Abstract
Chemical composition of volatiles emitted from fresh mouse carcasses (laboratory mice, Mus musculus) was studied using solid sample injection technique (solid-phase micro-extraction), two-dimensional gas chromatography with time of flight mass spectrometric detection and gas chromatography with electroantennographic detection. Electroantennography (EAG) and laboratory olfactometric behavioural observations were used to study the antennal sensitivity to identified infochemicals and their attractiveness for burying beetles Nicrophorus vespillo and Nicrophorus vespilloides (Silphidae: Nicrophorinae). Chemical analysis showed that immediately after death, emitted volatiles did not differ from those emitted by a living organism. However, in the course of time, sulphur-containing chemicals, specifically methanethiol, methyl thiolacetate, dimethyl sulphide, dimethyl disulphide and dimethyl trisulphide appear. EAG measurements revealed antennal sensitivity to these compounds. Behavioural tests in laboratory olfactometer showed that dimethyl sulphide, dimethyl disulphide and dimethyl trisulphide are highly attractive to both studied species. The data suggest that sulphur-containing chemicals are involved in mediating the fresh carcass attractiveness for N. vespillo and N. vespilloides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carrion beetles (Coleoptera: Silphidae) are part of a large group of scavengers that aid in the break down and recycling of organisms back into the ecosystem. So far, ca. 210 species are classified into two subfamilies (Silphinae and Nicrophorinae), and 13 genera have been described worldwide (Peck 1990; Růžička and Schneider 2004; Sikes 2005; Růžička 2007). The genus Nicrophorus Fabricius (Nicrophorinae), also called burying beetles, is well known for biparental care of its offspring, which is believed to have developed evolutionarily due to intense competition within scavengers for unpredictable and discrete food resources (Pukowski 1933; Milne and Milne 1976; Anderson 1982; Anderson and Peck 1984, 1985, 1986; Peck and Kaulbars 1987; Trumbo 1994; Eggert and Müller 1997; Scott 1998; Xu and Suzuki 2001). Burying beetles feed and reproduce on small vertebrate carcasses (rodents and birds). Males attract females by means of a pheromone released on a carcass suitable for reproduction (Barlett 1987; Eggert and Müller 1989a, b; Eggert 1992; Scott 1998; Haberer et al. 2008). When a female arrives, the beetles move the carcass to a suitable burying spot. During burial, the beetles excavate soil out from underneath the carcass, strip it of hair or feathers and mould it into a brood ball coated with oral and anal secretions to preserve and control the decay of the carcass (Pukowski 1933; Milne and Milne 1976; Halffter et al. 1983; Scott 1998). The pair mates repeatedly during the burial. A female lays around 10 to 30 eggs, depending on the carcass size (Müller et al. 1990; Trumbo 1994; Trumbo and Fernandez 1995; Nagano and Suzuki 2007). The parents feed hatched larvae (especially of the first instar) by regurgitated partially digested carcass (Pukowski 1933, 1934; Trumbo 1994; Xu and Suzuki 2001; Smisetf and Moore 2002).
Formation of a suitable brood ball requires carcasses, which are not yet degraded or occupied by other scavenger species. This depends critically on an ability of the burying beetle to find a carcass as soon as possible after an animal death. Nicrophorinae locate carrion with sensitive chemoreceptors on their antennal club (Abbot 1927; Boeckh 1962; Ernst 1972). They can recognise a dead animal within 1 day (Smith and Heese 1995) and can locate it from a distance of up to several kilometres away (Petruška 1975).
As soon as an animal dies, its decomposition begins. The breaking tissues emit volatiles, attracting the burying beetle to the source (Grassberger and Frank 2004). It is not known which compounds mediate the carcass’ attractiveness for different scavenger species though the volatiles of many different decomposing carcasses have been studied. Among the first odours to be produced by natural degradation are those released during aerobic decomposition of animal’s hairy or feathery surfaces. The ever present dermatophytes and saprophytes start to degrade keratin and other coat proteins (DeVault et al. 2004; Wawrzkiewicz et al. 1997; Jojola-Elverum et al. 2001; Kadota and Ishida 1972; Segal and Starkey 1969). The degradation of sulphur-containing amino acids cysteine and methionine lead to a production of a wide variety of sulphur-containing volatile organic compounds (S-VOCs; Janzen 1977). The principal S-VOCs produced are MeSH, thiolesters (especially methyl thiolacetate (MeSAc)), sulfane, dimethyl sulphide (DMS), dimethyl disulphide (DMDS), dimethyl trisulphide (DMTS) and dimethyl tetrasulphide (DMQS; López del Castillo-Lozano et al. 2008; Cuer et al. 1979). S-VOCs together with other foul-smelling amines produced during further protein decomposition (indole, skatole, putrescine, cadaverine etc.) contribute to the distinctive odour of decaying meat and are generally considered as unpleasant, putrid, faecal and sulphurous (Thomas and McMeekin 1981).
Many insect species are attracted by odorants produced by decomposition of vertebrate carcasses (Wasserman and Itagaki 2003; Pfrommer and Krell 2004). Their succession on the carcass is quite predictable and quite well known. Blow flies (Diptera: Calliphoridae) and burying beetles (Silphidae: Nicrophorinae) are usually the first to colonise a carcass. Then, beetle predators and scavengers, such as Histeridae, Staphylinidae, Silphidae and Scarabaeidae, move in and prey on the fly larvae as well as feed on the carcass (Watson and Carlton 2004; Pfrommer and Krell 2004). In spite of this general knowledge of carcass attractiveness and species succession, little is known about the perception of decaying volatiles in arthropods.
The goals of this project were to determine the composition of volatiles emitted by fresh rodent carcasses (laboratory mice, Mus musculus Linnaeus) and to identify the infochemicals mediating the carcass attractiveness for Nicrophorus vespillo (Linnaeus) and Nicrophorus vespilloides Herbst, the two most abundant Nicrophorinae species in Europe and Czech Republic (Šustek 1981). The rationale for this study was (1) to increase our knowledge of the infochemicals that affect behaviour of these ecologically important insects and (2) to acquire a theoretical basis for development of a suitable monitoring technique for burying beetles based on chemical/artificial bait.
Materials and methods
Beetles
The individuals used for experiments were wild-caught (Czech Republic, Prague, Suchdol area, N 50° 08′ E 014° 21′) during April—September 2006 and 2007. Pit-fall traps were used. Trap consisted of a 1-L bucket (Anderson 1982; Ratcliffe 1996; Kočárek 2000) baited with a 3-day-old mouse carcass held in a 100-mL glass jar with a screened lid. The bottom of each trap was covered with moistened soil to provide a hiding place for captured beetles. The traps were spaced 25 m apart and checked every morning. Captured beetles were removed and transferred individually into cylindrical plastic cups (10 cm dia × 8 cm height) with moistened soil. Cups were placed in a dark cool room. Beetles were used the next day for experimentation.
Chemicals
Commercially available standards of DMS, DMDS, MeSAc, DMTS and DMQS (Sigma-Aldrich and Oxford Chemicals) were used. For electroantennography (EAG) experiments, MeSAc, DMS, DMDS and DMTS were diluted in hexane to create a concentration series from 1 ng–1 µg/µL. For gas chromatography with time of flight mass spectrometric detection (GC×GC-TOFMS) and gas chromatography with electroantennographic detection (GC-EAD), 1 µL of neat standards was allowed to evaporate in a closed glass flask (10 mL). Respective volatiles were sampled by solid-phase micro-extraction (SPME) CAR/PDMS fibre (Supelco) for 30 s and analysed under the same GC×GC-TOFMS or GC-EAD conditions as authentic samples (described below). MeSH is not commercially available. Therefore, the compound was generated in situ from aqueous solution of sodium methanethiolate (Sigma-Aldrich) mixed with saturated aqueous solution of oxalic acid. Structures of the discussed compounds are depicted in Fig. 1.
Electroantennography
In EAG experiments, the dose–response relationships of MeSH, MeSAc, DMS, DMDS and DMTS were determined in both N. vespillo and N. vespilloides. In these experiments, standard EAG technique was used (Roelofs 1984): Isolated antennae were connected between two Ag/AgCl glass microelectrodes filled with Ringer solution. The reference electrode was connected with the antennal base, with the recording one positioned to make contact with the sensory epithelium on the last antennomere surface. The antennal preparation was placed in a continual air stream (1 L/min) blowing from a glass tube (8 mm in diameter). The electrical signal generated by the antennal preparation was led to a high impedance pre-amplifier (1014 Ω; SYNTECH Equipment and Research, Kirchzarten, Germany) and fed to a PC. Data were evaluated using Syntech EAG software. Odour stimuli (1 mL/0.5 s) were injected into the air stream through a small orifice (5 mm dia in the air delivering tube wall situated 5 cm from the tube outlet). Odour stimuli were injected from odour cartridges. Odour cartridges were prepared from Pasteur pipettes with filter paper discs (10 mm in dia) to which the respective stimuli were loaded on. Odour cartridges were prepared prior to each experiment in the following way; 10 µL aliquots of the respective solution was loaded onto filter paper discs. Then, Pasteur pipettes were sealed with Parafilm M® and allowed to equilibrate for about 1 h. Parafilm seal was removed just before stimulation. Each odour cartridge was used only once. Stimuli were delivered at 1-min intervals. The EAG amplitude during the stimulation was evaluated. Five antennae were used for each respective compound and concentration. As MeSH was available only as sodium salt (MeSNa), a specific procedure to study dose–EAG response relationship was adopted. Aqueous solutions of sodium methanethiolate (MeSNa) at different concentrations were freshly prepared and placed in closed glass (20 mL) vials and mixed with a stoichiometric excess of oxalic acid saturated aqueous solution. We assumed that the different amounts of MeSNa would produce gaseous MeSH of scalable concentrations (from 0.01 to 1 µg/mL) in the flask volume. Then, 1 mL of MeSH enriched air was drawn from each vial via PTFE septum by a Hamilton gas-tight syringe and was delivered to the antennal preparation in order of ascending concentration. The other details of EAG recordings remained the same as described. For graphic visualisation and evaluation of measured EAG activities, the doses in µg/µL were converted to nmol/µL units, and “on antenna-active” amount of tested compounds was corrected using relative volatilities (DMS as reference compound; for details, see Hoskovec et al. 1993, 2005).
Solid-phase micro-extraction sample collection
SPME experiments were performed at ambient temperature (23 ± 1°C). A laboratory mouse (M. musculus) was killed by breaking its spinal cord. Dead mouse was placed on a glass rectangular plate (10 cm × 10 cm) and covered with an oval glass cover lid (10 cm dia, 7 cm height). The centre of the glass lid protrudes up to form a standard glass screw joint (8 mm dia). The joint was closed using a corresponding plastic cap with a PTFE septum. The SPME holder with CAR/PDMS fibre (Supelco, previously desorbed for 5 min in GC injection port heated to 200°C) was inserted through the PTFE septum into the atmosphere surrounding the mouse carcass, and the fibre was exposed for 15 min and immediately GC×GC-TOFMS-analysed. Ten fresh mouse carcasses (M. musculus) were used. During the first 24 h, SPME samplings were performed at every hour. Later on, samplings were repeated at longer intervals (Table 1).
Gas chromatography with electroantennographic detection (SPME-GC-EAD)
The SPME-GC-EAD was used to determine whether and which compounds emanating from dead mice (1–3 days old) are perceived by burying beetles N. vespilloides or N. vespillo. In these experiments, isolated male antennae were used as biological detectors (EAD) along with flame ionisation detector (FID). The antennal preparation is described in the “EAG” section. A HP 5890A chromatograph equipped with a DB-5 (J & W Scientific, Folsom, CA, USA; 30 m × 250 µm ID × 0.25 µm film) column was used. To accommodate both FID and EAD detectors, GC column was split at the end by a four-arm splitter (Graphpack 3D/2 four-arm splitter). The splitter allows division of the effluent between FID and EAD detectors and introduction of N2 make up gas through the fourth arm, compensating for the flow reduction in the split column arms. The column arm for the EAD detector was led outside the GC into the continual air stream directed to the antennal preparation. The GC effluent was carried away by air stream to the preparation. When an active compound hits the antenna, it elicited negative sudden deflections of electrical signal continuously recorded by Ag/AgCl electrodes. The GC oven was operated at an initial temperature 40°C for 1 min and then ramped at a rate 10°C/min to 250°C (with 10-min hold). The temperatures of GC inlet and detector were set to 200°C and 260°C, respectively. The SPME samples were GC analysed split-less. Both antennal and FID signals were fed into a PC and analysed by Syntech GC-EAD software. A series of C8–C22 hydrocarbons were injected regularly between the analysed samples to allow retention time comparisons and calculation of Kovats retention indices. The same GC method was adopted for GC-EAD testing of the synthetic standards mixture. In these experiments, 10-mL glass vial containing 10 mg of all tested compounds (MeSH generated from MeSNa, DMS, DMDS, MeSAc and DMTS) were used instead of the animal’s carcass. The SPME holder with freshly desorbed CAR/PDMS fibre was inserted through the PTFE septum into a vial with an atmosphere saturated by vapours of standards, exposed for 10 s and immediately analysed.
Two-dimensional gas chromatography with time of flight mass spectrometric detection (GC×GC-TOFMS)
The analyses of the carcass volatiles were performed using a LECO Pegasus 4D instrument (LECO, St. Joseph, MI, USA). The key part of this GC×GC-TOFMS system is a cryomodulator located at the junction between the two columns. The cryomodulator is responsible for the trapping of compounds eluted from the first capillary column (first dimension GC) and injecting them into the second column (second dimension GC). The modulator was periodically heated and cooled. The duration and frequency of the hot-cool pulses allow adjustment of GC×GC chromatographic conditions according to the sample features with respect to its intensity and complexity. In the reported analysis, the hot-pulse and cool-time durations were set at 0.4 and 1.1 s, respectively. Both columns were mounted in an Agilent 6890N gas chromatograph oven (Agilent Technologies, Palo Alto, CA, USA). The second column was located in its specific oven with the temperature regimens programmable independently of the first oven. Helium (1 mL/min) was used as a carrier gas for both columns. The temperature programme for the primary GC oven was set as follows; 40°C for 1 min, then to 250°C at 5°C/min and finally for 5 min held at 250°C. The temperature programme for the secondary oven was set 7°C above the programme of the primary oven and operated in the iso-ramping mode. The inlet temperature was 200°C, and the purge time was 60 s, at a flow rate of 60 mL/min. The first dimension column utilised a weak-polar DB-5 (J & W Scientific; 30 m × 250 µm ID × 0.25 µm film), and the second dimension column was polar BPX-50 (SGE, Austin, TX, USA; 2 m × 100 µm ID × 0.1 µm film). The transfer line connecting the secondary column and the MS-TOF detector source was operated at a temperature of 260°C. The source temperature was 250°C with a filament bias voltage of −70 V. The data acquisition rate was 100 scans/s, along with a mass range of 29–400 amu and a detector voltage of 1,650 V. Data were processed and consecutively visualised on the 2D and 3D chromatograms using LECO ChromaTOF™ software. The compounds were identified based on the comparison of their MS spectra and Kováts together with commercially available standards.
Y olfactometer
The attractivity of carcasses and synthetic standards of DMS, DMDS and DMTS were tested in a laboratory olfactometer placed in dimly illuminated fumed space. The selection of tested compounds was influenced by several factors: (a) their commercial availability, (b) their physico-chemical properties (MeSH is gas at ambient temperature), (c) their EAG activities and (d) virtually only organic (oligo)sulphides were identified as key components of necrophagous insects attractants emitted by carcass-mimicking plants or fungi (Stensmyr et al. 2002; Ollerton and Raguso 2006; Jürgens et al. 2006; Stránský and Valterová 1999; Borg-Karlson et al. 1994). The olfactometer (Fig. 2) consisted of two glass Y-parts (4 cm dia, 30 cm length) connected together by their arms. The 7-cm-long arms were replaceable to aid control of contaminations. The olfactometer was connected by a round-shaped arena (35 cm dia, 15 cm height) into which the beetles were released. The bottom of the open arena was covered with moistened filter paper. The tested stimuli were placed in one olfactometer arm (randomly selected), with the other arm remaining empty. The charcoal cleaned humidified air (100 mL/s) was blown through both arms into the arena. Experimental session started with acclimatisation of beetles within the laboratory. During acclimatisation, beetles abandoned their soil shelters and started to explore the environment in plastic cups in which they were held. Only exploring beetles were used in behavioural experiments. Individual beetles were released into olfactometer arena. In empty clean olfactometer (i.e. without attractive stimuli), beetles explored arena and only seldom visited Y olfactometer (5% of N. vespillo and 3% of N. vespilloides). When an attractive stimulus was presented, majority of beetles abruptly changed their behaviour, oriented upwind, within a period < 25 s entered the olfactometer entrance and selected the arm with the attractive source. Beetles that did not find the olfactometer entrance within 2 min were discarded and were supposed as non-responding. Each beetle was tested only once. The natural activity of burying beetles peaks between 5:00 and 8:00 p.m. (Eggert 1992; Kočárek 1997; Podskalská unpublished data). Therefore, the olfactometer experiments were performed during this period. During one experimental session, about 20 beetles were tested. The species and sexes were released into the olfactometer randomly based on beetle exploring activity. Synthetic compounds were allowed to evaporate from 500 µL glass microvial. Neat respective compound (1 µL) was loaded onto cotton tissue packed within microvial. Dispensers were freshly prepared prior to each experiment. After death, the mouse carcasses were kept in the laboratory at ambient temperature (23 ± 1°C) in an open glass container. Twenty-four-hour-old carcasses were used in control experiments. Between experiments, both the olfactometer and the arena were washed with ethanol and distilled water and then dried up. Glass parts were heated for 20 min up to 200°C.
The generalised linear model (GLM) for data with binomial distribution was used for statistical analysis. In GLM model, two factors (bait type and sex) and their interactions were analysed. GLM analysis was followed by simple binominal Fisher test applied to every bait type to determine whether there were significant differences in beetle responses to different treatments.
Results
GC×GC-TOFMS analysis
GC×GC-TOFMS analysis disclosed that 0–30-min-old mice carcasses emit volatiles that did not differ from those emitted by a living mouse. In the course of time however, traces of low-molecular S-VOCs MeSH, MeSAc, DMS, DMDS, DMTS and DMQS appeared (Fig. 3, Table 1). The S-VOCs were identified based on mass spectra, and the identification was confirmed by full match of their mass spectra and GC×GC retention parameters with the corresponding synthetic standards. The relative quantities of emitted S-VOCs related to time elapsing from the death are depicted in Table 1. Table 1 shows the means from ten independent analyses. The relative quantification was based on peak areas provided by ChromaTOF® software at m/z 47. Small quantitative variations were observed in different analysis with respect to individual mouse carcasses depending on their weight, feeding history and other factors. Table 1 shows that dynamics of individual S-VOCs compounds were variable in the course of time. MeSAc and MeSH peaked at 24 and 36 h, respectively, and then declined. DMS peaked at 48–96 h and then declined. On the other hand, quantity of DMS, DMDS and DMTS increased steadily during the examined period. DMQS appeared in 2-day-old carcasses. Besides S-VOCs, no volatile products of degradation were detected in carcasses below 3 days of post mortem time. In older carcasses, however, protein enzymatic/bacterial degradation products like scatole, indole, amines, mercaptoacetic acid, methyl thiolpropanoate, methyl ethyl disulphide, 2,4-dithiapentane, thiopivalic acid and methyl (methylthio)methyl disulphide contributed to death odour of death. These compounds were identified based on NIST mass spectra libraries (with spectra match >95%).
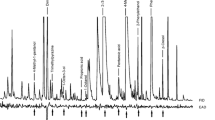
Two-dimensional GC×GC-TOFMS chromatogram of S-VOCs emanating from a 12-h-old (a) and a 72-h-old (b) M. musculus carcass displayed at specific ion mass 47 (CH3S· ion). Each spot in the graph represents a single compound, the intensity of which is colour-coded. White, yellow and red represent low, middle and high intensity, respectively. On the plane, the individual compounds are distributed based on their volatilities (X-axis) and polarities (Y-axis). The more polar and less volatile compounds elute at later retention times. Identified compounds (via standard’s mass spectra and GC×GC-retention behaviour): 1 MeSH, 2 DMS, 3 background contaminant, 4 MeSAc, 5 DMDS, 6 DMTS, 7 DMQS
GC-EAD
SPME and GC-EAD analysis of mouse carcass emanations showed that all identified S-VOCs are antennally active, i.e. they are perceived by antennae of the burying beetle (Fig. 4). SPME and GC-EAD analyses of synthetic S-VOCs elicit EAD responses at similar retention times as did the compounds emanated from the mouse carcasses. The retention times and antennal activities of authentic and synthetic compounds matched and supported the correctness of S-VOCs identification.
SPME and GC-EAD analysis of mouse carcass emanations and synthetic S-VOCs. A FID plot of volatiles emanating from 24-old mouse carcass. B FID plot of synthetic S-VOCs. C and D EAD plots of GC-EAD analysis with N. vespillo and N. vespilloides male antennae, respectively. Both species gave EAD responses at similar retention times
EAG
Burying beetles have large club-like antennae equipped with chemoreceptors capable of detecting a dead animal from afar. The last four segments (antennomeres) of their antennae are enlarged and flattened to increase their surface area. This enlarged surface enhances the beetle’s ability to detect the odours of decay (Fig. 5). EAG responses to carcass S-VOCs were obtained mainly from the intact surface of the last antennomere in males of both investigated species. The antennal responses to synthetic compound were dose dependent (Fig. 6) and ranged over several orders of concentration, suggesting the high probability that beetle antennae bear specific receptors for S-VOCs. Species-specific differences in dose–response curves were observed.
EAG responses curves of S-VOCs identified in volatile bouquet of mouse carcass in N. vespilloides and N. vespillo. Each dot represents a mean of five EAG recordings from male antennae; doses in µg/µL recalculated to nmol/µL and corrected using relative volatilities (p R; Table 2). Error bars represent SEM. Air and hexane stimuli were used as controls
Behavioural experiments
In an empty olfactometer, where no attractive stimuli were present, the beetles released in the olfactometer arena explored it and randomly visited Y olfactometer (5% of N. vespillo and 3% of N. vespilloides). When an attractive stimulus was presented, however, the beetle behaviour changed, they oriented upwind and within short period (t < 25 s) entered the Y entrance and selected the arm with the attractive source. Beetles that did not find Y entrance within a defined period of 2 min were discarded and were considered as non-responding. Twelve N. vespillo individuals out of a total of 259 and seven N. vespilloides individuals out of a total of 272 did not find the olfactometer entrance within 2 min and were discarded.
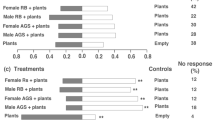
In N. vespillo, GLM analysis revealed non-significant sex-specific and bait-specific differences (both effects χ 2 < 1.99, P = 0.137) and marginally significant sex × bait interaction (χ 2 = 2.60, P = 0.0501). In N. vespilloides, GLM analysis indicated non-significant sex-specific differences (χ 2 = 0.03, P = 0.0.387), non-significant sex × bait interaction (χ 2 = 0.61; P = 0.435), but significant bait-specific differences (χ 2 = 4.04, P = 0.007). The Fisher test following GLM analysis aimed to determine significant differences in bait attractiveness was applied to pooled data from both sexes since no sex-specific differences were indicated by previous GLM analysis in either species investigated. In N. vespillo, the Fisher test showed that 24-h-old mouse carcass and DMTS were highly attractive (P = 0.003 and 0.002, respectively) followed by DMDS and DMS (P = 0.0236 and 0.0199, respectively). In N. vespilloides, mouse carcass was also highly attractive (P = 0.0001) followed by DMS (P = 0.003) and DMTS (P = 0.0163). Responses to DMDS were below statistical significance (P = 0.0731). The described data are displayed in Fig. 7. Bars represent percentages of beetles selecting the baited arm of the olfactometer. Numbers within bars represent the sum of all tested beetles (beetles that found the entrance of the Y olfactometer within the defined 2 min period), and numbers in brackets represent sum of those beetles, who found successfully the baited arm (numbers in brackets). Thus, for instance in N. vespilloides, 29 out of 31 tested females and 27 out of 32 tested males found the arm baited with mouse carcass. The same logic applies to all baits in Fig. 7. Asterisks above bars represent significant differences between the respective baits (as provided by Fisher test). Figure 7 shows that the most attractive stimulus for both studied species was, as expected, mouse carcass. Figure 7 also shows that, with exception of DMDS in N. vespilloides, all synthetic compounds tested reveal significant attractiveness for both species.
Attractiveness of DMS, DMDS and DMTS and 1-day-old mouse carcass for burying beetles N. vespilloides and N. vespillo as measured in laboratory Y olfactometer. Bars show percentages of responding beetles to respective stimuli. The upper numbers in bars represent number of beetles which found the odour source; the numbers in parentheses represent number of tested beetles for the respective bait. Slight differences in the responsiveness between the sexes are not statistically significant based on GLM analysis. Fisher's test for both sexes altogether: **P < 0.01, *P < 0.05, ns non significant
Discussion
In our experiments, we observed that the major products formed shortly after mouse death are mainly S-VOCs, specifically MeSH, MeSAc, DMS, DMSDS, DMTS and traces of DMQS. EAG experiments showed that both species were similarly sensitive to MeSH and DMDS, but they differ in their perception of MeSAc, DMS and DMTS. Slight differences were also observed in the attractiveness of DMS, DMSDS and DMTS in laboratory olfactometer experiments. N. vespilloides was found less responsive to DMDS. This compound elicited upwind orientation but beetles were less successful to correctly locate the odour source. It is possible that in N. vespilloides, DMDS alone is not enough for precise orientation to odour source and that a combination with other S-VOCs is required. Except for DMDS in N. vespilloides, we did not observe other differences in attractiveness of individual S-VOCs compared to fresh mouse carcass. However, in our olfactometric experiments, the compounds were presented only in one concentration, which cannot provide enough information about subtle differences in their attractiveness and their possible synergism. It is also necessary to keep in mind that there are huge differences in volatilities among tested S-VOCs that were not corrected in our behavioural experiments. Thus, further research is needed to specify more precisely the role of individual S-VOCs compounds in the orientation of N. vespillo and N. vespilloides beetles to fresh carcasses.
Our data show that both sexes of N. vespillo and N. vespilloides possess the ability to respond to tested S-VOCs. This suggests that both males and females might locate the fresh carcass based on the same cues. Wilson and Knollenberg (1984) make a distinction between Nicrophorus that were attracted to carrion as a food source (pre-reproductives and hungry reproductives) and those beetles that were searching for a fresh carcass (reproductives). Wilson and Knollenberg (1984) speculate that these different groups of beetles were using a different set of cues (pre-reproductives having some way of avoiding fresh carcasses). Our data do not allow to determine whether such a differentiation exist in N. vespillo and N. vespilloides since we did not select beetles based on Wilson and Knollenberg’s physiological criteria.
DMDS and DMTS were already reported as major emanations of fresh mouse carcasses by Woodard (2006). This author found that shaved carcasses produce lower amounts of these two oligosulphides than unshaven ones. MeSH, MeSAc and DMS as part of off odour have not been reported yet. Woodard (2006) speculates that the reason why burying beetles remove the carcass hairs or feathers during burying reflects an effort to decrease the carcass attractiveness for other competing scavengers (Xu and Suzuki 2001; Woodard 2006).
Production of S-VOCs also accompanies many other fermentation processes, and in some cases, their presence is considered desirable, for example in many cheeses (López del Castillo-Lozano et al. 2008; Cuer et al. 1979; Parliment et al. 1982), Sauvignon Blanc (Zoecklein 2005) and different brands of beer (Baxter and Hughes 2001). S-VOCs are also produced by many higher plants like carrion flowers (Aristolochiaceae, Asclepiadacae, Araceae; Kite and Hetterschieid 1997; Stránský and Valterová 1999; Stensmyr et al. 2002; Raguso 2004, 2008; Ollerton and Raguso 2006; Jürgens et al. 2006) and by stinkhorn fungi (Phallaceae; Borg-Karlson et al. 1994). Both plants and fungi simulate the odour of a rotting carcass to attract carrion beetles and a variety of flies, including blowflies, flesh flies and midges.
In spite of general knowledge of carcass attractiveness and species succession on it, little is known about the perception of decaying volatiles in arthropods. Two types of sulfane-sensitive receptors have been identified in the tropical bont tick, Amblyomma variegatum Fabricius (Ixodidae). Sulfane, which was identified as a part of human and animal breath, aroused 60% of resting ticks when tested at concentrations of ca. 0.02 and 1 ppm (Steullet and Guerin 1992). Some carrion beetles (Nicrophorus sp.) bear carrion sensilla excited by a wide variety of volatiles, including sulfane and butanethiol (Boeckh 1962; Waldow 1973). The larvae of Acrolepiopsis assectella (Lepidoptera: Acrolepiidae) and parasitoid Diadromus pulchellus (Hymenoptera: Ichneumonidae) are attracted by disulphides, thiosulfinates and thiosulfonates present in A. assectella faeces (Al Rouz and Thibout 1988; Auger et al. 1989a, b). Blowflies of the family Calliphoridae that are attracted to the inflorescence of “dead-horse arum” (Helicodiceros muscivorus) respond to three oligosulphides: DMS, DMSDS and DMTS (Stensmyr et al. 2002).
We observed that EAG responses to S-VOCs could be recorded mainly from the surface of the last antennomere. Dethier (1947), Waldow (1973) and Ernst (1972) found that in N. vespilloides, the terminal antennomere, which is essential for the ability of beetles to locate carrion, is innervated by specific sensilla coelosphaerica, while other segments are innervated by sensilla basiconica. Though both sensillar types contain ORNs sensitive to carrion, sensillae coelosphaericae are likely to have a unique function since its amputation fatally influences the ability of burying beetle to locate carrion (Dethier 1947). When terminal antennomere remained intact and other antennal segments are ablated, the orientation ability persists. Sensilla coelosphaerica was found to respond to sulfane and some other non-sulphur compounds (Ernst 1972).
Based on provided evidence that majority of S-VOCs identified in fresh mouse carcass are perceived by N. vespillo and N. vespilloides antennae and that major S-VOCs, e.g. DMS, DMSDS and DMTS, are attractive in laboratory olfactometric experiments, we hypothesise that S-VOCs might be responsible for the attractiveness of fresh carcasses in the studied species. This conclusion is in agreement with recent field experiments, where the pitfall traps were baited with individual DMS, DMDS and DMTS (Podskalská et al. unpublished results). In these experiments, DMS, DMDS and DMTS attracted considerable amounts of carrion beetles. Their attractiveness increase when DMS, DMDS and DMTS were mixed together. However, the blend of DMS, DMDS and DMTS was still less attractive than a 1-day-old mouse carcass, which suggests that perhaps other S-VOCs might be also involved.
In general, there has been very little research performed in the area of analysis of the volatile compounds that influence the behaviour of burying beetles. The research presented here is a small part that addresses the questions of host detection or practical applications for monitoring. Future research, including field experiments, is needed to understand the problem in its complexity.
References
Abbot CE (1927) Experimental data on the olfactory sense of Coleoptera, with special reverence to the Necrophori. Ann Entomol Soc Am 20:207–215
Al Rouz H, Thibout E (1988) Analyse en olfactomètre de l’attraction des larves d’Acrolepiopsis assectella par des substances allelochimiques. Entomol Exp Appl 47:231–237. doi:10.1007/BF00352209
Anderson RS (1982) Resource partitioning in the carrion beetle (Coleoptera: Silphidae) fauna of southern Ontario: ecological and evolutionary considerations. Can J Zool 60:1314–1325. doi:10.1139/z82-178
Anderson RS, Peck SB (1984) Bionomics of Nearctic species of Aclypea Reitter: phytophagous “carrion” beetles (Coleoptera: Silphidae). Pan-Pac Entomol 60:248–255
Anderson RS, Peck SB (1985) The insects and arachnids of Canada, part 13. The Carrion beetles of Canada and Alaska (Coleoptera: Silphidae and Agyrtidae). Research Branch, Agriculture Canada, Ottawa
Anderson RS, Peck SB (1986) Geographic patterns of colour variation in North American burying beetles (Coleoptera; Silphidae). J Nat Hist 20:283–297. doi:10.1080/00222938600770241
Auger J, Lecomte C, Paris J, Thibout E (1989a) Identification of leek-moth and diamondback-moth frass volatiles that simulate parasitoid, Diadromus pulchellus. J Chem Ecol 15:1391–1398. doi:10.1007/BF01014838
Auger J, Lecomte C, Thibout E (1989b) Leek odor analysis by gas chromatography and identification of the most active substance for the leek moth, Acrolepiopsis assectella. J Chem Ecol 15:1847–1854. doi:10.1007/BF01012271
Barlett J (1987) Evidence for a sex attractant in burying beetles. Ecol Entomol 12:471–472. doi:10.1111/j.1365-2311.1987.tb01028.x
Baxter D, Hughes PS (2001) Beer: quality, safety and nutritional aspects. RSC Paperback, Royal Society of Chemistry, London
Boeckh J (1962) Elektrophysiologische Untersuchungen an einzelnen Geruchsrezeptoren auf den Antennen des Totengräbers (Necrophorus, Coleoptera). Z Vgl Physiol Berl 46:212–248. doi:10.1007/BF00341551
Borg-Karlson AK, Englund FO, Unelius CR (1994) Dimethyl oligosulfides, major volatiles released from Sauromatum guttatum and Phallus impudicus. Phytochemistry 35:321–323. doi:10.1016/S0031-9422(00)94756-3
Cuer A, Dauphin G, Kergomard A, Dumont JP, Adda J (1979) Production of S-methylthioacetate by Brevibacterium linens. Appl Environ Microbiol 38:332–334
Dethier VG (1947) The role of the antennae in the orientation of carrion beetles to odors. J NY Entomol Soc 55:285–293
DeVault TL, Brisbin IL Jr, Rhodes OE Jr (2004) Factors influencing the acquisition of rodent carrion by vertebrate scavengers and decomposers. Can J Zool 82:502–509. doi:10.1139/z04-022
Eggert AK (1992) Alternative male mate-finding tactics in burying beetles. Behav Ecol 3:243–254. doi:10.1093/beheco/3.3.243
Eggert AK, Müller JK (1989a) Mating success of pheromone-emitting Necrophorus males: do attracted females discriminate against resource owners? Behaviour 110:248–257. doi:10.1163/156853989X00493
Eggert AK, Müller JK (1989b) Pheromone-mediated attraction in burying beetles. Ecol Entomol 14:235–237. doi:10.1111/j.1365-2311.1989.tb00774.x
Eggert AK, Müller JK (1997) Biparental care and social evolution in burying beetles: Lessons from the larder. In: Choe JC, Crespi BJ (eds) The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge, New York, pp 216–236
Ernst KD (1972) Sensillum coelosphaericum, die Feinstruktur eines neuen olfaktorischen Sensillentyps. Z Zellforsch Mikrosk Anat 132:95–106. doi:10.1007/BF00310299
Grassberger M, Frank C (2004) Initial study of arthropod succession on pig carrion in a central European urban habitat. J Med Entomol 41:511–523
Haberer W, Schmitt T, Peschke K, Schreier P, Müller JK (2008) Ethyl 4-methyl heptanoate: a male-produced pheromone of Nicrophorus vespilloides. J Chem Ecol 34:94–98. doi:10.1007/s10886-007-9406-y
Halffter G, Anduaga S, Huerta C (1983) Nidification des Nicrophorus (Col. Silphidae). Bull Soc Entomol Fr 88:648–666
Hoskovec M, Kalinová B, Konečný K, Koutek B, Vrkoč J (1993) Structure-activity correlations among analogs of the currant clearwing moth pheromone. J Chem Ecol 19:737–750. doi:10.1007/BF00985005
Hoskovec M, Grygarová D, Cvačka J, Streinz L, Zima J, Verevkin SP, Koutek B (2005) Determining the vapour pressures of plant volatiles from gas chromatographic retention data. J Chrom A 1083:161–172. doi:10.1016/j.chroma.2005.06.006
Janzen DH (1977) Why fruits rot, seeds mold, and meat spoils. Am Nat 111:691–713. doi:10.1086/283200
Jojola-Elverum SM, Shivik JA, Clark L (2001) Importance of bacterial decomposition, and carrion substrate to foraging, brown treesnakes. J Chem Ecol 27:1315–1331. doi:10.1023/A:1010357024140
Jürgens A, Dötterl S, Meve U (2006) The chemical nature of fetid floral odours in stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae). New Phytol 172:452–468. doi:10.1111/j.1469-8137.2006.01845.x
Kadota H, Ishida Y (1972) Production of volatile sulfur compounds by microorganisms. Annu Rev Microbiol 26:127–138. doi:10.1146/annurev.mi.26.100172.001015
Kite GC, Hetterschieid WLA (1997) Inflorescence odours of Amorphophallus and Pseudodracontium (Araceae). Phytochemistry 46:71–75. doi:10.1016/S0031-9422(97)00221-5
Kočárek P (1997) The occurrence of Silphidae and Leiodidae: Cholevinae (Coleoptera) in the Litovelské Pomoraví Protected Lanscape Area. Zprávy Vlastivěd Muz v Olomouci 275:17–29 (in Czech, English abstract)
Kočárek P (2000) A pitfall trap for carrion ecology studies. Biologia (Bratisl) 55:575–577
López del Castillo-Lozano M, Mansour S, Tâche R, Bonnarme P, Landaud S (2008) The effect of cysteine on production of volatile sulfur compounds by cheese-ripening bacteria. Int J Food Microbiol 122:321–327
Milne LJ, Milne M (1976) The social behavior of burying beetles. Sci Am 235:84–89
Müller JK, Eggert AK, Furlkröger E (1990) Clutch size regulation in the burying beetle Necrophorus vespilloides Herbst (Coleoptera: Silphidae). J Insect Behav 3:265–270. doi:10.1007/BF01417917
Nagano M, Suzuki S (2007) Effect of carcass size and male presence on clutch size in Nicrophorus quadripunctatus (Coleoptera: Silphidae). Entomol Sci 10:245–248. doi:10.1111/j.1479-8298.2007.00220.x
Ollerton J, Raguso RA (2006) The sweet stench of decay. New Phytol 172:379–381. doi:10.1111/j.1469-8137.2006.01903.x
Parliment TH, Kolor MG, Rizzo DJ (1982) Volatile components of limburger cheese. J Agric Food Chem 30:1006–1008. doi:10.1021/jf00114a001
Peck SB (1990) Insecta: Coleoptera Silphidae and the associated families Agyrtidae and Leiodidae. In: Dindal DL (ed) Soil biology guide. Wiley, New York, pp 1113–1136
Peck SB, Kaulbars MM (1987) A synopsis of the distribution and bionomics of the carrion beetles (Coleoptera: Silphidae) of the conterminous United States. Proc Entomol Soc Ont 118:47–81
Petruška F (1975) The effect of predominating winds on the flight of some species of beetles from the group of Silphidae into pitfall traps (Col. Silphidae). Acta Universitatis Palackianae Olomucensis. Facultas Rerum Natur 51:155–175
Pfrommer A, Krell FT (2004) Who steals the eggs? Coprophanaeus telamon (Erichson) buries decomposing eggs in western Amazonian Rain Forest (Coleoptera: Scarabaeidae). Coleopt Bull 58:21–27. doi:10.1649/585
Pukowski E (1933) Ökologische Untersuchungen an Necrophorus F. Z Morphol Oekol Tiere 27:518–586. doi:10.1007/BF00403155
Pukowski E (1934) Die Brutpflege des Totengräbers. Entomol Blatt 30:109–112
Raguso RA (2004) Flowers as sensory billboards: progress towards an integrated understanding of floral advertisement. Curr Opin Plant Biol 7:434–440. doi:10.1016/j.pbi.2004.05.010
Raguso RA (2008) Wake up and smell the roses: the ecology and evolution of floral scent. Annu Rev Ecol Evol Syst 39:549–569. doi:10.1146/annurev.ecolsys.38.091206.095601
Ratcliffe BC (1996) The carrion beetles (Coleoptera: Silphidae) of Nebraska. Bull Univ Nebr State Mus 13:1–100
Roelofs WL (1984) Electroantennogram assays: rapid and convenient screening procedures for pheromones. In: Hummel HE, Miller TA (eds) Techniques in pheromone research. Springer, New York, pp 131–159
Růžička J (2007) Fauna Europaea: Silphidae. In: Alonso-Zarazaga MA, Audisio P (eds) Fauna Europaea: Coleoptera, Beetles. Fauna Europaea version 1.3. http://www.faunaeur.org
Růžička J, Schneider J (2004) Family Silphidae Latreille, 1807. In: Löbl I, Smetana A (eds) Catalogue of Palaearctic Coleoptera, vol. 2: Hydrophiloidea–Histeroidea—Staphylinoidea. Apollo Books, Steensrup, pp 229–237
Scott MP (1998) The ecology and behavior of burying beetles. Annu Rev Entomol 43:595–618. doi:10.1146/annurev.ento.43.1.595
Segal W, Starkey RL (1969) Microbial decomposition of methionine and identity of the resulting sulfur products. J Bacteriol 98:908–913
Sikes DS (2005) Silphidae Latreille, 1807. In: Beutel RG, Leschen RAB (eds) Handbook of zoology, volume IV: Arthropoda: Insecta, Part 38: Coleoptera, Beetles. Volume 1: morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). Walter de Gruyer, Berlin, pp 288–296
Smisetf PT, Moore AJ (2002) Does resource availability affect offspring begging and parental provisioning in a partially begging species? Anim Behav 63:577–585. doi:10.1006/anbe.2001.1944
Smith RJ, Heese B (1995) Carcass selection in a high altitude population of the burying beetle, Nicrophorus investigator (Silphidae). Southwest Nat 40:50–55
Stensmyr MC, Urru I, Collu I, Celander M, Hansson BS, Angioy AM (2002) Pollination: rotting smell of dead-horse arum florets. Nature 420:625–626. doi:10.1038/420625a
Steullet P, Guerin PM (1992) Perception of breath components by the tropical bont tick, Amblyomma variegatum Fabricius (Ixodidae). J Comp Physiol [A] 170:677–685. doi:10.1007/BF00198977
Stránský K, Valterová I (1999) Release of volatiles during the flowering period of Hydrosme rivieri (Araceae). Phytochemistry 52:1387–1390. doi:10.1016/S0031-9422(99)00247-2
Šustek Z (1981) Key to identification of insects: Carrion beetles of Czechoslovakia (Coleoptera, Silphidae). Zprávy Československé Společnosti Entomologické při ČSAV, Klíče k určování hmyzu 2:1–47 (in Czech)
Thomas CJ, McMeekin TS (1981) Production of off odours by isolates from poultry skin with particular reference to volatile sulphides. J Appl Bacteriol 198:529–534
Trumbo ST (1994) Interspecific competition, brood parasitism, and the evolution of biparental cooperation in burying beetles. Oikos 69:241–249. doi:10.2307/3546144
Trumbo ST, Fernandez AG (1995) Regulation of brood size and cues employed to assess resource size by burying beetles. Ethol Ecol Evol 7:313–322
Waldow U (1973) Elektrophysiologie eines neuen Aasgeruchrezeptors und seine Bedeutung für das Verhaltern des Totengräbers (Necrophorus). J Comp Physiol 83:415–424. doi:10.1007/BF00696356
Wasserman SL, Itagaki H (2003) The olfactory responses of the antenna and maxillary palp of the fleshfly, Neobellieria bullata (Diptera: Sarcophagidae) and their sensitivity to blockage of nitric oxide synthase. J Insect Physiol 49:271–280. doi:10.1016/S0022-1910(02)00288-3
Watson EJ, Carlton CE (2004) Insect succession and decomposition of wildlife carcasses during Fall and Winter. J Med Entomol 42:193–203. doi:10.1603/0022-2585(2005)042[0193:ISADOW]2.0.CO;2
Wawrzkiewicz K, Ziókowska G, Wawrzkiewiczyna J (1997) In vitro biodegradation of hair from different animal species by Microsporum canis. Int Biodeter Biodegr 39:15–25. doi:10.1016/S0964-8305(96)00040-6
Wilson DS, Knollenberg WG (1984) Food discrimination and ovarian development in burying beetles (Coleoptera: Silphidae: Nicrophorus). Ann Entomol Soc Am 77:165–170
Woodard CBS (2006) Odor masking of a vertebrate carcass by a burying beetle (Nicrophorus marginatus). Master’s thesis, Texas Tech University, Lubbock, TX
Xu H, Suzuki N (2001) Effects of carcass size and parental feeding on reproductive success of the burying beetle Nicrophorus quadripunctatus (Coleoptera Silphidae). Entomol Sci 4:217–222
Zoecklein B (2005) The good volatile sulfur compounds. Enology Notes 101. http://www.fst.vt.edu/extension/enology/EN/101.html
Acknowledgement
We are highly indebted to Jarmila Titzenthalerová and to Ondřej Blažek (in memoriam) for their skilful technical assistance in insect handling and electrophysiological experiments. We also thank to Miroslav Šálek (Faculty of Environmental Sciences, Czech University of Life Sciences, Prague) for help in statistical calculations. Special thanks are addressed to Yvonne Kavanagh (Dublin and IOCB Prague) for the language check of this paper. We very much thank the anonymous referees for constructive criticism of the manuscript. This work was supported by research project Z4-055-905 (Institute of Organic Chemistry ASCR, v.v.i.) and by grant CULS-IGA 200741110031 (Faculty of Environmental Sciences, Czech University of Life Sciences, Prague).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalinová, B., Podskalská, H., Růžička, J. et al. Irresistible bouquet of death—how are burying beetles (Coleoptera: Silphidae: Nicrophorus) attracted by carcasses. Naturwissenschaften 96, 889–899 (2009). https://doi.org/10.1007/s00114-009-0545-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-009-0545-6