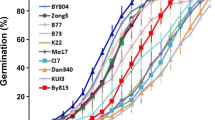
Temperature and light conditions during seed imbibition had large effects on distributions of phenotypic variation for germination traits of the RIL populations produced in three different environments (Davis, Yuma, and The Netherlands) (Fig. 1–). Seeds of the parental lines were produced only in the Davis environment. Seed from RILs grown in the Davis environment germinated well at 25°C (LTG) with 78 families germinating >90% and no RILs germinating <60% (Fig. 1). Parental germination means were 96 and 99% for L. serriola and cv. Salinas, respectively, and population mean germination was 92.5%. Yuma-grown seed exhibited a more uniform phenotypic distribution and lower mean population germination value at 25°C (60.1%) (Fig. 1). Sixty lines grown in Yuma had <70% germination in contrast to only one line grown in Davis.
Frequency distributions of germination percentages at 25°C (LTG), 35°C (HTG), and germination in darkness following 24-h exposure to far-red light (GFR) for seed from bulked samples of 6–8 plants each of RILs grown in Davis (A, C, and E), Yuma (B, D, and F) and The Netherlands (E). Filled circle (filled circle) represents trait value of the Salinas parent and inverted triangle (filled inverted triangle) is the trait value of the L. serriola parent. Parental data were available only for Davis-grown seed
A 10°C increase in temperature from 25 to 35°C (HTG) for seed from the Davis environment completely reversed the population distribution, with 49 lines (54%) germinating <10% due to thermoinhibition (Fig. 1). The phenotypic distribution of the RIL population could be characterized as bi-modal, with two peaks associated with widely dispersed parental means, 2% germination for cv. Salinas and 95% for L. serriola at 35°C. Most notably, 30 RIL families had germination values ≥70% at 35°C, far in excess of the population mean of 32.6%. The Yuma-grown seed also exhibited a skewed population distribution toward thermoinhibition, though not as extreme as observed in the Davis environment, with 28 of 78 families having <10% germination (Fig. 1). The bi-modal trend for HTG apparent in the Davis-grown population was absent in the Yuma-grown population and mean germination percentage (26.3%) was significantly lower in the latter. The phenotypic distribution for HTG from the Netherlands-grown population (Fig. 1) strongly resembled that of the Davis-grown population, being bimodal with 28 RILs germinating >70% and 43 lines germinating <10%. Mean germination percentages (33.3%) for the Netherlands- and Davis-grown populations did not differ significantly.
Histograms for germination after exposure to far-red light (GFR) showed ranges of 0–100% and 0–80% for seeds grown in the Davis and Yuma environments, respectively (Fig. 1). Distributions were highly skewed toward low germination percentages, with over half of the population in each environment germinating <10%. Mean germination of the Davis-grown RIL population was significantly higher (20.2%) compared to the Yuma-grown population (12.2%). When grown in the Davis environment, seeds of the Salinas parent germinated significantly better under these conditions (65%) compared to the L. serriola parent (1.2%), with very little transgressive segregation outside these parental means.
Following initial testing at 25 and 35°C, RILs produced in Davis were tested over two temperature ranges (27–33°C and 33–39°C in 2°C increments) to further characterize germination response to increasing temperature (Fig. 2, left column). The parental lines used to generate the mapping population exhibited large differences in their ability to germinate at high temperature. The Salinas parent germinated near 100% at 27°C, but germination declined to 43% at 29°C and was 0 at ≥31°C. Germination remained above 94% in L. serriola at temperatures up to 37°C before declining to 60% at 39°C and 0 at ∼40°C. Some transgressive segregation was present at the two lowest (27 and 29°C) and the highest (39°C) germination temperatures. Incremental increases in temperature (2°C) gradually shifted the population distributions from germinative to thermoinhibited, with mean germination of 94.6% (72 families germinating ≥90%) at 27°C declining to 12% (50 lines ≤10% germination) at 39°C. The bi-modal distribution previously observed at 35°C in the Davis- and Netherlands-grown populations (Fig. 1) was clearly evident at 31°C and more pronounced at 33°C, where 25 lines retained germination ≥90% and 40 lines were ≤10% (Fig. 2). These two categories coincided with the L. serriola and Salinas means, respectively, and defined 78% of the population at 33°C; the remaining families were classified as intermediate germination categories. The bi-modal distribution was not present at 39°C.
Frequency distributions of germination percentages for Davis-grown RILs over a 12°C temperature interval (left column of panels) and corresponding QTL LOD score profiles with permutated significance threshold (dashed lines) for Htg6.1 at the same temperatures (right column of panels). Filled circle (filled circle) represents trait value of the Salinas parent and inverted triangle (filled inverted triangle) is the value of the L. serriola parent
Frequency distributions of median germination inhibitory temperature (T50) values for all Davis-grown RILs (solid bars) and number of lines homozygous at L. serriola Htg6.1-linked AFLP marker 1A12-330<N> within each category (hatched bars). Open circle (open circle) represents trait value of the Salinas parent and inverted triangle (open inverted triangle) is the trait value of the L. serriola parent
The T50 values also demonstrated a bi-modal phenotypic distribution, with 31 families having T50 values between 29–31°C near the Salinas mean (29.2°C) and a second large group (23 RILs) having values between 37–41°C located nearer the L. serriola mean (39.8°C) (Fig. 3).
Phenotypic correlations among all measured traits were calculated for families within the RIL mapping population (Table 1). As expected, highly significant correlations were observed between T50 and HTG for the Davis-grown seeds. HTG values were also highly correlated among seeds produced in Davis, Yuma, and The Netherlands, indicating that RIL germination rankings at 35°C were conserved across production environments. While the correlation for LTG between Davis- and Yuma-grown populations was not significant, a significant correlation was observed for GFR between the Davis and Yuma environments (r=0.49, P<0.001). There was also a highly significant (P<0.01) correlation between LTG and HTG for Yuma-grown seed.
Significant genotypic (G), environmental (E), and G × E effects were observed for germination traits LTG, HTG, and GFR, with the exception of a nonsignificant environmental effect at high temperature. Heritabilities were 0.30, 0.84, and 0.74 for LTG, HTG, and GFR respectively.
Seedling traits
Phenotypic distributions for plant quality revealed differences in seed lots produced in Davis and The Netherlands. Davis-grown RILs ranged from 0–100% quality seedlings, and showed an even distribution across categories with a population mean of 50.4% (Fig. 4). In contrast, RILs grown in The Netherlands showed a highly skewed distribution, with 50 RILs having high quality (90–100%) and a population mean of 80.2% (Fig. 4).
Frequency distributions of plant quality (PQ) and hypocotyl length (HYL) of RILs produced in Davis and The Netherlands. Filled circle (filled circle) represents mean trait value of the Salinas parent and inverted triangle (filled inverted triangle) is the mean value of the L. serriola parent. No parental data were available for panels a and b
The hypocotyl length (HYL) attained after 72 h of imbibition in Davis-grown seed results from both the speed of germination and the seedling growth rate (Fig. 4). HYL varied by less than 1 mm between the parental lines. Among RILs, HYL ranged between 4 and 12 mm with a mean of 6.71 mm. Population mean values exceeded parental mean values by a large degree, indicating transgressive segregation.
QTL analyses
Composite interval mapping and multilocus QTL analysis revealed a total of 12 significant QTL for six traits measured using seed produced in Davis (Table 2). Individual QTL explained from 9 to 25% of the phenotypic variation of a given trait and were found on six different linkage groups (LG) (Fig. 5). Fewer phenotypic analyses were conducted on the seeds produced in Yuma and The Netherlands. However, three additional QTL for LTG were identified using the Yuma-grown seeds, one of which was coincident with a QTL identified using the Davis-grown seeds (Ltg7.1) (Table 2). A QTL for HTG (Htg6.1) was identified in both the Davis- and Netherlands-grown seeds and two additional QTL for PQ were also significant for the Netherlands-grown seeds (Table 2).
Genomic locations of QTL for seed and seedling traits detected using seeds grown in three environments. Numbers indicate locations (in cM) of genetic markers used in CIM analyses. Solid bars = Davis; diagonal lined bars = Netherlands; vertical and horizontal lined bars = Yuma. Lengths of bars denote 1-LOD confidence intervals.
Germination QTL
Germination-related QTL were detected using seed produced in multiple environments and were associated with more than one trait. CIM and multilocus QTL analysis of HTG using Davis-grown seed revealed Htg4.1,Htg6.1, and Htg7.1, collectively accounting for 37% of total phenotypic variation among RILs (Table 2). Htg6.1 alone explained 25% of the phenotypic variance for germination at 35°C, with the QTL allele being transmitted by the L. serriola parent. The appearance of Htg6.1 in Davis-grown seed was evident at 31°C and was highly significant over a 5°C interval (33–37°C) that peaked at 35°C (LOD 7.8) before declining and becoming non-significant at 39°C (Fig. 2, right column). When genotypes of RILs were examined at the Htg6.1-linked AFLP marker 1A12–330<N> and compared to T50values, all (22 of 22) of the lines with a T50 threshold above 37°C possessed the L. serriola allele (Fig. 3). Htg6.1 was detected in seeds produced in The Netherlands environment (R 2=0.23) within the same 1 LOD confidence interval, with the QTL allele of the same parental origin (Table 2; Fig. 5). The multilocus QTL analysis revealed significant epistatic interactions among Htg loci (Table 2). L. serriola alleles at Htg6.1 and Htg7.1 synergistically interacted to increase germination (58.1% compared to 65.1% in single and double homozygotes, respectively). Furthermore, the effect of Htg6.1 was significant when data for all three seed production environments were combined (P=0.03) and resulted in a 43% increase in germination (data not shown).
Two QTL for LTG were detected by CIM using seeds produced in Davis that together explained 21% of the phenotypic variation (Table 2). The alleles from L. serriola at Ltg2.1 and Ltg7.1 increased germination. Three QTL for LTG were detected using seed produced in Yuma, collectively explaining 39% of the phenotypic variation. Ltg7.1 was detected within the same 1 LOD genomic interval as Htg7.1, the QTL-linked locus being of the same parental origin and describing 13% of the phenotypic variation in the Yuma-grown seeds.
Three QTL for T50 values were identified among Davis-grown RILs, two of which, T506.1 and T507.1, collocated within previously identified 1 LOD QTL support intervals at Htg6.1 and Ltg7.1 (Table 2). Collectively, these QTL explained 28% of phenotypic variation for T50 values. RILs homozygous for the L. serriola allele at a T506.1-linked marker germinated at a median temperature of 36°C compared to 31°C for RILs homozygous for the Salinas allele at this marker.
Two QTL for GFR explaining 16% of total phenotypic variation were found using Davis-grown seeds (Table 2). The Gfr4.1-linked allele was from Salinas and the Gfr6.1-linked allele was from the L. serriola parent. Gfr6.1 co-located to the same 1-LOD confidence interval as Htg6.1 (Fig. 5); both Gfr6.1 and Htg6.1 alleles were transmitted from the L. serriola parent. A single small-effect QTL for GFR (Gfr6.2) was detected in Yuma-grown seed, accounting for 8% of the phenotypic variation.
To assess possible effects of temperature during seed maturation on seed germination behavior, DD from flowering to harvest were determined for RILs grown in the Davis environment. For the Yuma-grown seeds, since the seeds used represented different harvests among different RILs, the DD for 30 days prior to the final harvest were accumulated. An analysis of covariance for transformed HTG and LTG values and the combined Davis and Yuma production environments indicated that DD was not a significant source of variation for germination (P=0.23 and 0.07 for HTG and LTG, respectively). Degree days were a significant source of variation for GFR (P=0.02), but differences between adjusted and unadjusted mean germination values were very small and unlikely to affect QTL analyses. Insufficient degrees of freedom were available to test for DD by RIL interactions.
Seedling trait QTL
Two QTL for PQ were identified in the Netherlands-grown population and one in the Davis-grown population. For seed produced in The Netherlands, PQ QTL were located on LG2 and LG5, explaining 13 and 19% of the phenotypic variation, respectively. The PQ QTL for Davis-grown seed was located on LG2, explaining 12% of the phenotypic variation. QTL alleles that increased PQ originated from both Salinas and L. serriola. A single QTL for HYL was detected on LG5, explaining 13% of the phenotypic variation. Hyl5.1 and Pq5.2 co-located to the same 1 LOD genomic interval on LG5, but were of opposite effect, with Salinas alleles increasing HYL, and L. serriola alleles increasing PQ.
Discussion
Seed-related traits such as germination and seedling vigor are of considerable economic importance in lettuce. In particular, failure to germinate at warm temperatures (thermoinhibition) can result in reduced or delayed seedling emergence, poor crop stand establishment, and lower yield (Cantliffe et al. 1981; Valdes et al. 1985). Ten QTL associated with germination temperature were identified in the Salinas x L. serriola RIL population grown in three environments. The sensitivity of germination to temperature in L. sativa is known to be influenced by the temperature at which the seeds developed (Sung et al. 1998). Thus, it is not surprising that not all QTL were detected in seeds produced in all three environments. However, while the environment could contribute to the expression of seed thermoinhibition or light requirements for germination, covariate analysis indicated that temperature (DD) during seed maturation was not a major factor in the QTL analysis. Some QTL, particularly Htg6.1, showed strong effects on germination in two environments in the CIM analysis, accounting for 25 and 23% of the phenotypic variation for germination at 35°C in Davis- and Netherlands-grown seed, respectively (Table 2).
In many cases, loss of dormancy and a relaxation of environmental controls on germination are associated with domestication. However, in the present case, germination sensitivity to temperature was greater in the cultivated cv. Salinas (T50=30°C) than in the wild L. serriola accession (T50=39°C). The T50 for germination of this L. serriola accession is significantly higher than previously reported thermoinhibitory temperatures of 25–27°C for this species (Marks and Prince 1982; Small and Gutterman 1992). However, the high T50 and high germination percentage of L. serriola seeds is consistent with previous reports that seeds of this species lack primary dormancy (Weaver and Downs 2003)
Thermotolerance of lettuce seed germination is heritable and has been improved by selection in high-temperature environments (Guzman et al. 1992; Sung et al. 1998). L. serriola alleles promoted germination at high temperatures, and a small group of RIL families possessing the L. serriola allele at Htg6.1 consistently exhibited the ability to germinate at high temperature (Fig. 3). As thermotolerance was highly heritable (h2=0.84), Htg alleles originating from L. serriola could be utilized for introgression of this trait into cultivated lettuce.
Heritabilities for GFR and LTG were lower compared to HTG due to large environmental variances, especially for LTG. Significant differences were apparent in phenotypic distributions for LTG and HTG in Davis- and Yuma-grown seeds (Fig. 1). Differences could be due to diurnal temperature regimes, to variations in harvests from the different RILs due to the spread of flowering times or to post-harvest handling of the seeds. Notably, the phenotypic correlation between HTG and LTG was not significant for seeds grown in Davis but was highly significant for Yuma-grown seed. At 25°C, Yuma-grown seed also had significantly lower germination in comparison to Davis-grown seed, indicating that some members of the Yuma-grown population were thermoinhibited at this temperature. Consequently, the collocation of Ltg7.1 detected using both the Yuma- and Davis-grown populations with the Htg7.1 interval detected using Davis- and Netherlands-grown seed and the fact that the favorable alleles for both Ltg7.1 and Htg7.1 are derived from the L. serriola parent could indicate that Ltg7.1 is actually a Htg QTL detected at a lower germination temperature. Collocation of these QTL could be suggestive of a common site that is responsive to environmental factors and determines the maximum germination temperature in lettuce.
The multilocus QTL model-building methodology described in Gandhi et al. (2005) utilizes DNA markers as independent variables in a mixed model analysis of variance. The analysis takes advantage of raw data and greater degrees of freedom to increase statistical power beyond that available in CIM and can describe epistatic interactions and stability of QTL linked to DNA markers having significant intralocus effects across locations or years. The effect of the Htg6.1 locus on germination of lettuce seed at high temperature was significant using germination data for seed from three production locations, suggesting that it is stable across environments. This analysis also resulted in the detection of a significant 2-way epistatic interactions for Htg loci that functioned to increase germination in the Davis seed production environment, illustrating additional sources of variation for HTG that would likely be undetected using CIM. Specifically, L. serriola alleles at both Htg6.1 and Htg7.1 resulted in a 7% increase in germination over RILs homozygous for Htg6.1 alone. Multilocus epistatic interactions affecting seed dormancy have also been described in Arabidopsis (Alonso-Blanco et al. 2003).
Collocation of QTL for different traits can be an indication that a locus has pleiotropic effects on multiple traits due to a common mechanistic basis (Clerkx et al. 2004). A portion of the 1 LOD interval for Gfr6.1 collocated with the Htg6.1 interval, and favorable alleles at both QTL were from the L. serriola parent, suggesting a potential common regulatory mechanism for these traits. Such a regulatory mechanism could involve the action of GA or ABA. The red/far-red reversible phytochrome system controls the synthesis of gibberellins that are required for germination (Toyomasu et al. 1998). Roth-Bejerano et al. (1999) showed that the sensitivity of thermoinhibited seeds to ABA was increased and that light decreased this sensitivity, perhaps through the action of GA on ABA catabolism and catabolite export (Gonai et al. 2004). The collocation of T50 QTL within 1 LOD confidence intervals for QTL previously identified in single-temperature analyses was expected; however, T501.1 was not detected in 25 and 35°C tests and may indicate an additional genetic component regulating germination.
Collocation also occurred for QTL for HYL and PQ (an estimate of uniformity of growth and stand quality) on LG5 (Fig. 5), providing evidence for pleiotropic effects of this locus in controlling germination and early seedling growth. The QTL allele for hypocotyl length originated from the Salinas parent, and collocated to the same genomic region as a QTL allele previously identified from L. serriola for increased taproot length per gram of plant biomass at 30 days post-emergence (Johnson et al. 2000). Conceivably, alleles in cv. Salinas enhance early post-germinative seedling growth parameters such as hypocotyl length, while alleles from L. serriola enhance root architecture and growth later in development.
A more detailed analysis, and ultimately, isolation and functional analysis of candidate genes are necessary to understand the molecular functions of Htg and other QTL related to seed germination and seedling growth. Near-isogenic lines containing these introgressed QTL in a uniform Salinas background are being developed to confirm and characterize the effects of Htg loci. In addition, specific candidate genes related to germination and dormancy are being mapped to the lettuce consensus map using this RIL population.
References
Alonso-Blanco C, Bentsink L, Hanhart CJ, Blankestijn-de Vries H, Koornneef M (2003) Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics 164:711–729
Anderson JA, Sorrells ME, Tanksley SD (1993) RFLP analysis of genomic regions associated with resistance to preharvest sprouting in wheat. Crop Sci 33:453–459
Basten CJ, Weir BS, Zeng ZB (2001) QTL Cartographer. Dept of Statistics, North Carolina State University, Raleigh, NC
Bert PF, Jouan I, Tourvielle de Labrouche D, Serre F, Philippon J, Nicolas P, Vear F (2003) Comparative genetic analysis of quantitative traits in sunflower (Helianthus annus L.). 2. Characterization of QTL involved in developmental and agronomic traits. Theor Appl Genet 107:181–189
Bernardo R (2002) Breeding for quantitative traits in plants. Stemma Press, Woodbury, Minnesota
Bettey M, Finch-Savage WE, King GJ, Lynn JR (2000) Quantitative genetic analysis of seed vigour and pre-emergence seedling growth traits in Brassica oleracea. New Phytol 148:277–286
Botto JF, Sanchez RA, Whitelam GC, Casal JJ (1996) Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade in Arabidopsis. Plant Physiol 110:439–444
Cantliffe DJ, Sung Y, Nascimento WM (2000) Lettuce seed germination. Hortic Rev 24:229–275
Cantliffe DJ, Schuler KD, Guedes AC (1981) Overcoming seed thermodormancy in a heat-sensitive romaine lettuce by seed priming. HortScience 16:196–198
Clerkx EJM, El-Lithy ME, Vierling E, Gerda RJ, Blankestijn-De Veries H, Groot SPC, Vreugdenhil D, Koornneef M (2004) Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol 135:432–443
Csanadi G, Vollmann J, Stift G, Lelley T (2001) Seed quality QTLs identified in a molecular map of early maturing soybean. Theor Appl Genet 103:912–919
Cui KH, Peng SB, Xing YZ, Xu CG, Yu SB, Zhang Q (2002) Molecular dissection of seedling vigor and associated physiological traits in rice. Theor Appl Genet 105:745–753
Doganlar S, Frary A, Tanksley SD (2000) The genetic basis of seed weight variation: tomato as a model. Theor Appl Genet 100:1267–1273
Fennimore SA, Nyquist WE, Shaner GE, Doerge RW, Foley ME (1999) A genetic model and molecular markers for wild oat (Avena fatua L.) seed dormancy. Theor Appl Genet 99:711–718
Fielding A, Kristie DN, Dearman P (1992) The temperature of Pfr action governs the upper temperature limit for germination in lettuce. Photochem Photobiol 56:623–627
Foley ME (2002) Weeds, seeds, and buds-opportunities and systems for dormancy investigations. Weed Sci 50:267–272
Foolad MR, Lin GY, Chen FQ (1999) Comparison of QTLs for seed germination under non-stress, cold stress and salt stress in tomato. Plant Breeding 118:167–173
Gale MD, Flintham JE, Devos KM (2002) Cereal comparative genetics and preharvest sprouting. Euphytica 126:21–25
Gandhi S, Heesacker A, Freeman C, Argyris J, Bradford KJ, Knapp SJ (2005) The self-incompatibility locus (S) and quantitative trait loci for self-pollination and seed dormancy in sunflower. Theor Appl Genet 111:619–629
Gonai T, Kawahara S, Tougou M, Satoh S, Hashiba T, Hirai N, Kawaide H, Kamiya Y, Yoshioka T (2004) Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J Exp Bot 55:111–118
Gray D (1975) Effects of temperature on the germination and emergence of lettuce (Lactuca sativa L.) varieties. Hortic Sci 50:349–361
Guzman VL, Nagata RT, Datnoff LE, Raid RN (1992) ‘Florida 202’ and ‘Everglades’: New butterhead lettuce cultivars adapted to Florida. HortScience 27:852–853
Johnson WC, Jackson LE, Ochoa O, van Wijk R, Peleman J, St. Claire DA, Michelmore RW (2000) Lettuce, a shallow-rooted crop, and Lactuca serriola, its wild progenitor, differ at QTL determining root architecture and deep soil water exploitation. Theor Appl Genet 101:1066–1073
Kato K, Nakamura W, Tabiki T, Miura H (2001) Detection of loci controlling seed dormancy on group 4 chromosomes of wheat and comparative mapping with rice and barley genomes. Theor Appl Genet 102:980–985
Kesseli RV, Paran I, Michelmore RW (1994) Analysis of a detailed genetic linkage map of Lactuca sativa (lettuce) constructed from RFLP and RAPD markers. Genetics 136:1435–1446
Marks MK, Prince SD (1982) Seed physiology and seasonal emergence of wild lettuce Lactuca serriola. Oikos 38:242–249
Nascimento WM, Cantliffe DJ, Huber DJ (2000) Thermotolerance in lettuce seeds: association with ethylene and endo-beta-mannanase. J American Soc Hort Sci 125:518–524
Redoña ED, Mackill DJ (1998) Quantitative trait locus analysis for rice panicle and grain characteristics. Theor Appl Genet 96: 957–963
Reynolds T (1973) A temperature-dependent source of variability in estimates of germination behaviour of lettuce fruits. Planta 113:327–332
Roth-Bejerano N, Sedee NJA, van der Meulen RM, Wang M (1999) The role of abscisic acid in germination of light-sensitive and light-insensitive lettuce seeds. Seed Sci Res 9:129–134
Sako Y, McDonald MB, Fujimura K, Evans AF, and Bennett MA (2001) A system for automated seed vigour assessment. Seed Sci & Technol 29:625–636
Small JGC, Gutterman Y (1992) A comparison on thermo- and skotodormancy in seeds of Lactuca serriola in terms of induction, alleviation, respiration, ethylene and protein synthesis. Plant Growth Regul 11:301–310
Stam P (1993) Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant J 3:739–744
Sung Y, Cantliffe DJ, Nagata RT (1998) Seed developmental temperature regulation of thermotolerance in lettuce. J Amer Soc Hort Sci 123:700–705
Thompson PA, Cox AS, Sanderson RH (1979) Characterization of the germination responses to temperature of lettuce (Lactuca sativa L.) achenes. Ann Bot 43:319–334
Toyomasu T, Kawaide H, Mitsuhashi W, Inoue Y, Kamiya Y (1998) Phytochrome regulates gibberellin biosynthesis during germination of photoblastic lettuce seeds. Plant Physiol 118:1517–1523
Valdes VM, Bradford KJ, Mayberry KS (1985) Alleviation of thermodormancy in coated lettuce seeds by seed priming. HortScience 20:1112–1114
Van der Schaar W, Alonso Blanco C, Leon-Kloosterziel KM, Jansen RC, Van Ooijen JW, Koornneef M (1997) QTL analysis of seed dormancy in Arabidopsis using recombinant inbred lines and MQM mapping. Heredity 79:190–200
Weaver SE, Downs MP (2003) The biology of Canadian weeds. 122. Lactuca serriola L. Can J Plant Sci 83:619–628
Yoshioka T, Endo T, Satoh S (1998) Restoration of seed germination at supraoptimal temperatures by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol 39:307–312
Acknowledgements
This work was supported by the United States Department of Agriculture Initiative for Future Agriculture and Food Systems grant No. 00–52100–9609 and by National Science Foundation grant DBI-0421630.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. Paran
Rights and permissions
About this article
Cite this article
Argyris, J., Truco, M.J., Ochoa, O. et al. Quantitative trait loci associated with seed and seedling traits in Lactuca . Theor Appl Genet 111, 1365–1376 (2005). https://doi.org/10.1007/s00122-005-0066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-0066-4