Abstract
The bathymetric distribution of marine benthic invertebrates is likely governed by a combination of ecological and physiological factors. The present study investigates oxygen consumption and heartbeat rate in response to attempted feeding at 1, 100 and 150 atm in the shallow-water spider crab, Maja brachydactyla, from temperate European waters. No significant difference was evident between the resting heartbeat rate of specimens at 1 or 100 atm, which were 56 and 65 bpm, respectively (Mann–Whitney, U = 5382.0; n = 95, 98; p = 0.079). However, at 150 atm the resting heartbeat rate was significantly higher than that observed for 100 atm at 108 bpm (Mann–Whitney, U = 149.0; n = 45, 98; p < 0.001). At 150 atm, feeding was never observed and coupled with the elevated resting heartbeat rate; it is suggested by 150 atm continued survival is unfeasible. At 1 and 100 atm, feeding instigated a distinct increase in heartbeat rate, which remained elevated for over 30 h. This increase peaked within 1 h at 1 atm. At 100 atm, this required 4 h and postprandial oxygen consumption was significantly higher than at 1 atm (Kruskal–Wallis, H = 85.036; df = 2; p < 0.001). Elevated hydrostatic pressure is hypothesized to extend the duration and the total metabolic energy devoted to specific dynamic action. The metabolic requirements of feeding under hyperbaric conditions may even reach such a critical demand that feeding is entirely inhibited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrostatic pressure in the marine environment is characterized by a linear increase of one atmosphere for every 10 m of depth, reaching up to 1,100 atm in the deepest ocean trench. Organisms that live beyond continental shelf depth require physiological adaptations to hyperbaric conditions, which are often absent from shallow-water species (Somero 1992). For the approximately 80% of the global biosphere which occurs below a depth of 500 m, the effects of hyperbaric conditions may consequently be an influential factor in all biochemical and structural evolution (Jaenicke 1983; Jannasch and Taylor 1984; Somero 1990).
Selectivity towards pressure tolerant mechanisms in deep-living species may be such a necessity that a substitution in efficiency does not even convey a competitive loss. Specifically, in the enzyme lactate dehydrogenase, essential for anaerobic respiration, there is an apparent trade-off between adaptation to pressure and catalytic efficiency (Murray and Siebenaller 1987). However, without adaptation the effects of elevated hydrostatic pressures may impair enzymatic function entirely (Mozhaev et al. 1996). At the organismal level, these effects culminate in an obvious impairment in motor functioning and results in immobilisation as conditions exacerbate (MacDonald and Teal 1975; Jain 1994).
Tolerance to ambient environmental factors provides a fundamental limit to the habitable conditions in which any organism can survive. However, even minor forms of stress are capable of impacting upon the aerobic scope and optimum performance of an organism, as demonstrated for temperature stress (Frederich and Pörtner 2000). The mechanisms by which pressure and temperature act on chemical structures are different, since pressure effects result only from volume change, whereas temperature affects both energy and volume. The overall effect of increasing pressure or decreasing temperature on molecular structures may produce a similar outcome (Balny et al. 2002; Sébert et al. 2004). It has also been demonstrated that exposure to higher than native pressures may trigger an increase in the expression of heat shock proteins (Ravaux et al. 2003; Cottin et al. 2010), which are known to be involved in general stress situations (Feder and Hoffman 1999). It may thus be possible that the effects of temperature stress on aerobic scope may be equivalent to those for pressure (Balny et al. 1997). Specifically, as external conditions vary further from the optimal range for an organism, an increased homeostatic effort is required to maintain internal conditions within suitable boundaries. The increase in effort corresponds to an increase in mitochondrial activity, which is accompanied by an appropriate elevation in respiratory and circulatory functioning (Frederich and Pörtner 2000). This will continue until the maximum aerobic scope of the organism impedes further intensification of respiratory activity. Within this pejus tolerance range, growth in mitochondrial demand to counter stress increase is not equivalently matched by an increase in respiratory capacity (Pörtner 2001). When the mitochondrial oxygen demand, to normalise homeostatic processes, finally exceeds the respiratory capacity of the organism, there is a transition from aerobic to anaerobic mitochondrial metabolism (Stillman and Somero 2000). The point at which this mismatch occurs is termed the ‘critical tolerance’ and beyond this, the organism experiences an indefinitely sustainable level of respiration (Pörtner 2001). As a result, the Arrhenius break point in respiratory capacity may be used as an indicator of critical tolerance (Somero 2005).
Strongly linked to ventilation and both ambient oxygen concentrations and oxygen demand, cardiac activity in Crustacea has been used as a proxy for respiratory capacity (Frederich and Pörtner 2000). An Arrhenius response has been observed beyond 100 atm for the shallow-water European spider crab, Maja brachydactyla, suggesting this is the upper physiological limit of the species (Robinson et al. 2009). A similar response has also been observed for the vent-associated deep-sea species, Bythograea thermydron (Airriess and Childress 1994). However, in some species of crustaceans, the Arrhenius break points for mitochondrial respiration (Dahlhoff et al. 1991; Weinstein and Somero 1998) and enzymatic activity (O’Brien et al. 1991) may be considerably higher than those for observed cardiac activity (Stillman and Somero 2000). Functional failure in the cardiac muscle, prior to oxygen limitation, may therefore form a ‘weak link’ in an organisms’ physiological tolerance (Peck 1998). To accurately assess the true critical tolerance of an organism to a particular stress, both mechanistic and metabolic limits to performance must be measured.
Colonisation and speciation into the deep-sea is suggested to have primarily transpired through isothermal water masses, as found in the warmer waters of the Mesozoic and early Cenozoic (Young et al. 1997). Under such conditions, when migrating to depth does not incur strong transitions in temperature, pressure may instead become the dominant factor in restricting such movements. A tolerance to pressure may therefore define those species that are able to make such evolutionary transitions (Menzies et al. 1973). The capacity for evolutionarily rapid colonisation of deep-sea environments through such processes may even justify the close phylogenetic links between certain deep-sea species and their shallow-water counterparts (Distel et al. 2000; Hall and Thatje 2009).
Evidence that the warming of surface waters is reducing the physiological constraints that previously restricted emergence into shallow Antarctic waters could also relate to deep-sea environments (Thatje et al. 2005a; Aronson et al. 2007). If ambient temperatures rise beyond a species’ optimal range, as long as the increase in hydrostatic pressure does not incur greater energetic cost, migration to depth may provide a suitable action. Rising temperatures may even facilitate colonisation of deeper waters considering the antagonistic effects of pressure and temperature on molecular processes (Balny et al. 2002). In conclusion, if considered in conjunction with phylogenetic evidence, knowledge of species’ pressure tolerances may not only help to explain the distribution of extant aquatic species with respect to depth but also determine the potential of such species to respond to environmental change (Hall and Thatje 2009; Thatje et al. 2010).
The energetic requirements of feeding in particular are crucial when determining the overall metabolic capacity of an organism (Fig. 1a; McCue 2006). The postprandial increase in metabolism, termed specific dynamic action (SDA) reflects the energetic requirements of digestion; including the movement and handling of food, absorption and storage of nutrients, deamination of amino acids, synthesis of excretory products and elevated synthesis of proteins and lipids associated with growth (Jobling 1992). The cumulative impact of the various processes that form SDA may even exceed the metabolic cost of activity (Soofiani and Priede 1985). To facilitate these energetic requirements, feeding may elicit physiological responses as shown in the blue crab, Callinectes sapidus, which demonstrated an immediate increase in cardiac activity upon food detection (McGaw and Reiber 2000). Consequently, SDA may impact directly on the aerobic scope of an organism on a temporal basis by exerting additional demands on physiological systems (McGaw 2006). For the duration of the SDA, response critical tolerances may therefore be lowered and evidently feeding has been observed to increase mortality rate in osmoregulating marine Crustacea (Legeay and Massabuau 2000). The SDA scope can also be compared to the maximum metabolic scope of an organism, in order to estimate the residual energetic capacity during digestion (McCue 2006). If the metabolic scope is already nominal due to the impacts of additional stresses, the digestive processes that determine SDA could be impinged upon, even restricting successful feeding altogether (Peck et al. 2008).
The influence of SDA on aerobic scope. a The increased metabolic demand of postprandial processes reduces the aerobic scope (blood PO2) to adapt to stress (modified from Peck 1998; McGaw 2005a). b The effect of digestion temperature on SDA in poikilotherms. Lower temperatures reduce the peak metabolic cost but extend the duration of the SDA response (modified from Wang et al. 2002)
Environmental variables, such as temperature, have little impact on the overall metabolic cost of SDA in poikilotherms (Robertson et al. 2002). However, the duration and momentary size of the SDA response may be affected (Fig. 1b; Whiteley et al. 2001). At low temperatures, the induced reduction in the rate of the SDA response may indeed prove beneficial if metabolic scope under these conditions is also diminished (Bennett et al. 1999). This may be crucial in the deep sea, where temperatures are generally low and stable, and species mostly have a reduced rate of metabolism, relative to corresponding shallow-water species (Childress et al. 1990). The SDA in Crustacea has been measured in response to temperature (Whiteley et al. 2001), but has never actually been quantified for any species under hyperbaric conditions. Thus, to date, whether the role of ambient pressure in defining the SDA response aids or hinders the hypothesized lower metabolic scope of deep-sea adapted species remains unknown.
This study aims to determine the physiological limits of the shallow-water crab M. brachydactyla to hydrostatic pressure. By also determining the effects of SDA in response to pressure, it is possible to assess whether the effects of feeding are of importance in defining the capacity of organisms to migrate to depth. Such knowledge is instrumental to all further experimentation that assesses the critical tolerances of aquatic invertebrates to hydrostatic pressure.
Materials and methods
Sampling and maintenance
Male specimens of M. brachydactyla were collected using baited trap at depths of 4–12 m from Southampton Water, United Kingdom, in November 2008. Only males with a CL between 75-90 mm were used. Specimens of this size were solely suitable due to the size of the carapace in relation to the heartbeat sensor (Robinson et al. 2009) and to accommodate specimens inside the pressure chamber used in the experimental procedure (IPOCAMP™; Shillito et al. 2001). As maturity occurs at CL over 96 mm, these specimens were not sexually mature (Corgos and Freire 2006).
Individuals were transferred to outdoor flow-through tanks. These tanks were subject to ambient temperature fluctuations, natural light cycles and were supplied with seawater at salinity of 32 ± 1. After a minimum acclimation period of 1 week, specimens were cleaned and individually labelled with numbered tags. Labelled individuals were transferred to tanks in a constant temperature lab at 20 ± 3°C and subjected to diurnal light:dark cycles of equal periodicity (12:12 h). Tanks were stocked with 1-μm filtered seawater, which was aerated continuously and completely replenished on a weekly basis. Specimens were allowed to acclimate under these conditions for a minimum of a week. All organisms were fed twice weekly with squid. Upon the termination of each successful experiment, the tested individual was returned to temperature controlled-conditions for an additional week, to observe for any subsequent deterioration in health. After feeding, specimens were maintained for an additional day to grant a suitable period for the previous meal be completely processed and excreted (Hill 1976). Recorded experiments therefore started 2 days after the previous feeding. Prior to experimentation, the IPOCAMP™ system was filled with 1-μm filtered seawater and the system was allowed to circulate for several hours (see Shillito et al. 2001). All experiments were conducted at 20°C, which slightly exceeds the 8–17°C optimum temperature range for aerobic performance in this species (Frederich and Pörtner 2000).
Experimental pressure regimes and feeding under pressure
Experiments were used to test the effects of feeding at 1, 100 and 150 atm on M. brachydactyla. Specimens were fed squid equivalent of 1% of their wet mass (for description of feeding mechanism see Electronic Supplementary Material) on the second day of each treatment (Fig. 2). Under each pressure condition, three separate repeats were taken. For 1 atm experiments, the pressure was not adjusted over the length of the trail. For 100 and 150 atm experiments, in order to observe the basal heartbeat and respiration rate, pressure was always maintained at 1 atm on the first day until approximately 19:00. Pressure was then increased incrementally by 10 atm every 10 min until the appropriate pressure (100 or 150 atm) had been reached. If feeding was successful on the following day, pressure was maintained at the same level over the second day. On the third day, at approximately 20:00, pressure was reduced back to 1 atm at a similar rate to the pressure increase on the first day. Pressure was maintained at 1 atm until 08:00 on the fourth day when the experiment was ended (Fig. 2). Alternatively, if feeding was unsuccessful on the second day, pressure was decreased shortly after 12:00 on the day that the feeding mechanism was released.
Maja brachydactyla. Schematic overview of the pressure regimes used to compare the effects of 1 (red/bottom line), 100 (green/middle line) and 150 atm (blue/top line) on the feeding response. As feeding does not occur at 150 atm, the experiment runs over a shorter time frame. For clarity, the lines do not overlap, however, before and after pressurisation, all experiments were maintained at a uniform 1 atm. The first dotted vertical line represents the timing of food release. Pressure is reduced if feeding does not occur before the second dotted line. Incremental steps in pressure change are indicated as a straight line due to scale (cf “Materials and methods”)
Recording (cardiac) activity
Visual recording of the specimen was achieved using an endoscope and video recording over every light cycle during experimentation. As well as validating the onset of feeding, the timing and duration of any evident active behaviour was recorded. Cardiac activity was used as a measure of the circulatory capacity of the test organism. The movements of the cardiac muscle were recorded using the photoplethysmographic technique developed by Depledge and Andersen (1990) and subsequently adapted for hyperbaric use (Robinson et al. 2009). The change in current measured by the sensor, reflecting the beating motions of the cardiac muscle, was converted into voltage and passed to the voltage amplifier (Electrode Amplifier; Vernier). The analogue signal is digitalised by the Vernier LabPro system (Vernier) and recorded using the Vernier LoggerPro software (Version 3.4.2; Vernier). The raw signal was recorded at a sampling rate of 1,000 min−1. Cardiac activity was recorded continuously over the duration of the experiment; however, due the large amount of data collected, at 07:00 and 19:00 each day recording was stopped, saved and reset causing a 10–20 min gap in the heartbeat data.
To convert cardiac activity into heartbeat rate, computer analysis was required to identify the characteristic slope of a single heartbeat and this was achieved using Chart (Version 5.5.6; ADinstruments). As the strength and form of the signal depends on the exact placement of the sensor above the cardiac muscle, this varied between specimens. The parameters used to define individual heartbeats from the cardiac signal were therefore redefined when analysing each trial (see Robinson et al. 2009).
The heartbeat rate was then calculated for discrete 5 min samples over the entire length of the experiment. Samples were divided into separate categories, defined by different experimental and behavioural conditions. These categories were:
-
Resting during acclimation—all measurements taken at 1 atm on the first day of experimentation. Results from when the crab was in motion are excluded. Refers to acclimation to conditions inside the IPOCAMP™ and not experimental pressures.
-
Postprandial—starting at the 5-min sample that followed the onset of feeding, to exclude the direct locomotive impact of feeding. The postprandial increase in metabolism reflects the energetic requirements of digestion. The postprandial period is defined by elevated heartbeat level, before returning to levels equivalent of the acclimation period (resting during acclimation).
-
Resting at experimental pressure—all measurements taken on the third day of experimentation for those where feeding was successful. Pressure is now at experimental level (1, 100 or 150 atm). When feeding is not successful, measurements are taken prior to depressurisation on the second day. Results from when the crab was in motion are excluded.
-
Locomotion at experimental pressure—all measurements for when the crab was observed moving on the third day of experimentation. When feeding is not successful, measurements are taken prior to depressurisation on the second day.
-
Resting after depressurisation—all measurements at 1 atm taken on the fourth day of experimentation for those where feeding was successful. For 1-atm experiments, no such depressurisation occurs. Results from when the crab was in motion are excluded.
Only measurements during the light portion of the day were used to infer the average heartbeat rate at all the mentioned conditions. The data for each three specimens from each pressure experiment were grouped before means were calculated to determine the average heartbeat rate during each condition. This value is termed the ‘collective mean heartbeat rate’.
Oxygen consumption
The depletion of oxygen from the water of the IPOCAMP™ by the specimens was measured using high precision micro-optode sensors (Fibre-optic oxygen meter Microx TX 3; PreSens; Germany). Oxygen saturation of the water was recorded as it passed out of the IPOCAMP™ into a small reservoir, which drained back into the head tank, measured oxygen depletion in respect to the residence time of the water inside the IPOCAMP™ during flow-through (see Electronic Supplementary Material). On the first and fourth day of sampling a minimum of three separate oxygen measurements were taken. On the second and third day, oxygen measurements began at 07:30, to coincide the beginning of the light cycle, and were repeated every 1.5 h. On both days, this continued for the entire light cycle plus a single measurement during the dark cycle. A two-point calibration at 20°C was undertaken daily using fully aerated seawater (100% O2sat) and seawater over-saturated with sodium sulphite (0% O2sat) as standards. Using a constant value of salinity (S) at 32 and temperature (T) at 293°K, the maximum oxygen solubility was calculated using equation as given by Benson and Krause (1984):
Where CO is the concentration of oxygen in μmol/l at 100% saturation. Knowing the oxygen saturation of the sample (DO%sample) the actual concentration of oxygen in the sample (COsample) can then be calculated relative to 100% saturation using the equation:
Considering the residence time of water inside the IPOCAMP™ to be 1 h (for validation of method see Electronic Supplementary Material) and using the measured wet mass (g), this oxygen consumption can thus be converted into micromoles oxygen per gram hour. After conversion, the oxygen consumption rates from the first day of experimentation were averaged in order to indicate the basal oxygen consumption at 1 atm. Oxygen measurements on the subsequent 2 days were considered temporally in order to demonstrate the effects of SDA on oxygen consumption. Oxygen consumption measurements from the fourth day of experimentation were averaged.
Results
Feeding success at experimental pressure
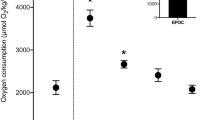
At 1 atm, specimens readily fed in all three experiments. At this pressure, the mean time for feeding to be observed after the release of the feeding capsule was 8.6 min (SD ± 9.07; Fig. 3). At 100 atm, feeding was instead only successful in three of four attempted experiments and when feeding did occur it took longer to instigate (32.67 ± 24.67 min, Fig. 3). However, this difference was not statistically significant (T test: t = −1.582, df = 4; p = 0.189). At 150 atm, no feeding attempt was observed.
Collective mean heartbeat data (left axis) for Maja brachydactyla at each categorised behaviour under each pressure regime. Time taken for feeding to occur after the feeding capsule was released (right axis). As feeding did not occur at 150 atm, there was no postprandial or resting after depressurisation response to measure
Heartbeat rate
Observations at 1 atm
The collective mean heartbeat rate during acclimation was 80.82 bpm (±15.45 SD; Fig. 3). By the onset of the light cycle on the second day and simultaneous with the occurrence of feeding, a distinct peak in the heartbeat rate was evident in both successfully recorded specimens. Reaching 108.43 bpm (SD ± 17.90), the postprandial increase of 27.61 bpm from acclimation was statistically significant (Mann–Whitney, U = 144.0; n = 12, 67; p < 0.001). In both cases, the heartbeat rate remained elevated for approximately 1 h, before returning to levels during acclimation (Fig. 4). This decline, lasting for over 60 h, actually continues far beyond acclimation levels and was matched by a reduction in standard deviation. As a result, the collective mean heartbeat rate during rest on the day following feeding was only 56.07 bpm (SD ± 10.43), significantly smaller than that observed postprandial (Mann–Whitney, U = 1.0; n = 12, 95; p < 0.001) or during acclimation (Mann–Whitney, U = 652.0; n = 67, 95; p < 0.001). However, intermittently associated with periods of movement, small peaks in the heartbeat rate do occur throughout the third day of experimentation. During these bouts of movement, the collective mean heartbeat rate was 74.02 bpm (SD ± 14.47), which was significantly higher than that observed the same day during resting (Mann–Whitney, U = 410; n = 25, 95; p < 0.001). While the heartbeat rate during movement does remain significantly lower than the postprandial heartbeat rate (Mann–Whitney, U = 41; n = 12, 25; p < 0.001), there was no significant difference between the heartbeat rate during movement or acclimation (Mann–Whitney, U = 1039; n = 25, 67; p = 0.025).
Response to feeding in heartbeat rate (bottom), oxygen consumption (top) and behaviour (middle) in three Maja brachydactyla specimens (a–c) for 1 atm. Food was released at the beginning of the light cycle on the second day. Heartbeat rate is indicated by grey lines representing continual 5-min samples. Black circles are the averages of these 5-min samples over a period of 30 min. Oxygen consumption measurements during the first and fourth day were averaged from a minimum of three discrete measurements. For the second and third day, each column represents single measurements taken at 1.5 h intervals. Behaviour was only assessed during the light period of the day. On the behaviour bar, periods of movement are indicated by grey L squares, grey F squares indicate feeding and D indicates postprandial digestion. White sections represent rest, indicated by a lack of movement. The uncharacteristically uniform single in c is attributed to a faulty sensor, and data were not considered in the analysis
The postprandial decline in heartbeat rate continues until the beginning of the dark cycle on the fourth day of experimentation and as a consequence the collective mean heartbeat rate during rest that day was the lowest recorded from all experiments at any pressure (Fig. 4). At 47.68 bpm (SD ± 17.27), it was even significantly different from the heartbeat rate during rest on the previous day (Mann–Whitney, U = 4716; n = 75, 95; p < 0.001).
Observations at 100 atm
Starting at 1 atm, the collective mean heartbeat rate during acclimation was 76.66 bpm (SD ± 14.98; Fig. 3). When pressure was increased to 100 atm, two of the three specimens demonstrated a steady and steep increase in heartbeat rate, which peaked at either 142.67 or 133.33 bpm depending on the specimen (Fig. 5). These peaks occurred after approximately 4 h, after which a decline was observed. In the single remaining specimen, the true cardiac response was hidden by a minor sensor malfunction, which extended over a period of 9 h (Fig. 5c).
Response to feeding in heartbeat rate (bottom), oxygen consumption (top) and behaviour (middle) in three Maja brachydactyla specimens (a–c) for 100 atm. The left-most black dotted line indicates when pressure was raised to 100 atm, the right-most black dotted line indicates when returned to 1 atm. Food was released at the beginning of the light cycle on the second day. Heartbeat rate is indicated by grey lines representing continual 5-min samples. Black circles are the averages of these 5 min samples over a period of 30 min. Oxygen consumption measurements during the first and fourth day were averaged from a minimum of three discrete measurements. For the second and third day, each column represents single measurements taken at 1.5 h intervals. Behaviour was only assessed during the light period of the day. On the behaviour bar, periods of movement are indicated by grey L squares, grey F squares indicate feeding and D indicates postprandial digestion. White sections represent rest, indicated by a lack of movement. The uncharacteristically uniform single in c (hours 6–14) is attributed to a faulty sensor, and data were not considered in the analysis
At 100 atm, feeding once again prompted an increase in heartbeat rate. This increase was observed 30 min after feeding was instigated and peaked closer to 6 h later. This response was accompanied by a marked reduction in the standard deviation as the peak was approached. The collective postprandial heartbeat rate was still significantly higher, at 112.56 bpm (SD ± 15.08), than that observed during acclimation (Mann–Whitney, U = 197.0, n = 12, 105; p < 0.001). Over the subsequent 30 h, a general reduction in the heartbeat rate is apparent although individual patterns vary prominently (Fig. 5). The average decrease in heartbeat rate over this time is enough to lower the collective mean on the third day during periods of rest to 65.14 bpm (SD ± 19.73), which is significantly lower than the postprandial (Mann–Whitney, U = 127.0; n = 12, 98; p < 0.001) or acclimation heartbeat rate (Mann–Whitney, U = 7261; n = 98, 105; p < 0.001). This period of rest is interspersed with bouts of movements, for which the collective mean heartbeat rate increases to 84.19 bpm (SD ± 33.36). This is significantly higher than the resting heartbeat rate that day (Mann–Whitney, U = 1747; n = 53, 98; p < 0.001) but lower than the postprandial rate (Mann–Whitney, U = 592.0; n = 12, 53; p < 0.001). Furthermore, there is no significant difference between the heartbeat rates when moving or during acclimation (Mann–Whitney, U = 282.5; n = 53, 105; p = 0.877).
In two of the three experiments at 100 atm, a distinct peak in heartbeat rate was observed within 4 h after decompression (Fig. 5a, b). This change only lasted between 5 and 15 h and by the onset of the light cycle on the following day, the collective mean heartbeat rate had already returned to 56 bpm (SD ± 18.99), which is not significantly different to the observed heartbeat rate under pressure (Mann–Whitney, U = 2614.5; n = 98, 120; p = 0.03). Only a single specimen did not demonstrate an obvious peak in the heartbeat rate after decompression (Fig. 5c).
Observations at 150 atm
The collective mean heartbeat rate during acclimation was 79.45 bpm (SD ± 12.28; Fig. 3). When pressure was raised to 150 atm a unanimous increase in heartbeat rate was observed, which peaked within 1 h of pressurisation. Over time, a decline from this peak heartbeat rate was only observed in two specimens (Fig. 6a, c). Nonetheless, the heartbeat remained relatively constant after these initial fluctuations, exhibiting minimal variation and approximating at 130 bpm in two specimens (Fig. 6a, b) and 95 bpm in the third specimen (Fig. 6c). Consequently, the collective mean heartbeat rate for rest at 150 atm, at 107.92 bpm (SD ± 13.18), is far greater than that observed during acclimation (Mann–Whitney, U = 256.5; n = 15, 11; p < 0.001). While pressurised conditions were maintained, the nominal variation in the heartbeat rate exhibited for the duration of the experiment meant that the minor increase in heartbeat rate to 111.53 bpm (SD ± 11.57) observed during period of movement was not significant (Mann–Whitney, U = 360.5; n = 32, 45; p = 0.030). As feeding did not occur at 150 atm, all three trials were terminated early (Fig. 6).
Response to feeding in heartbeat rate (bottom), oxygen consumption (top) and behaviour (middle) in three Maja brachydactyla specimens (a–c) for 150 atm. Food was released at the beginning of the light cycle on the second day. Heartbeat rate is indicated by grey lines representing continual 5-min samples. Black circles are the averages of these 5-min samples over a period of 30 min. Oxygen consumption measurements during the first and fourth day were averaged from a minimum of three discrete measurements. For the second and third day, each column represents single measurements taken at 1.5 h intervals. Behaviour was only assessed during the light period of the day. On the behaviour bar, periods of movement are indicated by grey L squares, grey F squares indicate feeding and D indicates postprandial digestion. White sections represent rest, indicated by a lack of movement. Feeding did not occur at 150 atm and thus all experiments were terminated after a period of 24 h
Comparison between pressure regimes
For all experimental procedures at 1, 100 and 150 atm, the collective mean heartbeat rate during acclimation ranged from 76.66 to 80.82 bpm, and there was no statistically significant difference between each pressure regime (Mann–Whitney, 1:100 atm U = 3184.0; n = 67, 105; p = 0.296; 1:150 atm U = 3920.0; n = 67, 111; p = 0.543; 100:150 atm U = 6629.0; n = 105, 111; p = 0.081). The only statistically significant difference between heartbeat rate at 1 and 100 atm experiments was observed during resting after decompression. In this instance, heartbeat rate was lower during 1 atm experiments (Mann–Whitney, U = 5574.0; n = 75, 120; p < 0.005).
Oxygen consumption
Response to feeding at 1 atm
The mean oxygen consumptions for each specimen during the light cycle of the first day were similar, only ranging between 2.89–4.15 μmol O2 g−1 h−1 with standard deviations from 0.63–1.21 (Fig. 4). Following feeding however, a general increase in oxygen consumption was evident. Only in a single specimen was this increase not consistent for the duration of the second day and began to decline after 8 h (Fig. 4c). In each instance, the final measurement that day, 10.5 h later, is almost double the initial measurement of the day. At this level, oxygen consumption is now beyond the range of the standard deviation for collective mean oxygen consumption during the first day. On the third day relative to the first day, oxygen consumption is still elevated. However, in comparison to the peak oxygen consumption observed on the second day it was reduced. In addition, no uniform pattern is observed between repeats and only a single crab demonstrated a general decrease in oxygen consumption. Mean oxygen consumption between specimens was contained by the fourth day, within the standard deviation of measurements from the first day. No statistical significance is observed between oxygen measurements on these days (t test, t = −0.985, df = 16; p = 0.339). Throughout, no distinct correlation between oxygen consumption measurements and any observation of movement was apparent (Fig. 4).
Response to feeding at 100 atm
The mean oxygen consumptions for each specimen during the light cycle of the first day were similar, only ranging between 4.09–4.94 μmol O2 g−1 h−1, with standard deviations from 0.23–1.03 (Fig. 5). After pressurisation to 100 atm, the first oxygen consumption measurement on the second day of the experiment had now amplified by 262.10% to a collective mean of 12.02 μmol O2 g−1 h−1. Although during the light cycle of this day these values fluctuated by up to 5.80 μmol O2 g−1 h−1, no measurement decreased to those observed on the first day at 1 atm. Still at 100 atm on the third day, oxygen consumption measurements remained equivalent to those of the second day. The same as on day two, there was no uniform pattern between specimens with one specimen indicating a decreasing trend (Fig. 5b) while another specimen indicated an increasing trend (Fig. 5c). After decompression on the fourth day, although mean oxygen consumption fell back to an average of 5.21 and 4.90 μmol O2 g−1 h−1 for two specimens (Fig. 5a, b), it remained high in a single specimen at 9.46 μmol O2 g−1 h−1 (Fig. 5c). Due to this third specimen, there was no statistically significant difference in oxygen consumption between the first and fourth day of experimentation for the specimens collectively (t test, t = −2.184, df = 16; p = 0.054).
Response to feeding at 150 atm
The mean oxygen consumption of each specimen during the light cycle of the first day was similar, ranging between 3.89 and 4.72 μmol O2 g−1 h−1 (SD ± 0.35–1.71; Fig. 6). After pressurisation, the lowest oxygen measurement for any specimen was 7.49 μmol O2 g−1 h−1. No other distinct temporal patterns in oxygen consumption prior to pressurisation were observed.
Comparison between pressure regimes
Between all trials during acclimation at 1 atm on the first day of experimentation, no statistical difference in oxygen consumption between each experimental pressure was observed (one-way ANOVA: F 2, 26 = 1.117; p = 0.334). Grouping the oxygen consumption measurements for separate experimental pressures (second and third day) demonstrates an evident statistical difference between regimes (Kruskal–Wallis: H = 85.036, df = 2; p < 0.001). Furthermore, comparing 100 and 150 atm directly indicates that this significant difference is not between these two regimes (Mann–Whitney, U = 229.0; n = 11, 51; p = 0.347).
Discussion
Functional limitations in the cardiac muscle
During both rest and activity, heartbeat rate of M. brachydactyla was significantly higher at 150 atm than at either 1 or 100 atm (Fig. 3). Two of the specimens used to determine this collective mean heartbeat rate consistently demonstrated heartbeat rates which approached on 130 bpm (Fig. 6a, b). The third specimen exhibited a heartbeat rate closer to 95 bpm, but still showed a similarly unvarying pattern in output (Fig. 6c). Evidently, the cardiac muscle in the later individual was not performing at the maximum rate possible under optimum conditions for this species. However, the lack of variation infers the cardiac muscle was performing at the maximum rate allowable under these conditions, specifically pressures of 150 atm. The heartbeat rate may have therefore passed an Arrhenius break point and was subsequently limited in exertion by the capacity for sufficient oxygen to perfuse into the cardiac muscle (Lannig et al. 2004). Specifically, if M. brachydactyla is unable to acclimate to pressures of 150 atm, the detrimental effects of hyperbaric conditions on biological membranes may instigate functional failure in membrane dynamics (Heinemann et al. 1987; Theron and Sébert 2003). The increase in heartbeat rate may therefore be a homeostatic response, to correspond to the increasing energetic demands of routine processes (Avent 1975). Indicatively, by 150 atm, all specimens demonstrated disjointed movements, which are often observed just prior to the complete immobilisation associated with increasing hydrostatic pressures (MacDonald 1972).
Oxygen limitation to performance
Grouped oxygen consumption measurements for M. brachydactyla were statistically higher at 100 atm than at 1 atm, whilst a direct comparison of 100 atm and 150 atm did not show statistically significant difference. Oxygen consumption has previously been linked to an increase in hydrostatic pressure beyond optimum tolerance ranges, and such changes are associated with an acclimation response and return to basal levels after time (Mickel and Childress 1982). In the present case, elevated oxygen consumption may instead be accredited to the effects of SDA at 100 atm. The lack of statistically significant difference between the oxygen consumption rates at 100 or 150 atm may suggest that digestion at 100 atm is eliciting an equivalent metabolic response to rest at 150 atm. Although no statistically significant increase in heartbeat rate is facilitating this increase in oxygen consumption, a minor elevation in the heartbeat rate for each of the three categorised behaviours (postprandial; resting at experimental pressure; locomotion) at 100 atm relative to 1 atm is apparent (Fig. 3). This may coincide with observations by Frederich and Pörtner (2000) that an elevation in haemolymph circulation often precedes an increase in heartbeat rate.
The lack of additional heartbeat rate increase at 150 atm may suggest a functional limitation of the cardiac muscle, which may occur prior to oxygen limitation. This supports previous work suggesting that mechanical failure in the cardiac muscle is the preliminary factor that restricts tolerance to adverse environmental conditions (Somero 2005). If the resting metabolic rate at 150 atm requires the maximum functional capacity of the cardiac muscle, then it is possible to suggest no further increase in heartbeat rate can occur. Unable to increase further to meet increasing mitochondrial demand, anaerobic respiration may be necessitated. The capacity of the heart to perform is subsequently limited by the available oxygen supply, as well as the likely accumulation of anaerobic by-products (Pörtner 2001). As mitochondrial oxygen demand increases, the effects of oxygen limitation on the cardiac muscle are amplified (Somero 2005). The accumulation of chemical by-products formed during anaerobic respiration is correlated to the length of time that the specimen was under the conditions that caused the stress response (Herreid 1980). The duration for continued survival at this level of metabolism would be limited.
Effects of hydrostatic pressure on SDA
In the supralittoral isopod, Ligia pallasii, the immediate excitement of the cardiac muscle was suggested to occur in response to chemosynthetic stimulus of the olfactory organs upon release of the feeding mechanism. The initial increase in heartbeat rate may also be attributed to the manipulation of food prior to ingestion and mechanical breakdown in the foregut (Carefoot 1990). During these processes, it has been observed in Cancer magister that blood flow is diverted to the musculature tissue, instigating an instantaneous increase in heartbeat rate (McGaw 2005a, b). In M. brachydactyla, at 1 atm the extent of this increase momentarily reaches 130 bpm, suggesting that the act of feeding is energetically demanding. While comparable trends are apparent in similar species, equivalently high cardiac responses to feeding appear uncommon (Whiteley et al. 2001). For M. brachydactyla it may be that the highest respiratory output is purposefully utilised in the process of feeding. As the extent of exertion is often correlated to the necessity of the action, then it may be advantageous for this species to respond to any available food source as quickly as possible.
Subsequent to active feeding, blood flow is diverted to the digestive organs and a peak in heartbeat rate is observed within 1 h under optimal conditions (McGaw 2005b). After this immediate increase, a shallow but consistent decline in heartbeat rate is apparent (McGaw 2006). This steady decline in heartbeat rate over the subsequent 30 h observed in the present study has also been observed in the blue crab C. sapidus (McGaw and Reiber 2000). In C. sapidus, the decrease in heartbeat rate was also matched by an increase in the stroke volume of the heart. Even though the heartbeat rate was decreasing, flow rate of haemolymph from the cardiac muscle remained consistently elevated for over 24 h (McGaw and Reiber 2000). For M. brachydactyla, an increased level of metabolism is also indicated by oxygen consumption rates, which remain elevated for a minimum of 12 h.
The length of the SDA response in crustaceans is correlated with meal size (McCue 2006). In this study, this was relatively small at 1% of the specimens wet mass, while comparative studies predominantly used a meal size of 3% wet body mass (Legeay and Massabuau 2000; McGaw 2006). It is therefore possible that the quantity of provided food plays an important factor in defining the physiological performance of ectotherms under hyperbaric pressure conditions. In addition to meal size, it remains unknown whether specimen size affects the SDA. However, specimen size should not have affected the present study because only specimens of comparable size had been used.
While the postprandial peak in heartbeat rate observed in M. brachydactyla occurred within 1 h at 1 atm, at 100 atm, this peak was observed after approximately 4 h. Furthermore, as the initial heartbeat rate prior to this increase was higher at 100 than 1 atm, yet the peak heartbeat rate was comparable, the overall increase in heartbeat rate at 100 atm was smaller. It has been previously observed in poikilotherms that the integrated SDA response incurs a relatively fixed metabolic cost, which is independent of environmental factors such as temperature (Robertson et al. 2002; Wang et al. 2002). Instead, the duration and peak size of the SDA response may be accordingly affected (Whiteley et al. 2001). It is therefore possible that the energetic demands of digestive processes at higher pressures may prolong the SDA response. As such, the effects of elevated hydrostatic pressure on the duration of SDA may have a similar effect to low temperatures (Brockington and Clarke 2001). Analogous effects of low temperatures and high pressure on physiological processes have been previously observed (Airriess and Childress 1994; Balny et al. 2002). While in the present study no distinct postprandial pattern was observed in the oxygen consumption at 100 atm, it remained statistically higher than that observed at 1 atm. This suggests that under hyperbaric conditions, the metabolic cost of digestion may actually be amplified in effect as well as prolonged. Consistently high oxygen demand during digestion at 100 atm could reduce the aerobic scope to deal with other impacting factors. The effects of feeding and digestion may therefore be increasingly important in defining aerobic scope at elevated pressures (McCue 2006). The lack of successful feeding at 150 atm will ultimately affect continued survival. This supports the necessity for considering both physiological and ecological factors when defining the capacity for an organism to tolerate impacting environmental factors.
Capacity for deep-sea invasion
M. brachydactyla can evidently survive short-term exposure to the pressures that are apparent at depths up to 1,500 m (Fig. 6). Considering the successful feeding events demonstrated in the present work, continuous survival at depths of 1,000 m might be possible (Fig. 5). As such, it is unlikely that tolerance to hydrostatic pressure alone is restricting the movements of this species into deeper waters. Indeed, the range of any marine organism is rarely restricted by a single environmental or ecological factor (Airriess and Childress 1994; Somero 2005). Hydrostatic pressure may therefore work in conjunction with other environmental factors, especially temperature, to instigate a collaborative limit to the distribution of this species. In particular, low temperatures may often reduce tolerance to elevated pressures (Kaye and Baross 2004; Mestre et al. 2009). Thus, the additive effects of lower temperature and higher pressure may restrict the vertical movements of the species. As such, colonisation of deeper waters by this species may be increasingly plausible in an isothermal water column. Such conditions, which predominated during the Mesozoic and Cenozoic, have already been hypothesised to assist past shallow-water faunal exchange with the deep-sea (Young et al. 1997; Thatje et al. 2005b). Movement to depth during such events could minimise the evolutionary adaptations required to colonise these environments. Once adapted to the deep sea, a tolerance to low temperature could evolve independently as the temperatures of the deep ocean cooled with time (Elderfield and Ganssen 2000).
Conclusion
Marine ectotherms are specifically adapted to particular hydrostatic pressures. The range of this tolerance is potentially attributed to the capacity of biological structures, at all levels of organisation, to withstand hydrostatic pressure and retain optimal functioning. The implications of hyperbaric conditions may therefore extend to physiological processes and SDA in particular may be affected. It is herein hypothesized that the energetic cost of SDA increases with hydrostatic pressure in both size and duration. The metabolic requirements of feeding under hyperbaric conditions may even reach such a critical demand that feeding is entirely inhibited. This emphasizes the importance of holistic studies that consider physiological limits in a variety of routine behaviours to more accurately define an organism’s true capacity to withstand or adapt to hyperbaric conditions.
References
Airriess C, Childress JJ (1994) Homeoviscous properties implicated by the interactive effects of pressure and temperature on the hydrothermal vent crab Bythograea thermydron. Biol Bull 187:208–214
Aronson RB, Thatje S, Clarke A, Peck LS, Blake DB, Wilga CD, Seibel BA (2007) Climate change and invasibility of the Antarctic benthos. Ann Rev Ecol Evol Syst 38:129–154
Avent RM (1975) Evidence for acclimation to hydrostatic pressure in Uca pugilator (Crustacea: Decapoda: Ocypodidae). Mar Biol 31:193–199
Balny CV, Mozhaev V, Lange R (1997) Hydrostatic pressure and proteins: basic concepts and new data. Comp Biochem Physiol 116A:299–304
Balny C, Masson P, Heremans K (2002) High pressure effects on biological macromolecules: from structural changes to alteration of cellular processes. Biochim Biophys Acta 1595:3–10
Bennett VA, Kukal O, Lee RE (1999) Metabolic opportunists: feeding and temperature influence the rate and pattern of respiration in the high Arctic wollybear caterpillar Gynaephora groenlandica (Lymantriidae). J Exp Biol 202:47–53
Benson B, Krause D (1984) The concentration and isotopic fractionation of oxygen dissolved in fresh-water and seawater in equilibrium with the atmosphere. Limnol Oceanogr 29:620–632
Brockington S, Clarke A (2001) The relative influence of temperature and food on the metabolism of a marine invertebrate. J Exp Mar Biol Ecol 258:87–99
Carefoot TH (1990) Specific dynamic action (SDA) in the supralittoral isopod, Ligia pallasii: identification of components of apparent SDA and effects of diety amino acid quality and content on SDA. Comp Biochem Physiol A 95:309–316
Childress JJ, Cowles DL, Favuzzi JA, Mickel TF (1990) Metabolic rates of benthic deep-sea decapod crustaceans decline with increasing depth primarily due to the decline in temperature. Deep Sea Res II 37:929–949
Corgos A, Freire J (2006) Morphometric and gonad maturity in the spider crab Maja brachydactyla: a comparison of methods for estimating size at maturity in species with determinate growth. ICES J Mar Sci 63:851–859
Cottin D, Shillito B, Chertemps T, Thatje S, Léger N, Ravaux J (2010) Comparison of heat-shock responses between the hydrothermal vent shrimp Rimicaris exoculata and the related coastal shrimp Palaemonetes varians. J Exp Mar Biol Ecol 393:9–16
Dahlhoff EP, O’Brien J, Somero GN, Vetter RD (1991) Temperature effects on mitochondria from hydrothermal vent invertebrates: evidence for adaptation to elevated and variable habitat temperatures. Phys Zool 64:1490–1508
Depledge MH, Andersen BB (1990) A computer-aided physiological monitoring system for continuous, long-term recording of cardiac activity in selected invertebrates. Comp Biochem Physiol A 96:473–477
Distel DL, Baco AR, Chuang E, Morrill W, Cavanaugh C, Smith CR (2000) Do mussels take wooden steps to deep-sea vents? Nature 403:725–726
Elderfield H, Ganseen G (2000) Past temperatures and δ18O of surface ocean waters inferred from foraminiferal Mg/Ca ratios. Nature 405:442–445
Feder ME, Hoffman GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Frederich M, Pörtner HO (2000) Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am J Phys 279:1531–1538
Hall S, Thatje S (2009) Global bottlenecks in the distribution of marine Crustacea: temperature constraints in the biogeography of the family Lithodidae. J Biogeogr 36:2125–2135
Heinemann SH, Conti F, Stühmer W, Neher E (1987) Effects of hydrostatic pressure on membrane processes: sodium channels, calcium channels, and exocytosis. J Gen Physiol 90:765–778
Herreid CF II (1980) Hypoxia in invertebrates. Comp Biochem Physiol A 67:311–320
Hill BJ (1976) Natural food, foregut clearance-rate and activity of the crab Scylla serrata. Mar Biol 34:109–116
Jaenicke R (1983) Biochemical processes under high hydrostatic pressure. Naturwissenschaften 70:332–341
Jain KK (1994) High-pressure neurological syndrome (HPNS). Acta Neurol Scand 90:45–50
Jannasch HW, Taylor CD (1984) Deep-sea microbiology. Annu Rev Microbiol 38:487–510
Jobling M (1992) Bioenergetics: feed intake and energy partitioning. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Chapman and Hall, London, pp 1–44
Kaye JZ, Baross JA (2004) Synchronous effects of temperature, hydrostatic pressure, and salinity on growth, phospholipid profiles, and protein patterns of for Halomonas species isolated from deep-sea hydrothermal-vent and sea surface environments. Appl Environ Microbiol 70:6220–6229
Lannig G, Bock C, Sartoris FJ, Pörtner HO (2004) Oxygen limitation of thermal tolerance in cod, Gadus morhua L. studied by magnetic resonance imaging and on-line venous oxygen monitoring. Am J Phys 287:902–910
Legeay A, Massabuau JC (2000) Effect of salinity on hypoxia tolerance of resting green crabs, Carcinus maenas, after feeding. Mar Biol 136:387–396
MacDonald AG (1972) The role of high hydrostatic pressure in the physiology of marine animals. Symp Soc Exp Biol 26:209–231
MacDonald AG, Teal JM (1975) Tolerance of oceanic and shallow water Crustacea to high hydrostatic pressure. Deep Sea Res II 22:131–144
McCue MD (2006) Specific dynamic action: a century of investigation: a review. Molec Integr Physiol A 144:381–394
McGaw IJ (2005a) Does feeding limit cardiovascular modulation in the Dungeness crab Cancer magister during hypoxia? J Exp Biol 208:83–91
McGaw IJ (2005b) Prioritization or summation of events? Cardiovascular physiology of postprandial Dungeness crabs in low salinity. Physiol Biochem Zool 79:169–177
McGaw IJ (2006) Feeding and digestion in low salinity in an osmoconforming crab, Cancer gracilis, I. Cardiovascular and respiratory responses. J Exp Biol 209:3766–3776
McGaw IJ, Reiber CL (2000) Integrated physiological response to feeding in the blue crab Callinectes sapidus. J Exp Biol 203:359–368
Menzies RJ, George RY, Rowe GT (1973) Abyssal environment and ecology of the world ocean. Wiley Interscience, New York
Mestre N, Thatje S, Tyler PA (2009) The ocean is not deep enough: pressure tolerances during early ontogeny of the blue mussel Mytilus edulis. Proc Roy Soc B 276:717–726
Mickel TJ, Childress JJ (1982) Effects of temperature, pressure, and oxygen concentration on the oxygen consumption rate of the hydrothermal vent crab Bythograea thermydron (Brachyura). Phys Zool 55:199–207
Mozhaev VV, Heremans K, Frank J, Masson P, Balny C (1996) High pressure effects on protein structure and function. Proteins Struct Funct Bioinf 24:81–91
Murray TF, Siebenaller JF (1987) Comparison of the binding properties of A1 adenosine receptors in brain membranes of two congeneric marine fishes living at different depths. J Comp Physiol B 157:267–277
O'Brien J, Dahlhoff E, Somero GN (1991) Thermal resistance of mitochondrial respiration: hydrophobic interactions of membrane proteins may limit thermal resistance. Phys Zool 64:1509–1526
Peck LS (1998) Feeding, metabolism and metabolic scope in Antarctic marine ectotherms. In: Pörtner HO, Playle R (eds) Cold ocean physiology. Cambridge University Press, Cambridge, pp 365–390
Peck LS, Webb KE, Miller A, Clark MS, Hill T (2008) Temperature limits to activity, feeding and metabolism in the Antarctic starfish Odontaster validus. Mar Ecol Prog Ser 358:181–189
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Ravaux J, Gaill F, Le Bris N, Sarradin P-M, Jollivet DB, Shillito B (2003) Heat-shock response and temperature resistance in the deep-sea vent shrimp Rimicaris exoculata. J Exp Biol 206:2345–2354
Robertson RF, Meagor J, Taylor EW (2002) Specific dynamic action in the common shore crab, Carcinus maenas (L.) in relation to acclimatization temperature and the onset of emersion response. Biochem Physiol Zool 75:350–359
Robinson NJ, Thatje S, Osseforth C (2009) Heartbeat sensors under pressure: a new method for assessing hyperbaric physiology. High Pressure Res 29:422–430
Sébert P, Theron M, Vettier A (2004) Pressure and temperature interactions on cellular respiration: a review. Cell Mol Biol 50:491–500
Shillito B, Jollivet D, Sarradin P-M, Rodier P, Lallier F, Desbruyères D, Gaill F (2001) Temperature resistance of Hesiolyra bergi, a polychaetous annelid living on deep-sea vent smoker walls. Mar Ecol Prog Ser 216:141–149
Somero GN (1990) Life at low volume change: hydrostatic pressure as a selective factor in the aquatic environment. Am Zool 30:123–135
Somero GN (1992) Adaptations to high hydrostatic pressure. Annu Rev Physiol 54:557–577
Somero GN (2005) Linking biogeography to physiology: evolutionary and acclimatory adjustments to thermal limits. Front Zool 2:1–9
Soofiani NM, Priede IG (1985) Aerobic metabolic scope and swimming performance in juvenile cod, Gadus morhua L. J Fish Biol 26:127–138
Stillman JH, Somero GN (2000) A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol Biochem Zool 73:200–208
Thatje S, Anger K, Calcagno JA, Lovrich GA, Pörtner HO, Arntz WE (2005a) Challenging the cold: crabs reconquer the Antarctic. Ecology 86(3):619–625
Thatje S, Hillenbrand CD, Larter R (2005b) On the origin of Antarctic marine benthic community structure. Trends Ecol Evol 20:534–540
Thatje S, Casburn LM, Calcagno JA (2010) Behavioural and respiratory response of the shallow-water hermit crab Pagurus cuanensis to hydrostatic pressure and temperature. J Exp Mar Biol Ecol 390:22–30
Theron M, Sébert P (2003) Hydrostatic pressure and cellular respiration: are the values observed post-decompression representative of the reality under pressure? Mitochondrion 3:75–81
Wang T, Zaar M, Vedel-Smith C, Overgaard J (2002) Effects of temperature on the metabolic response to feeding in Python molurus. Comp Biochem Physiol A 133:519–527
Weinstein RB, Somero GN (1998) Effects of temperature on mitochondrial function in the Antarctic fish Trematomus bernacchii. Mar Biol 168:190–196
Whiteley NM, Robertson RF, Meagor J, El Haj AJ, Taylor EW (2001) Protein synthesis and specific dynamic action in crustaceans: effects of temperature. Comp Biochem Physiol A 128:593–604
Young CM, Tyler PA, Fenaux L (1997) Potential for deep-sea invasion by Mediterranean shallow water echinoids: pressure and temperature as stage-specific dispersal barriers. Mar Ecol Prog Ser 154:197–209
Acknowledgements
This work was supported by a grant (Abyss2100) from the Total Foundation to Sven Thatje, and greatly benefitted from frequent discussions with Bruce Shillito, Chris Hauton, and Olaf Heilmayer. Neil Jenkinson, Ray Collins, and Stephen Hayward were a great help in resolving technical problems during experimental work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thatje, S., Robinson, N. Specific dynamic action affects the hydrostatic pressure tolerance of the shallow-water spider crab Maja brachydactyla . Naturwissenschaften 98, 299–313 (2011). https://doi.org/10.1007/s00114-011-0768-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-011-0768-1