Abstract
Despite efforts to understand marine organismal responses to ocean acidification (gradual change in pH/\({p_{{\text{C}}{{\text{O}}_2}}}\) over decades), there is a lack of information about the capabilities of coastal organisms to endure rapid and extreme pH change (often full units within hours). We predicted that gastropods faced with estuarine acidification avoid extreme pH exposure through isolation and/or escape behavior, and energetically compensate for feeding and energy uptake limitations by facultative metabolic depression (FMD). To test this, we studied behavioral (organism activity) and aerobic (cardiac performance) responses to acidification in two closely related tropical intertidal species, the estuarine Indothais gradata (two populations) and the open-shore Reishia bitubercularis. Snails were exposed in the laboratory to either acutely declining or stable low pH conditions, using two acidification modes (HNO3-acidification and CO2-aeration). Under acutely declining pH, aerobic performance was regulated to unexpectedly low pH levels (4.5), effectively extending the field pH range for activity. This pH performance threshold marked the onset of behavioral isolation and FMD (as opposed to respiratory stress) and was lower in Indothais than Reishia snails during mineral acidification. Behavioral (in isolated gastropods) and environmental hypercapnic acidosis complicates interpretation of lowered metabolic performance. Stable reduced pH exposures resulted in different behavioral and physiological responses by the Indothais populations, including more prominent escape from water in the seaward population. Overall, these results suggest that aerobic and behavioral flexibility are crucial to organismal fitness in widely fluctuating pH circumstances. They further warn against overgeneralizing marine acidification consequences across physiological dispositions, taxonomic levels, and ecological systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pH is one of the most important determinants of organismal performance and ecological patterns in aquatic ecosystems (Kroeker et al. 2013a, b; Økland and Økland 1986; Schindler 1988). For organisms that lack the physiological mechanisms to regulate their internal acid–base balance, shifts in environmental pH can rapidly translate into changes in protein function and metabolic rate. For organisms with more sophisticated physiological machinery for acid–base regulation, changes in external pH result in energy expenditures to maintain homeostasis, and even the most sophisticated acid–base regulatory systems have limits. Due to energetic costs and/or physiological impairment, fluctuations in environmental pH can drive important shifts in organismal fitness, population growth, and ultimately community structure and ecosystem function.
The importance of pH in the marine environment has come to the fore with the realization that anthropogenic carbon dioxide emissions have the capacity to radically alter the chemistry of the oceans (Kleypas et al. 1999; Feely et al. 2004). In just 15 years, the topic of ocean acidification (OA) rose from relative obscurity to become one of the top priorities in marine research (Rudd 2014). Early efforts to understand the potential consequences of OA relied to a large degree on published physiological studies that used extreme pH values. Such extremes were soon judged to be unrealistic given anthropogenic emissions scenarios (IPCC 2013; Caldeira and Wickett 2003; Kimmerer and Weaver 2013), and were supplanted by studies on pH effects within the range of ~8.1 (ambient) to ~7.3 (strongly acidified), with the strongest emphasis being placed on studies that reduced pH by 0.5 units or less (e.g., Kroeker et al. 2013a). From this rapidly growing body of research, we now know that ‘moderate’ (≤0.5 pH units) acidification can result in a host of impacts ranging from changes in growth and survival to shifts in species interactions to reductions in biodiversity (Kroeker et al. 2013a, b; Heuer and Grosell 2014; Brown et al. 2016).
Such pH ranges, while appropriate for well-mixed marine waters subjected primarily to CO2-induced acidification, do not capture the full range of pH variation in the coastal marine environment. In particular, estuarine waters can exhibit much lower pH values than what has been observed or can be expected in the near future for well-mixed marine surface waters. Acidified estuaries occur in regions where groundwater drains through acidic soils and/or where eutrophic waters support extraordinary metabolic elevation of \({p_{{\text{C}}{{\text{O}}_2}}}\) (Duarte and Agusti 1998; Grealish and Fitzpatrick 2013; Cai et al. 2011; Hu and Cai 2013). Estuarine acidification (pH often <7) represents a largely overlooked aspect of the marine pH environment, and one for which the pH range used in traditional studies of OA may not be entirely relevant. Despite the limited attention they receive, acidified estuaries represent important habitat for countless species, and provide ecosystem goods and services to many coastal communities. Because species can survive and even thrive in these systems, acidified estuaries serve as a useful testing ground for the mechanisms and limits of acclimation and adaptation to extreme and fluctuating environmental acidity.
Here, we investigated physiological and behavioral capacities and responses of tropical gastropods to rapidly changing and extreme coastal environmental acidification. We explored the strategies for avoidance of extreme exposures and for the maintenance of a balanced energetic status. In particular, we tracked aerobic performance (cardiac activity) during acutely declining pH, to assess the pH range at which performance is sustained, as well as to assess the general aerobic response to severe acidification. In addition, physiological performance and movement behavior were monitored over time in snails experiencing stable, extremely reduced pH conditions, to assess recovery and escape responses. Although several recent studies consider marine gastropod responses to reduced pH exposures, these mainly concern OA contexts (pH and \({p_{{\text{C}}{{\text{O}}_2}}}\) levels expected by year 2100; Bibby et al. 2007; Melatunan et al. 2011; Parker et al. 2013; Lardies et al. 2014; Garilli et al. 2015; Harvey et al. 2015). While they inform about long-term ecological consequences, they are uninformative about the responses and strategies of species to maintain viable populations in dynamically changing acidified coastal waters. Furthermore, by design, OA experiments poorly consider alternative metabolic states, which underlie behavioral isolation in gastropods which functions to temporarily limit extreme exposure (see Abele et al. 2002; Gnaiger 2009; Marshall et al. 2011; Verberk et al. 2015).
We assessed species-level responses using the estuarine Indothais gradata and the closely related open coastal Reishia bitubercularis (Rapaninae, Muricidae). Population responses were evaluated for two I. gradata populations, each naturally acclimatized to different pH regimes at opposite ends of the acidified Brunei Estuarine System (BES; Marshall et al. 2008, 2016). Acidification of this system derives from multiple sources, including from sulfuric acid (H2SO4) discharge from pyritic sediments (FeS2) and CO2-supersaturation in the productive upper estuarine reaches (Marshall et al. 2016); physiological responses were compared for each mode of acidification.
Materials and methods
Study area and environment
The BES in Brunei Darussalam (Borneo, South East Asia) incorporates the Brunei Bay and Brunei, Temburong, Limbang, and Trusan Rivers (Lambiase and Cullen 2013; Bolhuis et al. 2014; Hossain et al. 2014; Fig. 1). The water is typically brown and turbid and carries high suspended sediment and organic loads. Habitat salinity (4–33) is influenced by tidal forcing in the South China Sea, stochastic swell forcing and freshwater inflow—which significantly increases during monsoon periods (Marshall et al. 2008; see Table 1). Water temperature varies little around 28 °C. Acidification derives from direct anthropogenic release, and naturally from the weak buffering capacity of the hyposaline water, groundwater discharge from pyritic sediments (H2SO4; acid sulfate soils), and eutrophication and CO2-supersaturation in the upper estuary (Marshall et al. 2008, 2016; Bolhuis et al. 2014; Table 1). Habitat pH and \({p_{{\text{C}}{{\text{O}}_2}}},\) respectively, range between 5.8 and 8.3, and ≈7000 and 400 µatm (Fig. 1; Table 1; Grealish and Fitzpatrick 2013; Marshall et al. 2008). Whereas pH and \({p_{{\text{C}}{{\text{O}}_2}}}\) are highly variable within a single tidal cycle due to mixing of hyposaline CO2 supersaturated water in the upper estuarine reaches, extreme baseline shifts in the tidal pattern occur during monsoons, primarily due to increased discharge of mineral acidified groundwater (Fig. 1). The Brunei River displays clear carbonate undersaturation to a point where it enters the Brunei Bay (Fig. 1). Despite acidification of the BES, the system supports rich benthic marine communities, with the intertidal snail Indothais gradata (Jonas, 1846) being ubiquitous and abundant along its length (Marshall 2009).
Localities and physicochemistry of environments. Snail localities are encircled and triangles represent sites where physicochemical data were collected. a BD, Indothais, Bandar; PB, Indothais, Pulau Bedukang; EH , Reishia, Empire Hotel, and PK, Pulau Keingaron. b Salinity and pH recordings showing tidal and monsoonal effects at BD and PK. Thin line monsoon (24 November–11 December 2013) and bold line intermonsoon (26 February–15 March 2014). Red indicates BD and black PK
Snail collection and maintenance
Individuals of I. gradata (shell length = 20.6–35.9 mm, n = 385) were collected from concrete piers at Bandar (BD: 4°53′9.58″ N, 114°56′26.40″ E) and a rocky outcrop at Pulau Bedukang in the Brunei Bay (PB: 4°58′21.66″ N, 115°3′12.89″ E), between January and June 2014 (Fig. 1). For comparison, an open ocean, closely related species, R. bitubercularis (Lamarck, 1822) was also investigated; specimens (shell length = 31.2–40.3 mm, n = 14 individuals) were collected from a natural rocky shore at Jerudong (Empire Hotel and Country Club, EH, 4°58′6.72″ E, 114°51′34.02″ N) (Fig. 1). All snails were collected from a mid-tidal level (approximately 0.5 m chart datum for a 2.0 m tidal range) and were transported to the laboratory (Universiti Brunei Darussalam) within 2 h, in water-filled polyethylene bags. Estuarine water (or seawater) was collected from each site and used to maintain the snails in the laboratory, following adjustment. Snails were kept in aquaria (20–30 L) containing aerated, filtered, and recirculated water. Temperature was controlled at 28 ± 0.6 °C (glass thermostatic heater, 300W/HE-300/25W). Salinity was monitored daily [Hach multi-meter (model HQ40d) and Intellical probes (Hach Lange GmbH Headquarter, Düsseldorf, Germany)]. pHNBS was measured with a Mettler Toledo pH transmitter 2100e and probes (Mettler-Toledo GmbH, Giessen, Germany), calibrated with pH 4.0, 7.0, and 9.2 buffers. Snails were fed on fresh mussel tissue provided daily.
Prior to experiment 1 (see below), snails from each site (BD and PB) were kept overnight (or up to 4 days) in the average paired salinity and pH conditions for the site of collection as well as the opposite site (for PB and BD, the conditions were respectively, 25 salinity/pH 8 and 12 salinity/pH 7; see Marshall et al. 2008). Aquarium water was prepared by mixing field water with a distilled water solution of NaOH (0.1 M) and HNO3 (1 M). HNO3 was used in the experiments as a stronger alternative to H2SO4. Reishia snails (EH) were held in water collected with the snails, with no further adjustment. Holding salinity and pH were checked twice daily and adjusted where necessary to a rounded unit. Prior to experiments 2 and 3 (see below), Indothais snails were kept overnight in water collected from their natural habitat, adjusted to the average pH and salinity of that habitat.
Cardiac responses to acutely declining pH (experiment 1)
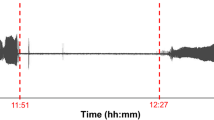
Heart beat rate (HR) was used as an index of aerobic performance, as it is easy to determine and functionally associates with oxygen delivery in gastropods (Marshall and McQuaid 1991, 2011; Marshall et al. 2011; Stenseng et al. 2005). HR is vastly easier to use compared to oxygen consumption or CO2 production, when tracking aerobic performance under continuously varying environmental conditions; this has proved to be the case in heating experiments involving aquatic invertebrates (Stillman and Somero 1996; Marshall et al. 2011; Polgar et al. 2014). Heart beat signals were detected non-invasively using optoelectronic (infrared) reflective sensors (CNY70, Vishay Semiconductors, Shelton, CT, USA) adhered to the shells near the mantle cavity (BluTac, Bostick, Australia, and Superglue, China) (see Marshall et al. 2011). The signals (cyclic traces in mV) were amplified and filtered with a custom-built bridge amplifier, and were digitally logged using a data acquisition system (PowerLab4/20 and LabChart v. 7, AD Instruments, Zenith Scientific, Australia). The number of heart beats per minute (BPM) was counted manually from traces (Fig. 2).
Heart beat rate (HR) parameter methods. a–c Effect of acutely declining pH (lines, right axis) on HR (symbols, left axis) of Indothais (BD), Indothais (PB), and Reishia (EH) snails. pH was lowered by HNO3 titration (see Fig. S1) and salinity is indicated in the figures. d–g Cardiac traces (for 1 min) at different times during acute acidification (see a above), symbols in f indicate periodicity of bradycardia. h–j Piecewise regressions fitted to plots for HR against pH (as shown in a–c), to determine slopes and pH performance thresholds (intersection)
During an experimental trial, water for each salinity (12 or 25) was acidified, starting from pH 8 or 7 (depending on the holding condition), using either HNO3 titration or CO2 aeration. An individual snail equipped with a HR sensor was allowed to settle for 20 min in a beaker (500 or 250 mL) of water of the same pH and salinity as the holding condition. The pH was lowered at a roughly constant rate (0.01–0.03 pH min −1) for 150–200 min (Fig. 2; Fig. S1). Given the logarithmic nature of pH, achieving a near-linear decline required constant monitoring and manual adjustment of aeration (tightening the screw clip on the feeder tube) or titration. At the end of an experiment, snails were returned to the same pH and salinity as at the start, to observe recovery over 20 min (Fig. 2a, b). Experimental beakers were held inside a temperature-controlled water bath set to a constant 28 °C (Fisher Grant W28). Beaker contents were continuously agitated with a submersible stirrer. HR and pH were simultaneously logged at 1-min intervals during the initial, experimental, and the recovery stages. pHNBS was monitored using a Nexsens pH electrode, calibrated using three buffers (pH 4.0, 7.0, and 9.2), and logged using WQS sensor software (NexSens Technology, Inc., Ohio, USA).
Preliminary experimental trials revealed that when pH was slowly lowered, HR was maintained at a near-constant level (termed aerobic regulation) down to a breakpoint (pH performance threshold; Fig. 2). This threshold was statistically assessed from piecewise regressions fitted to plots for HR against pH (Sigmaplot ver. 13; Fig. 2c). Computations were based on f = [(y 1 * (T 1 − t) + y 2 * (t − t 1))/(T 1 − t 1) for t 1 ≤ t ≤ T 1 (region 1(t)) and (y 2 * (t 2 − t) + y 3 * (t − T 1))/(t 2 − T 1) for T 1 ≤ t ≤ t 2 (region 2(t))], where t = pH, t 1 = min(t), t 2 = max(t). y 1, y 2, y 3, and T 1 (the pH breakpoint) are calculated parameters. Aerobic regulation was assessed from the slope of the left-side regression [region 1(t)]; a slope equal to zero represents perfect regulation. Capacity for aerobic recovery after exposure to reduced pH was assessed from HR performance after pH was normalized again at the end of a trial; initial (before) and final (after) HRs (each averaged over 20 min) were compared (Fig. 2). One-way ANOVAs were used to compare slopes and breakpoints for cardiac performances between treatments (species and populations—BD, PB, and EH), salinities, and acidification modes (CO2 and HNO3). Pre- and post-experimental HRs were compared using dependent t tests (Statistica 12, Statsoft, USA). We tested the predictions that snail species and populations that naturally experience more acidic conditions would, under declining pH, better regulate aerobic performance, have a lower pH performance threshold, and show more rapid recovery.
Cardiac responses to stable low pH and salinity (experiment 2)
We hypothesized that the Indothais populations should differ relative to natural acidity and salinity regimes experienced. Compared to BD snails, which experience more extreme tidally fluctuating conditions, PB snails were predicted to show greater aerobic depression and take longer to recover under chronically reduced pH. Snail HR was monitored every 1 min over 5 h for snails exposed to each of the following treatments: salinity 12/pH 6, salinity 12/pH 5, salinity 12/pH 4, salinity 25/pH 6, salinity 25/pH 5, and salinity 25/pH 4. At the start of an experiment, a snail supporting an HR sensor was placed in a beaker (500 or 400 mL) having the same water chemistry to that in which it was kept overnight. The beaker was held inside a Grant temperature-controlled water bath (28 °C). After monitoring the HR for 20 min, the snail was abruptly transferred to another beaker containing one of the six experimental treatments. The experiment was repeated seven times (n = 7 snails) for each treatment and each snail population. Experimental solutions were prepared from seawater, distilled water, and HNO3 1 M and NaOH 0.1 M solutions (see above). pH and salinity were checked hourly during the experiment (Mettler Toledo, pH Transmitter 2100e, Giessen, Germany; Hach, model HQ40d, Loveland, Colorado, USA). Effects of population, salinity, and pH on HR were determined for each hour from five separate generalized linear models (GLIMs) using a normal distribution and an identity-link function (Statistica ver. 12, Statsoft, USA). Data comprised untransformed averaged HR (BPM) for each individual collected over the last 10 min of each hour.
Behavioral responses to stable low pH and salinity (experiment 3)
We assumed that snails acclimated their behavior (adjustment during an individual’s lifetime) in accordance with the field acidity and salinity regime experienced. We predicted that PB (seaward) snails, when free to move, would attempt to escape from extremely low pH and salinity (Amaral et al. 2014). Because BD snails (landward) naturally experience more extreme and fluctuating water chemistry, we predicted that they would protect themselves (limit exposure) by isolating from the environment (withdrawal into shell), but would recover within the timeframe of a tidal cycle.
For each combination of source population (BD or PB), salinity (12 or 25), and acidity (4, 5 or 6 pH units), 7 individual snails (replicated 3 times) were placed together in an experimental beaker (500 mL), which was held in a Grant temperature-controlled water bath (28 °C). The position of each snail in the beaker was determined at 2-h intervals for 12 h. Movement was recorded when a snail moved from the bottom to the vertical sides of the beaker, but remained underwater. Surfacing (or escape behavior) was assessed from a snail breaking the air–water interface. Six separate GLIMs were run for a binomial distribution using a logit-link function (Statistica ver. 12, Statsoft, USA). These accounted for all effects (populations, salinity, pH, and time), as well as within-population effects (salinity, pH and time) for each population (BD and PB) and each behavior type (movement, surfacing). Three time intervals only were used (2, 6, and 12 h), and the response data comprised either moved or not moved (3 models) or either surfaced or not surfaced (3 models). Seawater chemistry was prepared as in experiment 2.
Results
Cardiac response to acutely declining pH (experiment 1)
HR response to acutely declining pH differed between the gastropod species, the Indothais populations, and between salinities and acidification modes. At the start of an experiment, individual snail HRs were variable (25 to 52 BPM), presumably relating to differences in size, sex, reproductive condition, and satiation level. HRs remained fairly constant initially when the pH was lowered, consistent with aerobic regulation. With progressive acidification there was a clear breakpoint, at which HR began to decelerate, until a stable bradycardia was eventually achieved (usually HR <10 BPM, Figs. 2, 3). Although the slopes of fitted piecewise regressions (before the breakpoint) tended to be relatively low for BD HNO3 snails, no significant differences were observed for any of the conditions (species, populations, acidification modes or salinities; mean slopes differed between 1.3 and 4.4, F = 1.86, P = 0.110, ANOVA; Cochran C = 0.28, Fig. 3). In contrast, breakpoints (pH performance thresholds) varied markedly among the conditions (F = 58.36, P < 0.001, Cochran C = 0.29, Fig. 3). For mineral acidification, thresholds were largely similar in the Indothais populations (4.54–5.14 pH), and were higher than in Reishia snails (6.7 pH; Fig. 3; Tukey HSD test, Fig. 3). CO2 acidification, however, raised the threshold in Indothais (BD) snails (to 6.4) to a level similar to that in Reishia snails for this acidification mode (Fig. 3). HR rapidly recovered (within min) when pH was normalized at the end of an experiment, after snails had experienced acidic water exposure and exhibited bradycardia. There was no significant difference between HRs before and after exposure for any of the conditions (Table 2). Post-experimental snails appeared healthy; they quickly emerged from their shells and remained attached to the walls of the recovery holding container for another 24 h.
Effects of species, population, and treatment on pH performance threshold and aerobic regulation under acutely declining pH. Populations are shown on bottom axis and treatments are given as symbols [mean ± 1SE; BD, Indothais, Bandar (landward); PB, Indothais, Pulau Bedukang (seaward); EH, Reishia)]. Circles indicate mineral acidification and triangles CO2-acidification. Closed symbols indicate 12 salinity, and open symbols 25 salinity. Different letters associated with symbols in the upper panel (pH threshold) indicate P < 0.05, and numbers show sample size. Aerobic regulation (lower panel) is assessed from the slope of HR versus pH, before the pH threshold (P = 0.110)
Physiological responses to stable low pH and salinity (experiment 2)
When snails were abruptly transferred to reduced pH conditions, HR decelerated rapidly (Fig. 4). Cardiac recovery over the following 5 h of exposure to stable reduced pH (and salinity) differed between the populations (Fig. 4; Table 3; Fig. S2). Population, salinity, and pH yielded significant effects (Table 3). Although there was no difference in HR performance among the populations initially (the first 3 h), BD snails (landward) generally showed better temporal recovery than PB snails (Fig. 4; Table 3; Fig. S2). Both populations showed limited to no recovery of HR at the lowest pH (4), for both salinities. BD snails recovered clearly better than PB snails in salinity 12 at the higher pHs (5 and 6; Fig. 4; Fig. S2). Recovery of PB snails was better in higher salinities at the higher pHs (5 and 6; Fig. 4). Snails of both populations recovered well in salinity 25 at pH 6.
Temporal effect of stable reduced pH exposure on HR for two populations of Indothais snails. BD landward population, is shown in bold/red and PB the seaward population, in thin/black. Lines indicate the mean of seven individual recordings taken every 1 min. Salinities are indicated above the figure panels
Behavioral responses to stable low pH and salinity (experiment 3)
When free to move, snails from the different populations clearly differed in movement behavior during 12-h exposure to extremely low pH and salinity (Fig. 5; Table 4). Generally, snails from the landward population (BD) showed reduced movement and surfacing compared to the seaward population (PB; Fig. 5). Analyses considering BD snails only, showed that pH had no effect on movement or surfacing, whereas salinity significantly affected surfacing (Table 4). Under the higher salinity conditions (25), unlikely to be naturally experienced by this population, snails initially suppressed all movement for 4 h in all pH treatments; this was followed by strong time-related movement behavior (Fig. 5). In contrast, separate analyses for PB showed significant effects of both pH and salinity on both movement and surfacing (Table 4). PB snails showed a great incidence of movement within the first 2 h of exposure in all treatments, though behavior was generally more readily induced in salinity 25 (similar to field conditions) compared to salinity 12 (which they never experience; Fig. 5). Notably, 100% surfacing was achieved in PB snails within 4 h in the apparently most benign of exposures for this population (salinity 25/pH 6).
Temporal effect of stable reduced pH exposure on behavior (movement and surfacing) of Indothais snails. Proportional responses (n = 21) for BD snails (landward population, red/open circles) and PB snails (seaward population, black/closed triangles). Movement is shown by dashed lines and surfacing by solid lines
Discussion
We found that in acidic estuarine water, gastropods modify their physiology and behavior in adaptive and plastic ways to limit exposure to extremes and to facilitate energy balance for improved individual fitness. Avoidance of extreme pH exposure is suggested by behavioral isolation or escape from the acidic water. Improved energy balance is achieved by extending the pH range for performance and hence the time for feeding and energy uptake under variable pH conditions. However, when resting or isolated, snails are also capable of conserving energy through facultative aerobic depression (FMD; steady-state downregulation of cellular aerobic processes). Acidification mode affected the pH performance threshold. This occurred at a higher pH during \({p_{{\text{C}}{{\text{O}}_2}}}\)-aeration than during mineral acidification, suggesting environmental hypercapnia as a mechanism triggering FMD, but also suggesting different physiological outcomes in estuaries and coastal systems in relation to the primary acidification source (mineral or CO2).
Maintenance of near-constant high aerobic performance under acutely declining pH was contrary to our expectation, given that marine molluscs are generally poor regulators of their body fluids (which increases energy consumption) compared to other marine animals (especially fishes and crustaceans; see Wittmann and Pörtner 2013). Active intertidal gastropods, nonetheless, have good capacities for intracellular ion and pH regulation (pHi), allowing near-optimal functionality under widely fluctuating conditions. Although pHi regulation requires significant metabolic demand (Mason and Nott 1981; Kapper et al. 1985; Sokolova et al. 2000; Wittmann and Pörtner 2013), this should be countered by the energetic benefit derived from extending the time and pH range for activity and feeding in highly variable pH circumstances. When comparing pH performance ranges with the naturally experienced pH range of the BES, all of the snails should be able to sustain activity most of the time (see Fig. 1, Table 1). Our pH habitat recordings, however, do not explain why pH performance thresholds were so low, especially those of Indothais snails during mineral acidification (4.54–5.14). Such capacity might derive from having experienced more extreme acidification within the timeframe of their Miocenic origin, or might represent trait conservation within a deeper lineage (Hönisch 2012; Claremont et al. 2013). Adaptive selection of this threshold is nonetheless suggested here by marked environmentally related differences between I. gradata (which experiences extremes in pH) and the closely related open-shore R. bitubercularis (which experiences relatively benign pH conditions) (see Tables 1, 2).
Should Indothais snails face (or have faced) naturally extreme circumstances in which estuarine water pH falls below their performance threshold (possibly during monsoon flooding), our data suggest that they are likely capable of undergoing behavioral isolation and FMD. When laboratory acidification progressed beyond the pH performance threshold, HR fell rapidly and then stabilized in the form of a bradycardia (usually <10 BPM). Features of this bradycardia indicate FMD, including (i) distinct heart beat periodicity (lacking arrhythmias and/or intermittent acardia that associate with respiratory stress; Marshall and McQuaid 1991; Marshall et al. 2004; Farrell 2016; see Fig. 2) and (ii) the ability to rapidly normalize cardiac performance when benign conditions are returned (a time lag usually accompanies molecular reconfiguration during recovery from stress; see Fig. 2; Hofmann and Somero 1995, 1996; Somero 2002, 2010). Whereas reduced cardiac and aerobic rates in marine animals often result from respiratory incapacity (insufficiency to extract oxygen through impaired functioning of the respiratory organs), in gastropods, individuals invoke FMD when isolating themselves from their environment (see Guppy and Withers 1999; Marshall and McQuaid 1991, 1993, 2011; Marshall et al. 2011). This metabolism effectively (i) reduces ROS production and oxidative tissue damage, (ii) lowers production and accumulation of acidic metabolites, and (iii) conserves energetic resources (Strahl et al. 2011).
The overall effectiveness of FMD in conserving energy, however, depends on the level of depression of total metabolism without involving compensatory anaerobic energy generation. Unlike many intertidal animals that have a limited capacity for total metabolic depression and compensate for reduced aerobic rates during air or hypoxia exposure, many gastropods can lower total metabolism to below 20% of the standard resting level (see Wieser 1980; Brinkhoff et al. 1983; Guppy and Withers 1999; Sokolova and Pörtner 2001, 2003; Marshall et al. 2011). Limited anaerobic involvement during aerobic depression (bradycardia) is suggested in our experimental animals by the weak and non-significant respiratory overshoot (elevated HR) observed when conditions were normalized after extreme acidic exposure (an overshoot would indicate repayment of an oxygen debt that might have incurred during anaerobiosis; Fig. 2; Table 2; Brown and Wynberg 1987; Marshall and McQuaid 1991, 1993). Furthermore, enhanced metabolic regulatory capacity of Indothais snails is suggested by field observations of aestivating individuals buried in muddy sediments for long periods (Marshall 2009). Although several other studies suggest enhanced capacities in the rapanine/muricid snail lineage for hypoxia tolerance and overall metabolic depression (Stramonita haemastoma; Stickle et al. 1989), anaerobic energy use is suggested in the case of Littorina littorea snails experiencing exceptionally long laboratory exposures to elevated \({p_{{\text{C}}{{\text{O}}_2}}}\) (Melatunan et al. 2011).
Behavioral isolation must go hand in hand with FMD in enabling snails to avoid extreme acid exposure, but we were unable (within our experimental context) to determine isolation behavior directly. This takes the form of either withdrawal of the entire animal into the shell (easily observed), or only mantle cavity exclusion, with the foot remaining extended. Regardless of the isolation form, impedance of mantle cavity ventilation leads to mantle water hypercapnia and a respiratory acidosis, which signals the onset of cellular aerobic downregulation (see Barnhart 1986a, b; Barnhart and McMahon 1987, 1988; Rees et al. 1991; Marshall and McQuaid 2011). Reduced perfusion (bradycardia) then becomes matched with the reduced cellular oxygen demand. Notably, an environmental hypercapnic acidosis is likely to have the same effect on mantle water pH and \({p_{{\text{C}}{{\text{O}}_2}}}\) (and the resulting physiology) as behavioral isolation, whatever the cause may be. The lower pH for behavioral isolation (implied from the lower performance threshold) during slow steady mineral acidification, compared to that during CO2-aeration, implies an additional effect of environmental hypercapnia on this isolation response. Different effects of the acidification mode on isolation should have ecological/evolutionarily implications, especially when these modes vary among environments. Ensuring a relatively broad pH range for activity in Indothais BD snails (landward) is consistent with the more severe mineral acidification they experience, especially during monsoonal downpours (Fig. 1). Similar pH performance thresholds of the two species during CO2-aeration (Fig. 3), suggests a phylogenetic constraint for hypercapnic-induced isolation and the associated FMD. Unraveling the interacting effects of environmentally reduced pH and elevated \({p_{{\text{C}}{{\text{O}}_2}}}\) on behavioral isolation and FMD in gastropods, thus, seems to be important to understanding their general metabolic and fitness responses to acidification (see also Michaelidis et al. 1999, 2005; Wittmann and Pörtner 2013). Arguably, more consideration should be given to the link between reduced oxygen uptake and behavioral isolation to avoid environmental stress, rather than only considering reduced metabolism in terms of stress-related, capacity-limited physiology. Adaptive FMD, rather than a stress-related (capacity-limited) response, is further confirmed in our study snails by cardiac recovery with an increase in the exposure period to a stable reduced pH (Table 3).
The Indothais populations differed with regard to cardiac and activity responses of snails when exposed to stable reduced pH and salinity. The BD snails (especially at pH 6) showed better cardiac recovery with time, suggesting possible anticipation of tidally changing/improving environmental conditions in preparation for activity and feeding. PB snails (from the less variable environmental conditions) on the other hand showed weak physiological recovery and persistent bradycardia, suggesting maintenance of isolation to limit extreme water chemistry exposure. When free to move, however, PB snails more commonly and rapidly initiated escape behavior, and crawled out of the water; this must ultimately be detrimental by exposing snails in air for longer. Initial inactivity of free-to-move BD snails suggests more refined acclimatized behavior to limit exposure (through inactivity) to the extreme water chemistry that they may occasionally experience during fluctuating tides and especially during monsoon flooding. The combination of patterns of inactivity of BD snails (acclimatized to extremes in water chemistry) and escape behavior of non-acclimatized PB snails contrasts with findings for the only other known similar estuarine gastropod study (Amaral et al. 2014). Bembicium auratum snails (Australia) that were naturally pre-exposed to acidic conditions moved more rapidly and in larger numbers out of experimentally acidified water, compared to snails sourced from (non-acidic) reference sites (Amaral et al. 2014). Differences between these studies should however be seen in the context of different habitat requirements of the study species. Unlike Indothais, which is functionally a mid-shore species with limited air exposure tolerance, Bembicium belongs to a taxonomic family (Littorinidae) predisposed for a high-shore and prolonged air exposure existence (Reid 1988).
Capacity to avoid extreme conditions (through behavioral isolation) and balance energy through metabolic flexibility, including energy-conserving FMD when resting, explains the observed establishment of flourishing Indothais populations in the highly acidic BES water. These traits are probably multifunctional, allowing snails to endure other physical estuarine extremes (salinity), as well as enduring limitation on food uptake that typically accompanies unpredictable and extreme environments (see Marshall 2009). Analogously, these traits which occur widely in gastropods are thought to permit ecological transitions across extreme marine and terrestrial environments (see Webb 2012; Marshall et al. 2015; Verberk et al. 2015). Because energy-conserving FMD when resting theoretically improves fitness (an alternative interpretation to the usually negative implication for stress-related, suboptimal performance), we need to understand better which gastropod lineages are predisposed for this physiology. The between- and within-species differences in physiological and behavioral responses of our study gastropods to acidification caution against overgeneralizing marine environmental acidification consequences across taxonomic levels and ecological systems. Finally, our findings lead to question the extent to which coastal systems, which already experience highly variable conditions and support appropriately adapted biota, are likely to be affected by the relatively subtle changes in pH and \({p_{{\text{C}}{{\text{O}}_2}}}\) that derive from atmospheric CO2 elevation (Meinshausen et al. 2011) and are having a major impact on open oceanic systems.
References
Abele D, Heise K, Pörtner HO, Puntarulo S (2002) Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J Exp Biol 205:1831–1841
Amaral V, Cabral HN, Bishop MJ (2014) Prior exposure influences the behavioral avoidance by an intertidal gastropod, Bembicium auratum, of acidified waters. Estuar Coast Shelf Sci 136:82–90
Barnhart MC (1986a) Control of acid–base status in active and dormant land snails, Otala lactea (Pulmonata, Helicidae). J Comp Physiol B 156:347–354
Barnhart MC (1986b) Respiratory gas tensions and gas exchange in active and dormant land snails, Otala lactea. Physiol Zool 59:733–745
Barnhart MC, McMahon BR (1987) Discontinuous carbon dioxide release and metabolic depression in dormant land snails. J Exp Biol 128:123–138
Barnhart MC, McMahon BR (1988) Depression of aerobic metabolism and intracellular pH by hypercapnia in land snails, Otala lactea. J Exp Biol 138:289–299
Bibby R, Cleall-Harding P, Rundle S, Widdicombe S, Spicer J (2007) Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol Lett 3:699–701
Bolhuis H, Schluepmann H, Kristalijn J, Sulaiman Z, Marshall DJ (2014) Molecular analysis of bacteria diversity in mudflats along the salinity gradient of an acidified tropical Bornean estuary (South East Asia). Aquat Biosyst 10:10
Brinkhoff W, Stockmann K, Grieshaber M (1983) Natural occurrence of anaerobiosis in molluscs from intertidal habitats. Oecologia 57:151–155
Brown AC, Wynberg RP (1987) Absence of an oxygen debt in the nassariid whelk Bullia digitalis (Dillwyn). J Molluscan Stud 53:289–290
Brown NEM, Therriault TW, Harley CDG (2016) Field-based experimental acidification alters fouling community structure and reduces diversity. J Anim Ecol 85:1328–1339
Cai WJ, Hu X, Huang JW, Murrell MC, Lehrter JC, Lohrenz SE, Chou WC, Zhai W, Hollibaugh JT, Wang Y, Zhao P, Guo X, Gundersen K, Dai M, Gong GC (2011) Acidification of subsurface coastal waters enhanced by eutrophication. Nat Geosci Lett. doi:10.1038/ngeo1297
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:356
Claremont M, Vermeij GJ, Williams ST, Reid DG (2013) Global phylogeny and new classification of the Rapaninae (Gastropoda: Muricidae), dominant molluscan predators on tropical rocky seashores. Mol Phylogenet Evol 66:91–102
Duarte CM, Agusti S (1998) The CO2 balance of unproductive aquatic ecosystems. Science 28:234–236
Farrell AP (2016) Pragmatic perspective on aerobic scope: peaking, plummeting, pejus and apportioning. J Fish Biol 88:322–334
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305:362–366
Garilli V, Rodolfo-Metalpa R, Scuderi D, Brusca L, Parrinello D, Rastrick SPS, Foggo A, Twitchett RJ, Milazzo M (2015) Physiological advantages of dwarfing in surviving extinction in high-CO2 oceans. Nat Clim Change 5:678–682
Gnaiger E (2009) Mitochondrial pathways and respiratory control, 2nd edn. OROBOROS MiPNet Publications, Innsbruck, p 43–53
Grealish GJ, Fitzpatrick RW (2013) Acid sulphate soil characterization in Negara Brunei Darussalam: a case study to inform management decisions. Soil Use Manag 29:432–444
Guppy M, Withers P (1999) Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol Rev 74:1–40
Harvey BP, McKeown NJ, Rastrick SPS, Bertolini C, Foggo A, Graham H, Hall-Spencer JM, Milazzo M, Shaw PW, Small DP, Moore PJ (2015) Individual and population-level responses to ocean acidification. Sci Rep 6:20194
Heuer RM, Grosell M (2014) Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol 307:R1061–R1084
Hofmann GE, Somero GN (1995) Evidence for protein damage at environmental temperatures: seasonal changes in levels of ubiquitin conjugates and hsp70 in the intertidal mussel Mytilus trossulus. J Exp Biol 198:1509–1518
Hofmann GE, Somero GN (1996) Interspecific variation in thermal denaturation of proteins in the congeneric mussels Mytilus trossulus and M. galloprovincialis: evidence from the heat shock response and protein ubiquitination. Mar Biol 126:65–75
Hossain MB, Marshall DJ, Venkatramanan S (2014) Sediment granulometry and organic matter content in the intertidal zone of the Sungai Brunei Estuarine System, northwest coast of Borneo. Carpathian J Earth Environ Sci 9:231–239
Hönisch B et al (2012) The geological record of ocean acidification. Science 335:1058–1063
Hu X, Cai WJ (2013) Estuarine acidification and minimum buffer zone—a conceptual study. Geophys Res Lett 40:5176–5181
IPCC (2013) Climate change 2013: Intergovernmental Panel on Climate Change. The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Kapper MA, Stickle WB, Blakeneyz E (1985) Volume regulation and nitrogen metabolism in the muricid gastropod, Thais haemastoma. Biol Bull 169:458–475
Kimmerer W, Weaver MJ (2013) Vulnerability of estuaries to climate change. In: Pielke RA (ed) Climate vulnerability, 1st edn. Academic, Oxford, p 271–292
Kleypas JA, Buddemeier RB, Archer D, Gattuso, J-P, Langdon C, Opdyke BN (1999) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284:118
Kroeker KJ, Kordas R, Crim R, Hendriks I, Ramajo L, Singh G, Duarte CM, Gattuso J-P (2013a) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19:1884–1896
Kroeker KJ, Micheli F, Gambi MC (2013b) Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat Clim Change 3:156–159
Lambiase JJ, Cullen AB (2013) Sediment supply systems of the champion “Delta” of NW Borneo: implications for deep water reservoir sandstones. J Asian Earth Sci 76:356–371
Lardies MA, Arias MB, Poupin MJ, Manriquez PH, Torres R, Vargas CA, Navarro JM, Lagos NA (2014) Differential responses to ocean acidification in physiological traits of Concholepas concholepas populations. J Sea Res 90:127–134
Marshall DJ (2009) Predatory and reproductive responses of the estuarine whelk Thais gradata (Caenogastropoda Muricidae) to novel colonization by Musculista senhousia (Bivalvia, Mytilidae). J Mar Biol Assoc UK 89:1387–1393
Marshall DJ, McQuaid CD (1991) Metabolic rate depression in a marine pulmonate snail: pre-adaptation for a terrestrial existence? Oecologia 88:274–276
Marshall DJ, McQuaid CD (1993) Differential physiological and behavioral responses of the intertidal mussels, Choromytilus meridionalis (Kr.) and Perna perna L., to exposure to hypoxia and air: a basis for spatial separation. J Exp Mar Biol Ecol 171:225–237
Marshall DJ, McQuaid CD (2011) Warming reduces metabolic rate in marine snails: adaptation to fluctuating high temperatures challenges the metabolic theory of ecology. Proc R Soc B 278:281–288
Marshall DJ, Peter R, Chown S (2004) Regulated bradycardia in the pulmonate limpet Siphonaria (Gastropoda: Mollusca) during pollutant exposure: implication for biomarker studies. Comp Biochem Physiol 139:309–316
Marshall DJ, Rezende E, Baharuddin N, Choi F, Helmuth B (2015) Thermal tolerance and climate warming sensitivity in tropical snails. Ecol Evol 5:5905–5919
Marshall DJ, Santos JH, Leung, KMY, Chak WH (2008) Correlation between gastropod shell dissolution and water chemical properties in a tropical estuary. Mar Environ Res 66:422–429
Marshall DJ, Dong Y, McQuaid CD, Williams GA (2011) Thermal adaptation in the intertidal snail Echinolittorina malaccana contradicts current theory by revealing the crucial roles of resting metabolism. J Exp Biol 214:3649–3657
Marshall DJ, Proum S, Hossain MB, Adam A, Lim L-H, Santos JH (2016) Ecological responses to fluctuating and extreme marine acidification: lessons from a tropical estuary (the Brunei Estuarine System). Sci Bruneiana 15:1–17
Mason AZ, Nott JA (1981) The role of intracellular biomineralized granules in the regulation and detoxification of metals with special reference to marine prosobranch Littorina littorea. Aquat Toxicol 1:239–256
Meinshausen M et al (2011) The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Change 109:213–241
Melatunan S, Calosi P, Rundle SD, Moody AJ, Widdicombe S (2011) Exposure to elevated temperature and pCO2 reduces respiration rate and energy status in the periwinkle Littorina littorea. Physiol Biochem Zool 84:583–594
Michaelidis B, Rofalikou E, Grieshaber M (1999) The effects of hypercapnia on force and rate of contraction and intracellular pH of perfused ventricles from the land snail Helix lucorum (L.). J Exp Biol 202:2993–3001
Michaelidis B, Ouzounis C, Paleras A, Pörtner H-O (2005) Effects of long-term moderate hypercapnia on acid–base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar Ecol Ser 293:109–118
Økland J, Økland KA (1986) The effects of acid deposition on benthic animals in lakes and streams. Experientia 42:471–486
Parker LM, Ross PM, O’Connor WA, Pörtner H-O, Scanes E, Wright JM (2013) Predicting the response of molluscs to the impact of ocean acidification. Biology 2:651–692
Polgar G, Khang TF, Chua T, Marshall DJ (2014) Gross mismatch between thermal tolerances and environmental temperatures in a tropical freshwater snail: climate warming and evolutionary implications. J Therm Biol 47:99–108
Rees BB, Malhotra D, Shapiro JI, Hand SC (1991) Intracellular pH decreases during entry into estivation in the land snail Oreohelix strigosa. J Exp Biol 159:525–530
Reid DG (1988) The genera Bembicium and Risellopsis (Gastropoda: Littorinidae) in Australia and New Zealand. Rec Aust Mus 40:91–150
Rudd MA (2014) Scientists’ perspectives on global ocean research priorities. Front Mar Sci 1:36
Schindler DW (1988) Effects of acid rain on freshwater ecosystems. Science 239:149–157
Sokolova IM, Pörtner H-O (2001) Physiological adaptations to high intertidal life involve improved water conservation abilities and metabolic rate depression in Littorina saxatilis. Mar Ecol Prog Ser 224:171–186
Sokolova IM, Pörtner H-O (2003) Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. J Exp Biol 206:195–207
Sokolova IM, Bock C, Pörtner H-O (2000) Resistance to freshwater exposure in White Sea Littorina spp. II: acid–base regulation. J Comp Physiol B 170:105–115
Somero GN (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr Comp Biol 42:780–789
Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213:912–920
Stenseng E, Braby CE, Somero GN (2005) Evolutionary and acclimation-induced variation in the thermal limits of heart function in congeneric marine snails (genus Tegula): implications for vertical zonation. Biol Bull 208:138–144
Stickle WB, Kapper MA, Liu L-L, Gnaiger E, Wang SY (1989) Metabolic adaptations of several species of crustaceans and molluscs to hypoxia: tolerance and microcalorimetric studies. Biol Bull 177:303–312
Stillman JH, Somero GN (1996) Adaptation to temperature stress and aerial exposure in congeneric species of intertidal porcelain crabs (genus Petrolisthes): correlation of physiology, biochemistry and morphology with vertical distribution. J Exp Biol 199:1845–1855
Strahl J, Brey T, Philipp EER, Thorarinsdóttir G, Fischer N, Wessels W, Abele D (2011) Physiological responses to self-induced burrowing and metabolic rate depression in the ocean quahog Arctica islandica. J Exp Biol 214:4221–4231
Verberk WCEP, Bartolini F, Marshall DJ, Portner H-O, Terblanche JS, White CR, Giomi F (2015) Can respiratory physiology predict thermal niches? Ann N Y Acad Sci 1365:73–88
Webb TJ (2012) Marine and terrestrial ecology: unifying concepts, revealing differences. TREE 27:535–541
Wieser W (1980) Metabolic end products in three species of marine gastropods. J Mar Biol Ass (UK) 60:175–180
Wittmann AC, Pörtner H-O (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Change 3:995–1001
Acknowledgements
Funding to DJM was through a Universiti Brunei Darussalam Grant (UBD/GRS/S&T/16). SP was funded through a bursary attached UBD/GRS/S&T/16 and through the International Science Program, Uppsala University, Sweden, and the Royal University of Phnom Penh (RUPP), Cambodia (IPICS CAB:1). CH visited Brunei with funding through iCUBE (Universiti Brunei Darussalam), and was supported by a Canadian NSERC Discovery Grant. The manuscript was improved by inclusion of comments of two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Responsible Editor: A. E. Todgham.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Proum, S., Harley, C.D., Steele, M. et al. Aerobic and behavioral flexibility allow estuarine gastropods to flourish in rapidly changing and extreme pH conditions. Mar Biol 164, 97 (2017). https://doi.org/10.1007/s00227-017-3124-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3124-y