Abstract
We report new dental remains of Mustelidae from the late middle Miocene of Mae Moh Basin, northern Thailand, improving the poor fossil record of the family in Southeast Asia. Siamogale thailandica is a poorly known mustelid, previously recorded from just a single tooth. Here we present over a hundred new specimens attributable to this species. S. thailandica shows a combination of primitive and convergent features of the dentition that makes its original subfamilial assignment to Lutrinae doubtful. Evidence from the dental morphology suggests that it belongs to a bunodont otter-like mustelid that evolved in convergence with “true” otters (Lutrinae) toward a semi-aquatic way of life. Autapomorphic features such as the height and the position of the m1 metaconid and the shape of the P4 lingual shelf make S. thailandica unique among Mustelidae. The morphology of this species is mostly similar to Mionictis species and Lartetictis dubia, reported in the Miocene of North America and Europe, respectively. These similarities could imply immigration events to Thailand in the early or middle Miocene. Alternately, the lineage leading to Siamogale might have deeper origins from an endemic early Miocene Southeast Asian mustelid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
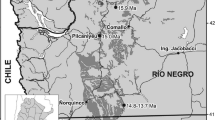
Northern Thailand comprises numerous intramontane basins that have yielded a rich and diversified Neogene mammalian fauna, including fossil hominoid (Chaimanee et al. 2003). One of these basins, the Mae Moh Basin, has provided one of the richest Miocene vertebrate fossil record of Southeast Asia, with several mammal taxa: mustelid and amphicyonid carnivorans, elephantoid, and deinothere proboscideans, a rhinocerotid, a cervid, a palaeochoerid, suids, rodents, a sivaladapid, and an undescribed tarsiid primate (Von Koenigswald 1959; Ginsburg et al. 1983; Ducrocq et al. 1994, 1995; Peigné et al. 2006; Chaimanee et al. 2008; Chavasseau 2008; Chavasseau et al. 2009). The basin is located in the Lampang province, approximately 26 km east of Lampang city (Fig. 1a). The Tertiary sedimentological filling of Mae Moh has been estimated by biostratigraphy to range from the middle part of the middle Miocene to the early late Miocene (Ginsburg and Tassy 1985; Ducrocq et al. 1995). Recent magnetic polarity stratigraphy investigations dated this sequence to the late middle Miocene, from 14.2 to 12.1 Ma (Benammi et al. 2002; Coster et al. 2010). The richest mammal-bearing formation of the basin, the Na Khaem Formation, is constituted by lacustrine and fluviatile claystones, mudstones, and siltstones, interbedded with fossiliferous layers of lignites (named R, Q, K, and J from the base to the top, Fig. 1b; see Benammi et al. 2002 and Coster et al. 2010 for more detailed sedimentology and stratigraphy). Fossil mammals mainly occur in the Q and K coal layers, estimated by magnetostratigraphy to 13.4 and 13.2 Ma, respectively (Coster et al. 2010).
Mae Moh Basin: location in northern Thailand (a) and simplified stratigraphic log representing the Siamogale bearing layers of the Na Khaem Formation. Modified from Coster et al. (2010; b)
With about 25 genera and 70 extant species, the Mustelidae is the largest and the most diversified family among Carnivora. The earliest mustelids Paragale and Plesiogale are reported from the early Miocene of Europe (Wolsan 1993; Wang et al. 2004). Although the Miocene fossil record of the family is abundant in Eurasia, Africa, and North America, especially in the Late Miocene (Savage 1978; Baskin 1998; Ginsburg 1999), it is still very scarce in Southeast Asia, where only two species are reported from Thailand: Siamogale thailandica and one indeterminate species (Ginsburg et al. 1983; Ducrocq et al. 1995). The description of S. thailandica was based on a single isolated tooth, a left m1. Due to this poor material, the anatomical comparison and the relationships of this species with other mustelids have been very limited since its discovery (Ginsburg and Tassy 1985; Willemsen 1992; Ginsburg and Morales 1996; Pickford 2007; Thewissen and Bajpai 2008). New field campaigns conducted by a Thai-French team (Department of Mineral Resources of Bangkok, Thailand and University of Poitiers, France) in the Mae Moh Basin yielded more than a hundred dental specimens belonging to S. thailandica in the Q and K lignite layers. This new material is particularly significant because it brings enough additional morphological information to throw light on the morphological affinities and the subfamilial assignment of S. thailandica within Mustelidae. This would ultimately improve our knowledge of the early evolutionary history of this family in Southeast Asia.
Material and methods
The specimens of S. thailandica, more than a hundred isolated teeth and mandible and maxilla fragments, are stored at the Department of Mineral Resources, Bangkok, Thailand (DMR). The comparative material includes specimens of Miocene, Pliocene, and Pleistocene Mustelidae housed in different institutions: American Museum of Natural History, New York, NY, USA; Muséum National d’Histoire Naturelle, Paris, France (MNHN); Institut International de Paléoprimatologie, Paléontologie Humaine: Évolution et Paléoenvironnements, Poitiers, France; and Staatliches Museum für Naturkunde von Stuttgart, Stuttgart, Germany (SMNS). Measurements of the Mae Moh specimens were taken with a digital caliper to the nearest 0.1 mm. Abbreviations for the dentition measurements are c, lower canine; Hhypo, hypoconid height; Hmeta, metaconid height; Hpara, paraconid or paracone height; Hproto, protoconid or protocone height; L, maximum mesiodistal length; Llab and Lling, labial and lingual maximum mesiodistal length of M1; LtrigoLab, labial maximum mesiodistal length of m1 trigonid; LtrigoLing, lingual maximum mesiodistal length of m1 trigonid; m, lower molar; M, upper molar; MlabDling, maximum distance between mesiolabial corner and distolingual corner of M1; MlingDlab, maximum distance between mesiolingual corner and distolabial corner of M1; p, lower premolar; P, upper premolar; W, maximum linguolabial width; Wtalo, maximum linguolabial width of m1 talonid; and Wtrigo, maximum linguolabial width of m1 trigonid. Taxonomy follows McKenna and Bell (1997).
Systematics
Order Carnivora Bowdich, 1821
Suborder Caniformia Kretzoi, 1943
Family Mustelidae Fischer, 1817
Genus Siamogale Ginsburg et al. 1983
Original diagnosis (translated in English from Ginsburg et al. (1983): pp. 954): Lutrine slightly smaller than the European otter; m1 with low and anteriorly tightened trigonid, hugely enlarged talonid, metaconid going down backward with a gentle slope as in Mionictis and extending toward the talonid back in a marked padding on top of the talonid hollow; low hypoconid extending backward by a thin crest.
Emended diagnosis: Mustelidae with double-rooted lower premolars and P3 that lack accessory cusps; p1 lost; broad and long basined talonid of m1; low trigonid; metaconid nearly at the same position and height as protoconid; metaconid directly connected to an entoconid crest that encloses the talonid until joining the low hypoconid; talonid outline rounded or subsquared; main cusp of p4 higher than m1 paraconid; P4 with a short shearing blade, a tiny parastyle, and a cup-shaped protocone mesially situated with respect to the paracone; lingual shelf moderately developed with a thin hypoconal crest distal to the protocone; M1 with a broad distolingual expansion, a crescent-shaped protocone, and a small metaconule just lingual to the metacone; enamel markedly crenulated on the m1 talonid basin and on the lingual parts of P4 and M1.
Type species: S. thailandica Ginsburg et al. 1983
S. thailandica Ginsburg et al. 1983
Holotype: TF 6316, left m1 (original figured in Ginsburg et al. (1983), pp. 954; cast in MNHN collections).
Type locality and stratigraphic ranges: Q and K coal layers of the Na Khaem Formation, age estimated between 13.4 and 13.2 Ma (Coster et al. 2010), Mae Moh Basin (Thailand), late middle Miocene (14.2–12.1 Ma; Benammi et al. 2002; Coster et al. 2010)—Tunggurian of the Asian Land Mammal Age (Qiu and Qiu 1995).
Other referred specimens: TF 6284, left mandible (c-m2; p2 alveolus, broken m1, and c); TF 6285, left maxilla (P4); TF 6286, right m1; TF 6287, left m1; TF 6288, left maxilla (P4); TF 6289, right P4; TF 6290, left M1; TF 6291, right m1; TF 6292, right mandible (p3–m1; broken m1 and m2 alveolus); TF 6293, left m1; TF 6294, left M1; TF 6295, right M1; TF 6296, left maxilla (P3–M1; broken P4); TF 6297, right P4; TF 6298, right mandible (p4–m1; p3 alveolus); TF 6299, right m2; TF 6300, right m2; TF 6301, left m2; TF 6302, left mandible (p3–m1; p2 and m2 alveoli); TF 6303, right m2; TF 6304, left m1; TF 6305, right P4; TF 6306, left maxilla (P2–P4; P3 alveolus and broken P4); TF 6307, right M1; TF 6308, left m2 (broken); TF 6309, right mandible (m1–m2); TF 6310, left mandible (p2–p4); TF 6311, left M1 (broken); TF 6312, left P4; TF 6313, right m1; TF 6314, left P4; TF 6315, right maxilla (P4–M1) plus other specimens of S. thailandica and fragments of isolated canines, premolars and mandibles of indeterminate Mustelidae stored at the DMR.
Remarks: These specimens are assigned to the same species in spite of some variations of tooth size (Table 1) and shape (e.g., the M1 distolingual border) because (1) these ranges of variations can agree with those of extant species of Mustelidae (Alcala et al. 1994; Wolsan et al. 1985; Wolsan 1988, 1989; Baryshnikov et al. 2003), (2) all these remains have been discovered in the same fossiliferous levels, separated by a maximum range of 200,000 years (Benammi et al. 2002; Coster et al. 2010).
Diagnosis: As for the genus.
Differential diagnosis: Differs from Mionictis and Lartetictis by a more prominent and more mesial m1 metaconid, a broader m1 talonid, a lower hypoconid, and a shorter P4 shearing blade. Differs from Mionictis by the position of the M1 metaconule, located lingually to the metacone. Differs from Lartetictis by a subequal importance of paracone and metacone of M1 and a less developed P4 hypoconal crest.
Description
Mandible: The ascending ramus displays a deep masseteric fossa, less marked than in Lutra but more developed than in Meles (Fig. 2 a). The coronoid process is rounded, as in the genera Enhydra and Meles, and wide at its dorsal rim. Its dorsal and rostral part, where inserts the temporalis muscle, is slightly convex in labial view. It is placed rostrally with respect to the angular process. This latter process is slender and curved lingually to form the ventral outline of the medial pterygoid muscle area, visible in lingual view (Fig. 2 b). The internal mandibular foramen is distal to the tooth row and located below the alveolar plane. A thin ventral groove for the attachment of the digastric muscle is visible below p4–m2 in the lingual side of the mandible (Fig. 2 b, c). The tip of the articular condyle is not preserved. The horizontal ramus bears two mental foramina: one below the position of p2 and another below the position of the mesial part of p4.
S. thailandica, photographs and drawings of the most significant specimens. a TF 6309, right mandible (m1–m2) in labial view; b TF 6292, right mandible (p3–m1) in lingual view; c TF 6284, left mandible (c-m2) in lingual view; d TF 6302, right mandible (p3–m1) in occlusal view; e TF 6313, right m1 in occlusal (e1), labial (e2) and lingual (e3) views; f TF 6299, right m2 in occlusal view; g TF 6296, left maxilla (p3–M1) in labial view; h P4 in occlusal (h1) and lingual (h2) views; i TF 6307, right M1 in occlusal views. Scale bar = 1 cm
Lower teeth: The dental formula for the lower dentition is ?.1.3.2. The teeth are closely set together (Fig. 2 a–d). Only one mandible is preserved with the base of a canine crown (i.e., TF 6284; Fig. 2 c). This canine is slightly less long than the p4, and its root is curved toward the labial side of the mandible. There is no p1. The premolars, doubled-rooted, lack accessory cusps. They are asymmetrical in lateral view: The main cusp is located mesially, the distal cingulum is more developed than the mesial one, and the former has a swelling shelf shape. These extended cingula create a broad occlusal surface. The size of these teeth and the height of the main cusp increase from p2 to p4 (Table 1). The p4 main cusp is higher than the m1 paraconid. The m1 are often preserved on the mandibles, but most of the teeth are isolated (Fig. 2 e1–e3). The m1 paraconid is the smallest cusp of the trigonid, and the protoconid is slightly higher than the metaconid (Table 1). The paraconid is nearly lingually oriented. The protoconid is slightly mesial with respect to the metaconid. The three cusps of the m1 trigonid are very close to each other. The carnassial notch and the lingual opening (between the paraconid and the metaconid) are weak. The metaconid and the protoconid are separated by a shallow notch. A distal crest extends with a gentle slope from the tip of the metaconid to join the entoconid crest of the talonid. The talonid is broader than the trigonid. The former is basined and has a rounded to subsquared shape. It is enclosed by a low and smooth entoconid crest which appears continuous from the distal crest of the metaconid to the labial hypoconid. This hypoconid is low and it is the unique cusp of the talonid. It is located at the distal base of the protoconid, from which it is separated by a weak notch. Enamel crenulations of m1 are mainly visible on the hollow talonid. The m2 is single-rooted (Fig. 2 b, d). Its root is concave in labial view. Its crown base arises slightly above the level of the premolars and the m1 (Fig. 2 a, c). The protoconid is the unique visible cusp (Fig. 2 f). It is labial and medial to slightly mesial. A small crenulated basin extends lingually to the protoconid. The outline, triangular to subcircular in shape, is formed by a wide and smooth crest, lower at the region of the basin.
Maxilla: Only TF 6296 (Fig. 2 g) shows an infraorbital foramen. This foramen is extended above the region of contact between P3 and P4 until the mesial part of P4 in lateral view. It has a wide diameter and is oval in mesial view.
Upper teeth: Within all the specimens, only P3, P4, and M1 are preserved. The P3 is double-rooted (Fig. 2 g). Like the lower premolars, it is asymmetrical, with a single cusp located mesially and a weak distal cingulum, which protrudes toward lingual part of the maxilla. Teeth are also closely set together. P4 lacks a carnassial notch, a derived feature find in most mephitids and mustelids (Wolsan 1993). The shearing blade is short. The paracone is the highest cusp of the tooth, nearly twice higher than the protocone (Fig. 2 h1–h2; Table 1). Two crests extend from its tip: One is oriented mesiolabially in direction of a low parastyle, and another is oriented mesiolingually in direction of the protocone basis. In mesial view, this last crest forms a V-shaped notch together with the mesiolabially crest coming down from the protocone. The protocone, more mesial than the paracone, is cup-shaped (i.e., the labial surface is roughly plane whereas the lingual portion is convex). The lingual shelf is moderately developed. It shows a slight padding distal to the protocone, which is considered in this study as a hypoconal crest/crestiform hypocone (Fig. 2 h2). A crest that originates from the distolingual face of the protocone joins the hypoconal crest at the level of the paracone position. The enamel is markedly crenulated on the lingual cingulum part near the shearing blade. The M1 has a prominent distolingual border. It forms a broad heel across the lingual third of the tooth (Fig. 2 i). As a result of this expansion, the distal border of the tooth shows like a medial constriction, and the maximal length of the M1 is more important than the P4 one (Table 1). The lingual cingulum is thick. There is no hypocone but a crescent-shaped protocone, which elongates distally and ends approximately to the middle of the tooth. A thin crest originates from the paracone for ending to the middle of the protocone. The paracone is slightly higher than the metacone, and its intracingular shelf (i.e., region located labially to the paracone) is slightly more extended than the metacone one. The two labial cusps are linked by a thin oblique crest. The enamel is mostly crenulated on the lingual part of the M1.
Comparisons
S. thailandica displays a hypocarnivorous dentition (e.g., low m1 trigonid, wide and long basined talonid, lingual expansion of the upper teeth). Within Mustelidae, several taxa show a tendency of M1 enlargement, one of the most remarkable dental features of Siamogale. This is notably the case of Gulolinae and some Galictinae, Lutrinae, and otter-like or meline-like genera. The stratigraphic distributions of the main compared genera are reported in Fig. 3.
The distolingual expansion of M1 is particularly visible in the extinct Gulolinae (Gray, 1825) Dehmictis, Plesiogulo, and Iberictis from the Miocene and Pliocene of Eurasia and in the extant genus Martes, known in Asia since the middle Miocene (Ginsburg 1999). In spite of the M1 shape, these taxa have a clearly more trenchant dentition than Siamogale, with, for example, a weak or absent P4 lingual shelf and a long opened trigonid with a low or nearly absent metaconid.
Some genera of the Galictinae (Reig, 1956) show a M1 with a distolingual expansion and a lingual shelf on P4 giving to the tooth a triangular shape. Baskin (1998) defined this group to have a wide m1 basined talonid and a metaconid connected to the entoconid crest of the talonid, as in Siamogale. They also show a tendency toward the loss of the p1 (García et al. 2008). The main difference between the galictines and S. thailandica is the longer and more opened m1 trigonid, with its slightly lower and more distal metaconid. This group includes the semi-aquatic Mustelidae Lutravus, from the Mio-Pliocene of North America after Baskin (1998) and the Plio-Pleistocene Eurasian Enhydrictis and Pannonictis after Pilgrim (1932a). The American Pliocene genera Trigonictis and Sminthosinis are also often considered as close to the extant galictines (e.g., Baskin 1998; see Fig. 3 for temporal and geographical distributions).
Lutravus is described by Baskin (1998) to have a short P4 shearing blade, a prominent M1 lingual cingulum, and a p4 without distal accessory cusp, as in Siamogale. But Lutravus differs from Siamogale in its M1, wider than long, because of the weak distolingual expansion of its tooth.
Siamogale resembles Trigonictis in its P4 triangular shape, which exhibits an incipient hypocone, the presence of a M1 metaconule just lingual to the metacone, the thick lingual cingulum, and the lack of accessory cusps on lower premolars. Nevertheless, in Trigonictis, the m1 trigonid is longer, with a reduced and more distal metaconid, the P4 protocone is nearly conical, the paracone is higher, the lingual shelf is less developed, and the M1 distolingual expansion is less prominent (Bjork 1970).
Sminthosinis is closely related to Trigonictis (Bjork 1970). The metaconid of m1 is approximately at the same position as the protoconid, like in Siamogale. P4 protocone is cup-shaped and wide but very reduced compared to Siamogale. It also differs from our Thai genus in its very moderate lingual shelf on P4, its lack of metaconule on M1, and its long m1 trigonid.
The genus Enhydrictis is similar to Siamogale with regard to its cup-shaped P4 protocone. Moreover, in Enhydrictis galictoides, the metaconid and protoconid are located about the same position as in S. thailandica (García et al. 2008). But the M1 is nearly rectangular; the metacone and its labial shelf are reduced; the m1 metaconid is low, more vertically connected to the talonid basin; the hypoconid is higher; and the p2 is single-rooted.
Pannonictis displays the same general morphological characters as Enhydrictis. Pannonictis nestii presents a P4 hypoconal crest and a more developed M1 distolingual expansion than the other species of the genus, making it closer to Siamogale. Enhydrictis and Pannonictis display, however, two roots on M1 whereas Siamogale M1 shows three roots.
Paralutra, known from the early middle Miocene of Europe, is the earliest representative of Lutrinae (Ginsburg 1999). This genus and notably the species Paralutra jaegeri (Fraas, 1862) have certainly the most important distolingual expansion on M1 within otters and shows a crenulated enamel, as in Siamogale. But the m1 talonid is shorter and narrower; the metaconid is smaller, more distal; and its distal part is not connected to the entoconid crest of the talonid. The P4 shows a high paracone and a distinct hypocone very close to the protocone. These features are clearly different from those observed in Siamogale.
Some Miocene otter-like or meline-like forms of the Old and the New World are also compared to Siamogale. Strong similarities with the otter-like genus Mionictis have been suggested (Ginsburg et al. 1983; Willemsen 1992; Ginsburg and Morales 1996, 2000). Mionictis was originally represented in the Miocene of both North America and Europe. European species (Mionictis dubia de Blainville, 1841; Mionictis artenensis Ginsburg 1968; and Mionictis ginsburgi Alcala et al. 1994) were placed in this genus before being moved to the genera Lartetictis, Trochictis, and Adroverictis, respectively (Ginsburg and Morales 1992, 1996).
Adroverictis ginsburgi is represented by a first upper molar from Spain (Alcala et al. 1994). The genus shows both a M1 mesiolingual and distolingual expansion, in conjunction with a crescent-shaped protocone, extended from the base of the paracone to the metaconule. These features are very different from those observed in Siamogale.
The American species of Mionictis and the European genera Lartetictis and Trochictis display several similarities with Siamogale: a low m1 trigonid, a metaconid connected with the entoconid crest of the talonid, a basined talonid surrounded by an entoconid crest and presenting a low hypoconid, a slightly reduced M1 metacone, a crescent-shaped protocone, and a distolingual expansion with thick lingual cingulum. The teeth have also enamel crenulations, notably visible on the m1 talonid and on the lingual development of the upper teeth. The lower and upper teeth measurements of Siamogale, Mionictis, Lartetictis, and Trochictis are reported in Fig. 4a, b and in Tables 2 and 3.
The monospecific genus Lartetictis extends from the early to the middle of middle Miocene in Europe (Heizmann and Morlo 1998; Ginsburg and Morales 2000). Its dental dimensions are close to those of Siamogale (Tables 2 and 3; Fig. 4a, b). In addition to the similarities cited above, the m1 talonid of Lartetictis is wide, and its M1 shows a metaconule, although slightly smaller than in Siamogale and a M1 distal border which is constricted just lingual to this metaconule, as in Siamogale. Lartetictis differs from it in a slightly smaller and more distal m1 metaconid, a longer P4 shearing blade, a more prominent P4 hypoconal crest, and a more extended intracingular shelf of the M1 paracone.
The genus Trochictis is represented by two species, Trochictis artenensis and Trochictis carbonaria, from the early Miocene of France and Switzerland (Ginsburg 2002). Their teeth are smaller than those of Siamogale (Tables 2 and 3; Fig. 4a, b). The m1 talonid is narrower than in Siamogale, the metaconid is lower and located more distally, and the entoconid crest appears less smooth. The M1 lacks a metaconule and a medial constriction at the distal border of the tooth, so that it is nearly straight, unlike in Siamogale.
From the middle Miocene of France, Rhodanictis depereti was removed from the genus Trochictis (Ginsburg and Morales 2000). Although the m1 has a similar morphology and proportions with the other species of Trochictis (Tables 2 and 3; Fig. 4a), the M1 is nearly subsquared, with a moderate distolingual expansion, and the crescent-shaped protocone is connected to the metaconule, unlike in Siamogale. The P4 protocone, cup-shaped, is similar to the Siamogale one, but the shearing blade is longer and the lingual shelf is reduced, no hypoconal crest being visible. Rhodanictis also differs from Siamogale by its interdental gap between premolars and the presence of a p1 and a distal accessory cusp on p4.
In the American species of Mionictis, only the lower dentition has been figured (Matthew 1924; Cook and McDonald 1962). The type species Mionictis incertus lacks p1, distal accessory cusps on premolars, and interdental gap between premolars, as Siamogale. However, the m1 talonid is narrower, the metaconid is lower and more distal, and the hypoconid is prominent. Harrison (1981) referred an undescribed partial skull from Texas to Mionictis sp. (F:AM 63296). Recently, the P4 and M1 of this specimen have been illustrated and briefly described in a taxonomical comparison (Tseng et al. 2009). These teeth are similar to the upper teeth of Siamogale in having a weak P4 hypoconal crest, a M1 paracone and metacone intracingular shelf nearly similarly developed, and a metaconule. Moreover, the M1 dimensions are close to those of Siamogale (Tables 2 and 3; Fig. 4b). But the P4 shearing blade is longer, the M1 paracone is higher, and the metaconule is more prominent and more labial. The associated mandible (F:AM 63298) shows a wide m1 talonid, as in Siamogale, but unlike M. incertus. Moreover, the m2 is more rounded than in Siamogale. These specimens of Mionictis sp. lack crenulated enamel, unlike M. incertus and Siamogale.
Thus, S. thailandica appears to be morphologically closer to the European Lartetictis dubia and the American Mionictis sp. However, several features as the height and position of the m1 metaconid and the development of the P4 make it distinctive and difficult to link with other known Mustelidae.
Discussion
Apomorphies such as the lack of m3 and M2, and the lack of a carnassial notch on P4 in S. thailandica are present in Mustelidae and Mephitidae families (e.g., Tedford 1976; Wozencraft 1989; Baskin 1998). We suspect that the Thai species belongs to mustelids because they lack extra roots on m1 and of a postprotocrista on M1, two derived features occurring in mephitids (Wang et al. 2004).
Concerning the subfamily, Ginsburg et al. (1983) placed S. thailandica within the Lutrinae as a specialized shellfish-eating otter. They based their attribution on the morphological features of the holotype, close to those present in Lutrinae (i.e., low closed trigonid, wide-basined talonid). They also took into account the morphological similarities of Siamogale with the genus Mionictis, considered as an otter, and notably with the European species M. artenensis. This hypothesis will be followed in the few later publications related to Siamogale (Ginsburg and Tassy 1985; Willemsen 1992; Ginsburg and Morales 1996; Pickford 2007; Thewissen and Bajpai 2008). However, the morphology of the other dental remains of S. thailandica, especially the upper teeth, and the taxonomical changes of the European “Mionictis” species (Ginsburg and Morales 1992, 1996) make the subfamilial assignment of Siamogale doubtful and more complicated to establish.
According to recent molecular studies, Mustelidae are distributed into height extant subfamilies: Taxidiinae (American badgers), Mellivorinae (honey badger), Melinae (badgers), Gulolinae (wolverine, martens), Helictidinae (ferret-badger), Galictinae (grisons and their allies), Mustelinae (weasels and their allies), and Lutrinae (otters; e.g., Koefli et al. 2008; Sato et al. 2009; Wolsan and Sato 2010). The fossil group Leptarctinae is also considered as a mustelid subfamily (e.g., McKenna and Bell 1997; Baskin 1998). The crushing dentition tendency is observed in Leptarctinae, in badgers sensu lato (Taxidiinae, Helictidinae, Melinae), and in some Lutrinae and Galictinae.
The Miocene North American and Eurasiatic fossil group Leptarctinae includes members generally characterized by a more hypocarnivorous dentition than that of Siamogale, with notably quadrate and complex P4s and M1s (e.g., Lim 1999; Wang et al. 2004). The more singular genera Kinometaxia and Schultzogale, from the early Miocene of China and Nebraska, respectively, have a clearly more trenchant dentition compared to the other genera of the group but also compared to the Thai species (i.e., no lingual shelf and a long shearing blade on P4, transversely broad M1 with a small metacone and a wide intracingular shelf of the paracone; Lim and Martin 2000; Wang et al. 2004). Thus, Siamogale appears to be morphologically far from this subfamily.
Badgers sensu lato include relatively diverse Old World and New World forms. The Taxidiinae are known since the late Miocene and includes only three genera. S. thailandica differs from the extant Taxidea and the extinct Pliotaxidea in which the P4 has a well-developed hypocone and protocone, the M1 and m1 talonid have numerous cuspules, and the p4 shows an accessory cusp (Baskin 1998). The earliest genus of Taxidiinae, Chamitataxus, displays a long P4 shearing blade, a wide metaconule more distally located than in Siamogale, and lacks a crescent-shaped protocone. Morphological differences with Siamogale are too numerous to assign it to this subfamily.
The Helictidinae, currently represented by the Asian ferret-badger Melogale, are recognized as a distinct group of badgers but have no clear fossil representatives (e.g., Petter 1971; Sato et al. 2009). The teeth show a voluminous P4 similar to that of mephitids, a M1 wider than longer, without distolingual development, and bearing a longitudinal protocone, unlike in S. thailandica. Moreover, the m1 has a narrower and shorter talonid compared to the Thai species.
Considering only the dental material, Siamogale shows several features resemble those of Lutrinae, Melinae, and Galictinae. The connection of the m1 metaconid with the entoconid crest or entoconid is a morphological feature present in musteline-like forms including Galictinae, although this connection is often more abrupt than in Siamogale. This feature is absent in the Lutrinae, except in the genus Mionictis, its subfamilial position being doubtful. Other characters which are observed in Siamogale, as the closed low trigonid of m1, the elongated basined talonid, and the unreduced metaconid are convergent morphological tendencies seen in Lutrinae and Melinae (Baskin 1998). However, the talonid of Melinae shows numerous cuspules on its rim, and the metaconid is often located more distally with respect to the protoconid, unlike in Siamogale. Some Galictinae also show a wide-basined talonid, but the metaconid is lower and more distal than in Lutrinae and in Siamogale. A P4 lingual shelf and a short shearing blade are developed by Melinae and Lutrinae. The Galictinae also present a lingual shelf but the protocone is generally more prominent and the shearing blade is longer than in Siamogale. The distolingual development of the M1 is a feature of Melinae and some Galictinae, but this development is generally in conjunction with a mesiolingual expansion for Melinae and the metacone tends to be reduced in Galictinae. Moreover, the oldest record of a distinctive badger, Taxodon sansaniensis from the early middle Miocene of France, shows a m1 talonid rim with several cuspules and a long P4 shearing blade while it has no M1 distolingual development yet. Paralutra is the unique genus of Lutrinae that displays a similar M1 development, but the lower dentition is more hypercarnivorous (see comparisons).
The dental pattern of the hypercarnivorous Martes is often considered as an ancestral morphological type for Mustelidae. Several groups of the family have evolved independently toward a crushing dentition. For Siamogale, this step implicated notably a reduction of the protoconid of m1 with an enlargement and lengthening of the talonid, a development of a lingual shelf and a cup-shaped protocone on P4, a reduction of the P4 shearing blade length, and an enlargement of the M1 metacone. Because of the lack of paleontological data and the problematic subfamilial assignment of fossil mustelids (notably for Helictidinae, Melinae, and Galictinae) and because of the primitive and convergent dental features of S. thailandica, we failed to find a subfamily for this species. A cladistic analysis integrating extant and extinct mustelids should be performed in a near future to test its position within the family. Until more data, notably cranial, are available, we suggest that Siamogale is an otter-like mustelid, certainly strongly dependent of water. Actually, most of the Carnivora specimens discovered in the lacustrine deposits of the Na Khaem Formation belong to this species. Its bunodont dental morphology and its horizontal worn tooth pattern suggest the consumption of hard items. We remark that mollusks and crabs are frequent in the sediments of the fossil coal layers, which could constitute a possible diet. Unfortunately, no postcranial elements are attributable to Siamogale, but its morphological affinities with Lartetictis, for which the postcranial bones seem to be adapted for a semi-aquatic way of life (Ginsburg 1968, 1999; Heizmann and Morlo 1998), can follow the otter-like hypothesis. According to the mustelid fossil record of Mae Moh, competition between S. thailandica and bunodont Lutrinae was unlikely, the only Miocene otters from Asia being confined in the Indian Subcontinent and in China (Lydekker 1884; Pilgrim 1932b; Qiu and Qiu 1995). Interestingly, several otter-like mustelid lineages, such as those of Siamogale, Lutravus, and Enhydrictis, occur in the Miocene and Plio-Pleistocene epochs, whereas the extant mustelids that inhabit freshwater and marine environments are nearly all included in the Lutrinae subfamily. Currently, the few semi-aquatic mustelids, such as the Mustelinae Mustela vison living near rivers and stream waters, can be forced to shift their diet when they are in competition with lutrines feeding on the same aquatic items (Larivière 1999; Bonesi et al. 2004). This could be partly explained by the more efficient aquatic adaptations for swimming and hunting preys underwater that might have been acquired by the Lutrinae during their evolution.
The geographic origin of Siamogale lineage is also questionable. The Mae Moh fauna usually appears close to taxa coming from other Miocene localities of northern Thailand and from the Siwaliks province of Pakistan (e.g., Ginsburg and Tassy 1985; Ducrocq et al. 1995; Chaimanee et al. 2008; see also Chavasseau 2008). However, some genera show possible close relatives in northern China and in North America or Europe (Peigné et al. 2006; Chavasseau 2008). Within the compared mustelids, the American Mionictis sp. and the European Lartetictis display the most similar morphology with Siamogale, thus suggesting a European or a North American immigration event before 13.4 Ma. Other geographic occurrences of Mionictis sp. are reported in the middle Miocene from Al-Sarrar (Saudi Arabia) and from Tunggur (China). However, the paucity of the material and the discovery, at Tunggur, of Sthenictis neimengguensis, a mustelid which might have been around the same size as Mionictis, render these occurrences very uncertain (Thomas et al. 1982; Qiu and Qiu 1995; Tseng et al. 2009; Wang, personal communication). According to the palynological studies and the Mae Moh fossil record, especially the rodents and the sivaladapid primate, Southeast Asia appears as a distinct biogeographic province in the middle Miocene (e.g., Sépulchre 2003; Chaimanee et al. 2007, 2008). Added to the scarcity of the Mustelidae fossil record in that region and to the dental peculiar features of S. thailandica, a Southeast Asian endemic origin for Siamogale lineage cannot be ruled out.
Conclusions
Many additional specimens of S. thailandica were collected in the Mae Moh Basin of northern Thailand. We suggest that this species is a bunodont otter-like mustelid, whose taxonomical position within the family is still unclear. After comparisons of its dental morphology with those of extinct and extant mustelids, we conclude that Siamogale displays more similarities with the early and middle Miocene genera Mionictis and Lartetictis. If these genera are close relatives, it implies a European or a North American immigration event to Thailand before 13.4 Ma. Nevertheless, Siamogale shows peculiar morphological features, such as the shape of the P4 lingual shelf and the m1 metaconid position and height. Due to the scarcity of the fossil record of Mustelidae in Southeast Asia and the degree of endemism of this fauna, further fieldworks are necessary to find other representatives of this peculiar lineage.
References
Alcala L, Montoya P, Morales J (1994) New large mustelids from the Late Miocene of the Teruel Basin (Spain). C R Acad Sci II 319(9):1093–1100
Baryshnikov GF, Puzachenko AY, Abramov AV (2003) New analysis of variability of cheek teeth in Eurasian badgers (Carnivora, Mustelidae, Meles). Russ J Theriol 1(2):133–149
Baskin JA (1998) Mustelidae. In: Janis CM, Scott KM, Jacobs LL (eds) Evolution of tertiary mammals of North America, vol 1: terrestrial carnivores, ungulates, and ungulatelike mammals. Cambridge University Press, Cambridge, pp 152–173
Benammi M, Urrutia-Fucugauchi J, Alva-Valdivia LM, Chaimanee Y, Triamwichanon S, Jaeger JJ (2002) Magnetostratigraphy of the Middle Miocene continental sedimentary sequences of the Mae Moh Basin in northern Thailand: evidence for counterclockwise block rotation. Earth Planet Sci Lett 204(3–4):373–383. doi:10.1016/S0012-821X(02)01002-6
Bjork PR (1970) The Carnivora of the Hagerman local fauna (Late Pliocene) of southwestern Idaho. Trans Am Phil Soc Phila New Ser 60:1–54
Bonesi L, Chanin P, McDonald DW (2004) Competition between Eurasian otter Lutra lutra and American mink Mustela vison probed by niche shift. Oikos 106:19–26
Chaimanee Y, Jolly D, Benammi M, Tafforeau P, Duzer D, Moussa I, Jaeger JJ (2003) A Middle Miocene hominoid from Thailand and orangutan origins. Nature 422(6927):61–65. doi:10.1038/nature01449
Chaimanee Y, Yamee C, Marandat B, Jaeger JJ (2007) First Middle Miocene rodents from the Mae Moh Basin (Thailand): biochronological and paleoenvironmental implications. Bull Carnegie Mus Nat Hist 39:157–163. doi:10.2992/0145-9058(2007)39[157:FMMRFT]2.0.CO;2
Chaimanee Y, Yamee C, Tian P, Chavasseau O, Jaeger JJ (2008) First middle Miocene sivaladapid primate from Thailand. J Hum Evol 54(3):434–443. doi:10.1016/j.jhevol.2007.10.001
Chavasseau O, Chaimanee Y, Yamee C, Tian P, Rukbumrung M, Marandat B, Jaeger JJ (2009) New Proboscideans (Mammalia) from the middle Miocene of Thailand. Zool J Linn Soc 155(3):703–721. doi:10.1111/j.1096-3642.2008.00456.x
Chavasseau O (2008) Les grands mammifères miocènes d’Asie du Sud-Est: biochronologie et biogéographie. Ph.D. thesis, Université Montpellier 2, p 299
Cook HJ, McDonald JR (1962) New Carnivora from the Miocene and Pliocene of Western Nebraska. J Paleontol 36:560–567
Coster P, Benammi M, Chaimanee Y, Yamee C, Chavasseau O, Emonet EG, Jaeger JJ (2010) A complete magnetic-polarity stratigraphy of the Miocene continental deposits of Mae Moh Basin, northern Thailand, and a reassessment of the age of hominoid-bearing localities in northern Thailand. Geol Soc Am Bull 122:1180–1192. doi:10.1130/B26568.1
Ducrocq S, Chaimanee Y, Suteethorn V, Jaeger JJ (1994) Ages and paleoenvironment of Miocene mammalian faunas from Thailand. Palaeogeogr Palaeoclimatol Palaeoecol 108:149–163. doi:10.1016/0031-0182(94)90027-2
Ducrocq S, Chaimanee Y, Suteethorn V, Jaeger JJ (1995) Mammalian faunas and the ages of the continental tertiary fossiliferous localities from Thailand. J Southeast Asian Earth Sci 12(1–2):65–78. doi:10.1016/0743-9547(95)00021-6
García N, Arsuaga JL, Bermúdez de Castro JM, Carbonell E, Rosas A, Huguet R (2008) The Epivillafranchian carnivore Pannonictis (Mammalia, Mustelidae) from Sima del Elefante (Sierra de Atapuerca, Spain) and a revision of the Eurasian occurrences from a taxonomic perspective. Quat Int 179:42–52. doi:10.1016/j.quaint.2007.09.031
Ginsburg L (1968) Les mustélidés piscivores du miocène français. Bull Mus Natn Hist Nat 40(1):228–238
Ginsburg L (1999) Order Carnivora. In: Rössner GE, Heissig K (eds) The Miocene land mammals of Europe. Verlag Dr. Friedrich Pfeil, Münich, pp 109–148
Ginsburg L (2002) Les carnivores fossiles des sables de l’Orléanais. Ann Paleontol 88:115–146. doi:10.1016/S0753-3969(02)01042-X
Ginsburg L, Tassy P (1985) The fossil mammals and the age of the Lignite beds in the intramontane basins of northern Thailand. J Geol Soc Thail 8(1–2):13–27
Ginsburg L, Morales J (1992) Contribution à la connaissance des Mustelidés (Carnivora, Mammalia) du Miocène d’Europe Trochictis et Ischyrictis, genres affines et genres nouveaux. C R Acad Sci II 315:111–116
Ginsburg L, Morales J (1996) Lartetictis et Adroverictis, nouveaux genres de Melinae (Mustelidae, Carnivora, Mammalia) du Miocène de l’Ancien monde. Bull Mus Natn Hist Nat 18(4):663–671
Ginsburg L, Morales J (2000) Origine et évolution des Melinae (Mustelidae, Carnivora, Mammalia). C R Acad Sci II 330:221–225. doi:10.1016/S1251-8050(00)00139-7
Ginsburg L, Invagat R, Tassy P (1983) Siamogale thailandica, nouveau Mustelidae (Carnivora, Mammalia) néogène du Sud-Est asiatique. Bull Soc Géol Fr 7(25):953–956
Harrison JA (1981) A review of the extinct wolverine, Plesiogulo (Carnivora: Mustelidae), from North America. Smithson Contr Paleobiol 46:1–27
Heizmann EPJ, Morlo M (1998) Die semi-aquatische Lartetictis dubia (Mustelinae, Carnivora, Mammalia) vom Goldberg/Ries (Baden-Württemberg). In: Grimm KI, Grimm MC, Morlo M (eds) Festschrift zum 70. Geburtstag von Prof. Dr. Karlheinz Rothausen Mainzer naturwissenschaftliches Archiv, Beiheft, vol 21., pp 141–153
Koenigswald GRD von (1959) A Mastodont and other fossil Mammals from Thailand. Report of Investigation of Royal Department of Mines, Bangkok 2, pp 25–28
Koefli KP, Deere KA, Slater GJ, Begg C, Begg K, Grassman L, Lucherini M, Veron G, Wayne RK (2008) Multigene phylogeny of the Mustelidae: resolving relationships, tempo and biogeographic history of a mammalian adaptive radiation. BMC Biol 6(10):1–22. doi:10.1186/1741-7007-6-10
Larivière S (1999) Mustela vison. Mamm Species 608:1–9
Lim JD (1999) Systematics and functional morphology of Leptarctus (Mammalia: Mustelidae). Ph.D. thesis, University of Kansas, p 151
Lim JD, Martin LD (2000) A new primitive Leptarctinae (Mustelidae) from the North American Miocene. Neues jahrb Geol Paläontol Monatshefte 10:632–640
Lydekker R (1884) Indian tertiary and post-tertiary vertebrata: Siwalik and Narbada Carnivora. Palaeontol Indica 10:178–355
Matthew WD (1924) Third contribution to the Snake Creek Fauna. Bull Am Mus Nat Hist 50:59–210
McKenna MC, Bell SK (1997) Classification of mammals above the species level. Colombia University Press, New York, p 631
Peigné S, Chaimanee Y, Yamee C, Tian P, Jager JJ (2006) A new amphicyonid (Mammalia, Carnivora, Amphicyonidae) from the late middle Miocene of northern Thailand and a review of the amphicyonine record in Asia. J Asian Earth Sci 26(5):519–532. doi:10.2517/1342-8144-13.3.245
Petter G (1971) Origine, phylogénie et systématique des blaireaux. Mamma 35(4):567–597. doi:10.1515/mamm.1971.35.4.567,//1971
Pickford M (2007) Revision of the Mio-Pliocene bunodont otter-like mammals of the Indian Subcontinent. Est Geol 63(1):83–127
Pilgrim GE (1932a) The genera Trochictis, and Trocharion, with remarks on the taxonomy of the Mustelidae. Proc Zool Soc London 4:845–867
Pilgrim GE (1932b) The fossil Carnivora of India. Palaeontol Indica 18:1–232
Prothero DR (1998) The chronological, climatic, and paleogeographic background to North American mammalian evolution. In: Janis CM, Scott KM, Jacobs LL (eds) Evolution of tertiary mammals of North America terrestrial carnivores, ungulates, and ungulatelike mammals, vol 1. Cambridge University Press, Cambridge, pp 9–36
Qiu Z, Qiu Z (1995) Chronological sequence and subdivision of Chinese Neogene mammalian faunas. Paleogeogr Palaeoclimatol Paleoecol 116:41–70. doi:10.1016/0031-0182(94)00095-P
Sato JJ, Wolsan M, Minami S, Hosoda T, Sinaga MH, Hiyama K, Yamaguchi Y, Suzuki H (2009) Deciphering and dating the red panda’s ancestry and early adaptative radiation of Musteloidea. Mol Phyl Evol 53(3):907–922. doi:10.1016/j.ympev.2009.08.019
Savage RJG (1978) Carnivora. In: Maglio VJ, Cooke HBS (eds) Evolution of African mammals. Harvard University Press, Cambridge, pp 249–267
Sépulchre P (2003) Paléoenvironnements d’Asie du Sud-Est: Apports de l’analyse palynologique de deux sites miocènes de Thaïlande. Unpublished DEA Mémoire, Université de Montpellier II, Montpellier, France
Steininger FF (1999) Chronostratigraphy, geochronology and biochronology of the Miocene “European Land Mammal Mega-Zones (ELMMZ)” and the Miocene “Mammal-Zones (MN-Zones)”. In: Rössner GE, Heissig K (eds) The Miocene land mammals of Europe. Verlag Dr. Freidlich Pfeil, Münich, pp 9–24
Tedford RH (1976) Relationship of pinnipeds to other carnivores (Mammalia). Syst Zool 25:363–374
Thewissen JGM, Bajpai S (2008) New Oligocene mustelid from western India. J Vertebr Paleontol 28(2):565–567. doi:10.1671/0272-4634(2008)28[565:NOMFWI]2.0.CO;2
Thomas H, Sen S, Khan M, Battail B, Ligabue G (1982) The lower Miocene fauna of Al-Sarrar (Eastern Province, Saudi Arabia). Atlal J Saudi Arab Archaeol 5:109–136
Tseng ZJ, Wang X, Stewart JD (2009) A new immigrant mustelid (Carnivora, Mammalia) from the middle Miocene temblor formation of central California. PaleoBios 29(1):13–23
Wang X, Zx Q, By W (2004) A new leptarctine (Carnivora: Mustelidae) from the early Miocene of the northern Tibetan Plateau and implications of the phylogeny and zoogeography of basal mustelids. Zool J Linn Soc 142:405–421. doi:10.1111/j.1096-3642.2004.00135.x
Willemsen GF (1992) A revision of the Pliocene and Quaternary Lutrinae from Europe. Scr Geol 101:1–115
Wolsan M, Sato JJ (2010) Effects of data incompleteness on the relative performance of parsimony and Bayesian approaches in a supermatrix phylogenetic reconstruction of Mustelidae and Procyonidae (Carnivora). Cladistics 26(2):168–194. doi:10.1111/j.1096-0031.2009.00281.x
Wolsan M (1993) Phylogeny and classification of early European Mustelida (Mammalia: Carnivora). Acta Theriol 38:345–384
Wolsan M (1988) Morphological variations of the first upper molar in the genus Martes (Carnivora, Mustelidae). In: Russell DE, Santoro JP, Sigogneau-Russell D (eds) Teeth revisited: proceedings of the VIIth international symposium on dental morphology, Paris 1986, 53(série C). Mémoire du Muséum National d’Histoire Naturelle, Paris, pp 241–254
Wolsan M (1989) Dental polymorphism in the genus Martes (Carnivora: Mustelidae) and its evolutionary significance. Acta Theriol 34(40):545–593
Wolsan M, Ruprecht AL, Buchalczyk T (1985) Variation and asymmetry in the dentition of the pine martens (Martes martes and M. foina) from Poland. Acta Theriol 30(3):79–114
Wozencraft WC (1989) The phylogeny of the recent Carnivora. In: Gittleman JL (ed) Carnivore, behavior, ecology and evolution. Cornell University Press, Ithaca, pp 495–535
Acknowledgments
We would like to thank Electricity Generating Authority of Thailand for providing scientific assistance and access to the mine, the DMR of Bangkok, the Thai-French TRF-CNRS Biodiversity Project, the ANR-05-BLAN-0235, and the CNRS-Eclipse II Program for their financial support. We thank Xavier Valentin and Mana Rugbumrung for the preparation of the material and Sabine Riffaut for the drawings. The first author is grateful to Stéphane Peigné for his help and advice on this study and Xiaoming Wang and Zhigie Jack Tseng for their help concerning the specimen of Mionictis sp. from Texas. C. Grohé also would like to thank Basil Thüring, Elmar Heizmann, Begoña Sánchez Chillón, Ruth O’Leary, and Carl Mehling for providing comparison material of Mustelidae. Finally, we would like to thank Sven Thatje, Robert J. Asher, Xiaoming Wang, Mieczyslaw Wolsan, and one anonymous reviewer for their helpful comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Robert Asher
Rights and permissions
About this article
Cite this article
Grohé, C., Chaimanee, Y., de Bonis, L. et al. New data on Mustelidae (Carnivora) from Southeast Asia: Siamogale thailandica, a peculiar otter-like mustelid from the late middle Miocene Mae Moh Basin, northern Thailand. Naturwissenschaften 97, 1003–1015 (2010). https://doi.org/10.1007/s00114-010-0721-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-010-0721-8