Abstract
Associative learning of host-associated chemical cues was studied in Nasonia vitripennis, a parasitoid of fly pupae in nests of hole-nesting birds. When females encountered a fly pupa and performed one sequence of host recognition behaviour including drilling the ovipositor into the host in the presence of the artificial odour furfurylheptanoate (FFH), they were afterwards arrested by FFH in olfactometer experiments. The response vanished after 4 days and could be blocked after 3 days by feeding wasps with ethacrynic acid prior and after the training. This indicates the formation of an intermediate form of memory by one host experience in N. vitripennis. Interestingly, the trained wasps avoided odours that were not present during the host encounter, although naive wasps did not react to these odours. This unique behaviour probably causes wasps to focus during host searching on those chemical cues they have experienced in the host environment. Studies in nests of hole-nesting birds revealed that about 30% of all nests contained only one fly pupa, and laboratory studies showed that N. vitripennis females are able to parasitise around 100 fly pupae in their life. It is discussed that under these conditions, the formation of a non-permanent intermediate memory for host-associated odours after one host encounter is adaptive to avoid costs involved with formation and maintenance of memory for misleading cues. The demonstration of associative olfactory learning in N. vitripennis, the first parasitoid species with sequenced genome, opens the gate to study molecular mechanisms of memory formation and its ecological adaptation in parasitoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Associative learning is common throughout the animal kingdom, from invertebrates to vertebrates, and it is assumed that this learning ability has evolved to adjust the behavioural response of animals to changing ecological conditions (Stephens 1993). During classical conditioning as a form of associative learning a stimulus, the unconditioned stimulus (US), which induces an innate reaction (the unconditioned reaction, UR), is paired with a stimulus that normally does not induce any reaction, the so-called conditioned stimulus (CS; Menzel 1995). As a consequence of one or several of these pairings, a reaction can also be induced by the CS. In all animals where this aspect has been studied, memory after learning consists of different phases, which are remarkably similar between species (DeZazzo and Tully 1995). The most important phases are short-term memory (STM), which lasts only for minutes to hours, and protein-biosynthesis-dependent long-term memory (LTM), which lasts for several days or the whole life of the organism. Between these two phases, an intermediate memory phase often termed medium-term memory (MTM) has been described in many species (Stough et al. 2006). Insights into the proximate molecular mechanisms behind these memory phases were provided by many studies with rodents, chicken, Aplysia, Drosophila melanogaster Meigen and Apis mellifera L. (Rose 2000; Kandel 2001; Müller 2002; Davis 2005; Barco et al. 2006). With respect to the ultimate evolutionary reason for these memory phases, however, only little is known. Menzel (1999, 2001) suggested that memory phases represent an adaptation to the ecology of an animal and the variable reliability of available information. According to this idea, potentially unreliable information is stored only in the transient STM or MTM. Reliable information, on the other hand, is kept in the LTM for a longer period of time or the entire life. One prediction from this hypothesis is that two species, which obtain information of differing reliability during their life due to their ecology, should also differ in their memory structure and, consequently, also in the molecular mechanisms (or its expressions) which are responsible for the formation of the memory phases.
Hymenopterous parasitoids, i.e. parasitic wasps, are one group of animals where learning has been demonstrated in many species (Steidle and van Loon 2003). Parasitoids are insects which lay their eggs on or in other organisms, mostly other insects. Per definition, a parasitoid kills and consumes its host for development (Lafferty and Kuris 2002). To find hosts, many parasitoid females orient to chemical cues from their hosts, their hosts’ food, or the hosts’ environment (Turlings et al. 1993). During this host finding process, many parasitoids learn to react to chemical host-associated cues either during the development (Gandolfi et al. 2003), during emergence from a certain host (van Emden et al. 1996), or during the first host encounters (Steidle and van Loon 2002). To test the hypothesis that memory phases represent an ecological adaptation and to study the underlying molecular mechanisms, parasitic wasps seem to be highly suitable for two reasons: (1) Parasitoids are not able to reproduce without hosts, which causes a strong selection pressure on all traits connected to successful host location, including learning behaviour and memory structure and (2) even related species of parasitic wasps can be ecologically very different, which enables the comparative study of species. In fact, recent studies with the congeneric braconid wasps Cotesia rubecula and Cotesia glomerata Marshall (Bleeker et al. 2006; Smid et al. 2007) revealed remarkable differences in the memory structure of these species consistent with their ecology (Collett 2007), thus supporting the hypothesis by Menzel (1999, 2001).
One group of parasitic wasps, which is suitable for comparative behavioural and molecular studies to test this hypothesis, are Pteromalids. Among these, associative learning and LTM formation has been intensively studied in Lariophagus distinguendus Förster, a parasitoid of the granary weevil Sitophilus granarius L. (Steidle and Schöller 1997; Steidle 1998; Müller et al. 2005; Collatz et al. 2006). A suitable species for comparison is Nasonia vitripennis (Walker 1836), a parasitoid of fly pupae in carcasses or nests of hole-nesting birds (Merwe van der 1943; Abraham 1985). N. vitripennis has a comparable longevity as L. distinguendus; it is easy to rear and many aspects of its biology have been studied (Whiting 1967; Steiner et al. 2006; Beukeboom et al. 2007; Ruther et al. 2007; Sykes et al. 2007). Recently, the genome of N. vitripennis (Hymenoptera: Pteromalidae) was sequenced and is available (Werren et al. 2005). However, only little is known on the ecology and the behaviour of N. vitripennis in the context of host finding, e.g., the size of the host patches, the use of chemical cues for host finding and the ability to learn chemical cues associated with hosts. Learning behaviour has been studied so far only with respect to colour learning (Oliai and King 2000; Baeder and King 2004).
To test the hypothesis that memory structure is adapted to the ecology of a species, the current study aims to provide data on the learning ability of N. vitripennis and important aspects of the ecology of its hosts for a comparison with L. distinguendus and the two Cotesia species. In detail, we studied (1) associative olfactory learning in N. vitripennis, (2) the duration of olfactory memory formed after one single host experience and (3) the patch size of N. vitripennis hosts in carcasses and nests of hole-nesting birds as compared to the number of hosts that can be attacked by one female of N. vitripennis.
Materials and methods
Insects
Insect cultures were kept at 25°C and a photoperiod of 16:8 h (L/D). Deep-frozen fly pupae of Lucilia sp. obtained from a pet shop were used as hosts for N. vitripennis. To rear N. vitripennis, about 30 parasitoids were placed into a Petri dish (9-cm diameter, 1-cm height) with about 200 fly pupae and kept there until their death. After 13 days, ten parasitised fly pupae were separated and kept individually in glass vials. After 14–15 days of development time, parasitoids emerged from the fly pupae and were collected daily. Until training, they were kept in Petri dishes on filter paper. The mean lifetime of N. vitripennis in the presence of hosts is about 14 days, comparable to the longevity of L. distinguendus.
Training procedure
Parasitoids were trained individually 2–3 days after emergence and were tested individually at certain intervals afterwards. The training confirmed to the typical paradigm of classical conditioning and aimed to form an association between an unconditioned stimulus (the host recognition sequence, US) and the conditioned stimulus [the odour source: furfurylheptanoate (FFH), CS].
To study the general ability of N. vitripennis to learn host-associated odours, the wasps were released individually in a chamber with 30 dead, unparasitised pupae of Lucilia sp. and a glass capillary (1 cm, diameter 1.56 mm) containing pure FFH (98+% Sigma-Aldrich). The capillary was fixed in a block of paraffin (1 × 1 × 1 cm), allowing the FFH to evaporate. FFH is an artificial volatile substance and was chosen to avoid any potential pre-adaptation by the parasitoids. The wasps were allowed to move freely in the training chamber for 1 h, and care was taken that each wasp at least once drilled into one host pupa during the training phase. After training, wasps were kept in Petri dishes until testing in the olfactometer 24 h later. As controls, we tested wasps that had the same drilling experience in a fly pupa during 1 h but without FFH and naïve wasps that had no experience with fly pupae.
To test if learning already occurs after one drilling event, the same setup was used. However, wasps were removed from the training chamber after they had withdrawn their ovipositor following the first drilling into one fly pupa. This training procedure could last between few minutes to 1 h. To check how long memory after one drilling experience is retained, wasps were tested at 1, 5, 12, 24, 48, 72, 96 and 144 h afterwards.
General bioassay method
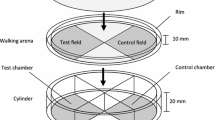
The response of the naïve and trained wasps towards FFH was tested in an olfactometer (Fig. 1). This is made of heat-resistant synthetic material and consists of a cylinder (4 cm high, diameter 19 cm) divided by vertical plates into four chambers. On the top of the cylinder, a walking arena (1 cm high, diameter 19 cm) is placed consisting of metal gauze (mesh 0.5 mm) with a rim of heat-resistant synthetic material (0.9 cm high) and covered with a glass plate. From earlier experiments, it was known that the metal gauze has no influence on the behaviour of the wasps. No airflow was generated. A glass capillary (2 cm, diameter 1.5 mm) containing pure FFH was placed in one chamber (test chamber). The opposite chamber contained an empty glass capillary (control chamber), and the two other chambers remained empty as transition zones. The olfactometer was illuminated from above. At the beginning of each experiment, one wasp was placed in the center of the walking arena. Then the behaviour (walking, resting) and the position of the wasp were recorded for 600 s using the computer software “The Observer 5.0” (Noldus, Wageningen). The time the wasp spent walking in the field of the walking arena above the test chamber with the odour (test field) or in the opposite field (control field) was compared with the Wilcoxon matched pairs test for non-parametric data using the software package Statistica for Windows 5.5 (StatSoft Inc. 1999). Walking was chosen as parameter to compare arrestment in the test and control fields because arrestment to chemical odours in insects involves orthokinetic and klinokinetic behaviour, i.e. a decrease in walking speed and an increase in turns during walking. Both cause the insect to remain in the area with higher odour concentration and lead to an increased probability to find the odour source (Wyatt 2003). Resting, i.e. not moving, is not part of olfactory searching behaviour. To avoid biased results due to side preferences, the position of the olfactometer was rotated clockwise after every wasp. Each wasp was tested only once.
Blocking of memory with ethacrynic acid
To examine if memory formed after one single training experience can be chemically blocked, wasps were continuously fed ethacrynic acid (EA) from 24 h before training until 24 h after training. This was done by placing an Eppendorff vial with a piece of filter paper soaked with EA dissolved in sugar–water (5 mM EA in 1% sugar–water) into Petri dishes where wasps were kept in. EA was described to deactivate the Na+/K+-ATPase and therefore to block a memory phase preceding long-term memory in Drosophila, rats and chicken (Gibbs and Ng 1977; Frieder and Allweis 1982; Xia et al. 1998). The wasps were trained as described above and tested at 24 and 72 h afterwards. Control wasps were treated identically, but were only fed sugar–water without ethacrynic acid. To study the influence of EA on the general odour discrimination ability, we tested the reaction of female wasps which had been fed EA for 48 h to extracts of male N. vitripennis wasps. The tests were performed 48 h after feeding. Therefore, this treatment is identical to the treatment in the 72-h experiment to block the memory and is therefore appropriate as a control. As demonstrated recently, extracts of male N. vitripennis wasps contain a sexual pheromone which is highly attractive to unmated N. vitripennis wasps (Ruther et al. 2007, 2008). The used female wasps were unmated and were fed EA as described above. The extract was obtained by placing 50 male wasps which were killed by freezing in 250 μl dichloromethane for 30 min. The extract was stored at −22°C until testing. Extract (5 μl) and solvent (5 μl) were applied on fresh filter paper and the solvent was allowed to evaporate for 90 s. Subsequently, the filter papers were placed in the olfactometer (filter paper with extract in one chamber, filter paper with solvent in the opposite chamber) for 30 s, enabling the formation of an odour gradient. The extract and the solvent control were renewed after each individual. In contrast to previous FFH experiments, the olfactometer in this bioassay was smaller (10-cm diameter, 3-cm height) and the walking time was shorter (300 s).
Avoidance behaviour
To investigate the avoidance behaviour of the control wasps towards the odour source, the wasps were trained with drilling experience in the absence of any odour. After 24 h, the reaction of these wasps to ethyl vanillin (Sigma-Aldrich) and cinnamon (retail industry) was tested in the olfactometer. Additionally, wasps were trained to FFH as described above and tested to FFH and cinnamon. During the bioassay, 50 mg of the odours (ethyl vanillin, cinnamon) was offered in a cap of an Eppendorff vial in the smaller olfactometer type. The walking duration was 300 s.
Size of host patches for N. vitripennis
The host patch size, i.e. the number of hosts available in one patch, was investigated using two potential patch types of N. vitripennis: mouse carcasses and nest boxes of hole-nesting bird species. Nine carcasses were placed in plastic buckets filled with soil in the park of the University of Hohenheim in Stuttgart in the southwest of Germany from 14 August 2006 until 16 November 2006. After decay of the carcasses, the fly pupae were collected from the ground, frozen and counted. Nesting material was collected from 19 bird nests of hole-nesting birds from a park area on the campus of the University of Hohenheim by the end of the breeding season in October 2006 and frozen. Afterwards, the fly pupae were collected from the nest material and counted.
Parasitisation rate of N. vitripennis
The parasitisation rate of N. vitripennis was measured by keeping 1-day-old mated females in Petri dishes together with 150 or 200 fly pupae until their death. After emergence of the offspring, the parasitised fly pupae were counted. Each treatment was repeated five times. Only those replicates were included in the analysis in which wasps did produce offspring.
Results
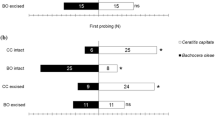
Wasps that were kept on fly pupae for 1 h in the presence of FFH were observed to perform intensive drumming and drilling on the fly pupae. After 24 h, they showed a significant preference for the field with FFH in the olfactometer (Wilcoxon matched pairs test, T = 7, Z = 4.18, n = 25, p < 0.001; Fig. 2). In contrast, naïve control wasps did not show any preference for the test field with FFH compared to the control field in the olfactometer (Wilcoxon matched pairs test, T = 104, Z = 1.57, n = 25, p = 0.115; Fig. 2). Interestingly, wasps that had experience with fly pupae only significantly avoided FFH (Wilcoxon matched pairs test, T = 15, Z = 3.97, n = 25, p < 0.001; Fig. 2).
Walking duration (mean + SD) of trained females of N. vitripennis in the odour fields of an olfactometer with one field containing odour of furfurylheptanoate (FFH; hatched bars) and the other left empty as control (empty bars). cond +: Females that were kept in a training chamber for 1 h on fly pupae in the presence of FFH; cond −: Females that were kept in a training chamber for 1 h on fly pupae without FFH; naïve: Females without any experience with FFH or fly pupae. Tests were done 24 h after training
When wasps were removed after one drilling experience from the fly pupa in the presence of FFH and tested in the olfactometer afterwards, they showed a significant preference for the FFH field at 1 h (Wilcoxon matched pairs test, T = 13, Z = 4.13, n = 26, p < 0.001; Fig. 3), 5 h (Wilcoxon matched pairs test, T = 14, Z = 3.89, n = 24, p < 0.001; Fig. 3), 12 h (Wilcoxon matched pairs test, T = 37, Z = 3,23, n = 24, p = 0.001; Fig. 3), 24 h (Wilcoxon matched pairs test, T = 52, Z = 2.97, n = 25, p = 0,003; Fig. 3), 48 h (Wilcoxon matched pairs test, T = 2, Z = 4.32, n = 25, p < 0.001; Fig. 3), 72 h (Wilcoxon matched pairs test, T = 28, Z = 3.62, n = 25, p < 0.001; Fig. 3) and 96 h (Wilcoxon matched pairs test, T = 71, Z = 2.26, n = 24, p = 0.024; Fig. 3) after the training. At 144 h (Wilcoxon matched pairs test, T = 92, Z = 0.49, n = 20, p = 0.627; Fig. 3), no attraction to FFH was found.
Walking duration (mean + SD) of trained females of N. vitripennis in the odour fields of an olfactometer with one field containing odour of furfurylheptanoate (FFH; hatched bars) and the other left empty as controls (empty bars). Females were kept on fly pupae in the presence of FFH until they had finished their first drilling. The olfactometer test was performed at different time intervals between 1 and 144 h afterwards
To test which memory phase is established after one single drilling experience, wasps were fed EA (5 mM in 1% sugar–water) or sugar–water (1%) 24 h before until 24 h after training and tested in the olfactometer at 24 and 72 h afterwards (Fig. 4a). After 24 h, the EA wasps (Wilcoxon matched pairs test, T = 5, Z = 3.62, n = 19, p < 0.001; Fig. 4a) and the sugar–water wasps (Wilcoxon matched pairs test, T = 11, Z = 3.51, n = 20, p < 0.001; Fig. 4a) showed a significant preference to FFH. After 72 h, only the control wasps that were fed sugar–water preferred FFH (Wilcoxon matched pairs test, T = 29, Z = 2.84, n = 20, p = 0.005; Fig. 4a). EA wasps did not show a preference for any field in the olfactometer (Wilcoxon matched pairs test, T = 68, Z = 1.09, n = 19, p = 0.277; Fig. 4a). Additionally, we tested the reaction of unmated EA wasps to a male sex pheromone extract in order to exclude any influence of EA on the general odour discrimination ability. The wasps showed a significant preference to the pheromone extract (Wilcoxon matched pairs test, T = 0, Z = 3.92, n = 20, p < 0.001; Fig. 4b).
Walking duration (mean + SD) of trained females of N. vitripennis in the odour fields of an olfactometer. a One field contained odour of furfurylheptanoate (FFH; hatched bars) and the other field was left empty as control (empty bars). Sugar: Wasps were fed 1% sugar water from 24 h before until 24 h after the training; EA: Wasps were fed ethacrynic acid in sugar water from 24 h before until 24 h after the training. The olfactometer test was performed 24 and 72 h after the training. The total observation time was 600 s. b One field contained odour of male pheromone and the other contained solvent (dichloromethane) as control (empty bars). Wasps were fed ethacrynic acid in sugar water for 48 h and the olfactometer test was performed 48 h after feeding. Therefore, the treatment is comparable to the 72-h experiments from a. The total observation time was 300 s
The avoidance of FFH in the control experiments was tested with two additional odours, ethyl vanillin, and cinnamon. Both compounds did not cause a reaction in the olfactometer in naïve wasps (ethyl vanillin: Wilcoxon matched pairs test, T = 143, Z = 0.82, n = 26, p = 0.409; cinnamon: Wilcoxon matched pairs test, T = 160, Z = 0.07, n = 25, p = 0.946; Fig. 5). However, 24 h after training with drilling, but in the absence of any artificial odour, the wasps showed a significant aversion to both odours (ethyl vanillin: Wilcoxon matched pairs test, T = 56, Z = 2.87, n = 25, p = 0.004; cinnamon: Wilcoxon matched pairs test, T = 39, Z = 3.17, n = 24, p = 0.002; Fig. 5). Cinnamon was also avoided 24 h after training in the presence of FFH (Wilcoxon matched pairs test, T = 40, Z = 2.62, n = 21, p = 0.009; Fig. 5), whereas FFH was significantly preferred (Wilcoxon matched pairs test, T = 3, Z = 3.81, n = 20, p < 0.001; Fig. 5).
Walking duration (mean + SD) of naïve and conditioned females of N. vitripennis in the odour fields of an olfactometer with one field containing odour of ethyl vanillin, cinnamon, or FFH (hatched bars) and the other left empty as controls (empty bars). Naïve: Females without any experience with FFH or fly pupae; cond −: Females that were kept in a training chamber on fly pupae without any artificial odour until they had finished their first drilling; cond +: Females that were kept in a training chamber on fly pupae in the presence of FFH until they had finished their first drilling. The olfactometer test was performed at 24 h afterwards
The patch size of N. vitripennis, i.e. the number of potential hosts per patch varied considerably. In mouse carcasses, the number of fly pupae ranged from 21 to 127. In nesting material of hole-nesting birds, it ranged from 0 to 148, with 30% of all the nests containing only one pupa. The number of fly pupae parasitised by N. vitripennis females during their lifetime was 82 ± 29 (mean ± SD) in the presence of 150 fly pupae and 96 ± 50 in the presence of 200 pupae.
Discussion
When females of N. vitripennis were kept for 1 h in the presence of host fly pupae together with the artificial odour FFH, they preferred this odour in subsequent olfactometer experiments. Control wasps, which had only experience with fly pupae in the absence of FFH as well as naïve wasps, which had no experience with fly pupae or FFH, showed no preference for FFH. As far as we know, this is the first demonstration of olfactory learning of host finding cues for N. vitripennis, with FFH acting as conditioned stimulus that is associatively learned by the females upon their encounters with fly pupae. Thus, N. vitripennis agrees with many other wasp species studied so far, which have been shown to learn to react to host associated chemical cues during the first host experience (Steidle and van Loon 2003). It is reasonable to assume that due to this learning, the females of N. vitripennis are able to improve their ability to locate hosts as has been shown in other parasitoids earlier (Lewis and Martin 1990; Papaj and Vet 1990; Steidle 1998).
Generally, it is assumed that learning of host finding cues is especially important in generalist parasitoids. In these species, hosts and host environment vary between generations which also results in a variation of host-associated odours. Therefore, learning should be more adaptive than a genetically fixed response to olfactory host finding cues (Vet and Dicke 1992). In fact, a meta-analysis revealed that learning was more frequently found in generalist as compared to specialist parasitoids (Steidle and van Loon 2003). N. vitripennis seems to fit into this concept as well. For oviposition, females use a number of different fly species including blowflies, flesh flies, and houseflies occurring in or around carcasses of different animals (Putman 1977; Grassberger and Frank 2004) and in nests of hole-nesting birds. The latter seem to be more important as host patches for N. vitripennis than carcasses (Abraham 1985; Peters 2006). Because nesting material varies between bird species (Bezzel 1993) and birds have been shown to actively collect aromatic plant parts to repel parasites (Clark and Mason 1988; Petit et al. 2002), it is reasonable to assume that at least on a species level, bird nests represent unique odourous environments. Thus, chemical cues vary between host patches and learning of these cues is adaptive.
Naïve wasps without host experience showed no response to FFH, whereas control wasps, which had only experience with fly pupae, significantly avoided FFH in most experiments. This difference is surprising because both groups of wasps did not have any contact with FFH before. In additional experiments on this phenomenon, a significant repellent effect of otherwise neutral odours after host experience was also found with ethyl vanillin and cinnamon. Furthermore, when wasps had one host experience in the presence of FFH, they were afterwards arrested by FFH but avoided cinnamon, which they had never contacted before. We are not aware that this kind of negative reaction after positive experience has been described before in other parasitoid species or in other animals in general. Obviously, females of N. vitripennis not only learn to react to odours present during host encounters but at the same time learn to avoid any absent odour. The involved type of learning could be sensitisation and the negative reaction could be facilitated by the high concentration of chemicals. Generally, the described negative response to unknown odours could cause the wasp to focus on only those chemical cues it has encountered upon host contact and enables to discriminate these cues from the background of numerous other environmental odours, which are generally present (Hilker and McNeil 2008). However, more studies are required to study this new phenomenon.
Females of N. vitripennis encountering fly pupae perform a behavioural sequence of host recognition on the pupae, which lasts for several minutes and is comparable to the behaviour of other pteromalid wasps, e.g. the granary weevil parasitoid L. distinguendus (Steidle 2000). The sequence consists of drumming with the antennae, tapping with the tip of the abdomen, drilling with the ovipositor and sometimes host feeding on the fly pupae (Edwards 1954). Because oviposition occurs inside the pupa when the ovipositor is inserted, it could not be separated from drilling in our experiments. When wasps were allowed to perform one sequence of host recognition behaviour up to drilling but without host feeding on a fly pupa in the presence of FFH, a preference for FFH was observed afterwards. This demonstrates that in the used training, the US which is associated with the FFH as CS must be encountered during the short phase of host recognition. Further studies are required to identify the US and the UR within the host recognition sequence. In the related parasitoid L. distinguendus, drilling behaviour serves as UR and no oviposition is required (Collatz et al. 2006).
The reaction to FFH lasted for up to 96 h (4 days) after the training and was gone after 144 h (6 days). Obviously, the memory for the CS formed during one host encounter is not permanent and might therefore be an intermediate memory phase between STM and protein-synthesis-dependent LTM. Analogous phases could be late short-term memory (lSTM) and MTM in honeybee (Menzel and Giurfa 2001; Eisenhardt 2006) as well as MTM (holding up to 4 h) and anesthesia-resistant memory (ARM; holding up a few days) in Drosophila (DeZazzo and Tully 1995; Isabel et al. 2004), all of which are independent from protein synthesis. To test the idea that one sequence of host recognition behaviour causes an intermediate memory phase in N. vitripennis equivalent to ARM in Drosophila, the learning experiments were repeated with wasps that were fed EA. EA inhibits the Na+/K+-ATPase and thereby interferes with the transport of sodium and potassium across the cell membrane (Banerjee et al. 1971). Probably due to this effect, EA blocks ARM in Drosophila (Xia et al. 1998) and MTM in rats and chicken (Gibbs and Ng 1977; Frieder and Allweis 1982), i.e. memory phases which precede protein-synthesis-dependent LTM and follow STM. In fact, our experiments revealed that wasps treated with EA did not react to FFH at 72 h after training but at 24 h. Because discrimination ability of EA-treated wasps was not affected after 24 h and the walking activity was the same as in other experiments were no preference was found, we conclude that this absence of a reaction is due to the blocking of the memory at that time. Furthermore, in control experiments, female wasps significantly preferred extracts of male N. vitripennis containing recently identified sexual pheromones (Ruther et al. 2007, 2008). This shows that EA treatment does not impair the general odour discrimination ability of the wasps. Thus, an intermediate memory phase for host-associated odours starting after 24 h and lasting for up to 96 h was induced in N. vitripennis in our experiments. Because this phase is probably too late for lSTM, we hypothesise that it is comparable to either MTM in honeybees or ARM in Drosophila. Further studies will address this question.
Among other wasp species studied so far, the formation of an intermediate memory phase after one experience was demonstrated in the braconid C. rubecula, a solitary parasitoid of the small cabbage white Pieris rapae (Smid et al. 2007). In contrast, a protein-dependent LTM for host-associated odours is formed after one single, short learning event in C. glomerata (Bleeker et al. 2006; Smid et al. 2007), a parasitoid of the gregarious large cabbage white Plasmodiophora brassicae, and L. distinguendus, a parasitoid of stored grain beetles which also occur in large patches (Collatz et al. 2006). Whereas C. rubecula and C. glomerata are closely related and both belong to the family Braconidae, L. distinguendus is related to N. vitripennis (Krogmann and Vilhelmsen 2006) both belonging to the family Pteromalidae. The difference in memory formation between C. rubecula on the one hand and C. glomerata and L. distinguendus on the other hand is explained by their ecology. In C. rubecula, hosts are solitary feeding on various plant species and therefore co-occur with varying and unreliable plant cues. Thus, the induction of a transient, intermediate form of memory for host-patch-associated cues after one learning event is adaptive to avoid costs involved with formation and maintenance of memory for misleading cues (Bleeker et al. 2006; Collett 2007; Smid et al. 2007). In C. glomerata and L. distinguendus, on the other hand, hosts occur in patches that are large enough for a female to lay all the eggs it is able to produce during its lifetime. Therefore, already during the first encounter with a host, the females perceive cues, which reliably indicate host presence for the rest of their lives. Under these conditions, the establishment of a LTM for host-associated cues after one single host contact is considered to be adaptive (Bleeker et al. 2006; Collatz et al. 2006; Collett 2007; Smid et al. 2007).
To examine if the establishment of an intermediate memory phase after one host experience can be interpreted as adaptation to the ecology of N. vitripennis, we studied the size of potential host patches and the lifetime fecundity of wasp females. It turned out that host patches consisting of carcasses contained between 20 and 120 fly pupae of different fly species as Calliphoridae, Sarcophagidae and Muscidae. In contrast, in nests of hole-nesting birds, 30% of patches contained only one fly pupa and 75% contained ten pupae or less. Similar data were obtained in a recent field study which revealed a high number of nest boxes without any fly pupae. In nests with fly pupae, the number ranged from six to 82 (Grillenberger et al. 2008). The average number of pupae of Lucilia flies that could be parasitised by one N. vitripennis female was between 80 and 100, and more detailed studies revealed that even up to 170 hosts can be parasitised when more hosts are offered (Schurmann, unpublished data). Similarly, Chabora (1970) reported that 100 to 120 Phaenicia fly pupae can be parasitised by one N. vitripennis female. Thus, although under natural conditions the fecundity of Nasonia females might be lower, the host patch situation in N. vitripennis seems to be intermediate between the three parasitoid species mentioned above. Many host patches only contain one or a few hosts, as it is the case for C. rubecula, but other patches might be large enough to cover the lifetime fecundity of one N. vitripennis female, as it is the case in C. glomerata and L. distinguendus. In analogy to the reasoning above, we assume that under these conditions, one host encounter should only induce the formation of a non-permanent memory because wasps should not be prevented from leaving to continue to search for other host patches. In agreement with this scenario, the recent study by Grillenberger et al. (2008) revealed that some N. vitripennis females visit more than one patch during their life. Only after a larger number of host encounters a LTM should be formed to retain parasitoids in the host patch for the rest of their life. Future studies will have to address the question which amount of experience is required for LTM in N. vitripennis.
In conclusion, our study demonstrates for the first time associative learning and the formation of an intermediate memory phase for olfactory cues by one host experience in the parasitoid N. vitripennis. In addition, this parasitoid seems to possess a novel learning mechanism which causes wasps to avoid unknown odours and further helps the parasitoids to focus on relevant chemical cues during host searching. Because the genome in this parasitoid species has recently been sequenced, these results open the gate to study the molecular mechanisms of learning and memory formation in parasitoids. Furthermore, our data support the hypothesis that memory dynamics in animals is an adaptation to the ecology of a species. However, this aspect has been studied so far only with four species of parasitic wasps, which is not enough to draw any final conclusions.
References
Abraham R (1985) Nasonia vitripennis an insect from birds’ nests. Entomol Gen 10:121–124
Baeder JM, King BH (2004) Associative learning of color by males of the parasitoid wasp Nasonia vitripennis (Hymenoptera: Pteromalidae). J Insect Behav 17:201–213. doi:10.1023/B:JOIR.0000028570.93123.dc
Banerjee SP, Khanna VK, Sen AK (1971) Inhibition of sodium- and potassium-dependent adenosine triphosphatase by ethacrynic acid: Ligand-induced modifications. Biochem Pharm 20:1341–1737
Barco A, Bailey CH, Kandel ER (2006) Common molecular mechanisms in explicit and implicit memory. J Neurochem 97:1520–1533. doi:10.1111/j.1471-4159.2006.03870.x
Beukeboom LW, Kamping A, Louter M, Pijnacker LP, Katju V, Ferree PM, Werren JH (2007) Haploid females in the parasitic wasp Nasonia vitripennis. Science 315:206. doi:10.1126/science.1133388
Bezzel E (1993) Kompendium der Vögel Mitteleuropas. AULA, Wiesbaden
Bleeker MAK, Smid H, Steidle JLM, Kruidhof M, van Loon JJA, Vet LEM (2006) Differences in memory dynamics between two closely related parasitoid wasp species. Anim Behav 71:1343–1350. doi:10.1016/j.anbehav.2005.09.016
Chabora PC (1970) Studies of parasite-host interaction using geographical strains of the blow fly Phaenicia sericata and its parasite Nasonia vitripennis. Ann Entomol Soc Am 63:495–501
Clark L, Mason JR (1988) Effect of biologically active plants used as nest material and the derived benefit to starling nestlings. Oecologia 77:174–180. doi:10.1007/BF00379183
Collatz J, Müller C, Steidle JLM (2006) Protein-synthesis dependent long-term memory induced by one single, non-spaced training in a parasitic wasp. Learn Mem 13:263–266. doi:10.1101/lm.192506
Collett TS (2007) Insect behavior: learning for the future. Curr Biol 18:131–134. doi:10.1016/j.cub.2007.11.052
Davis RL (2005) Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci 28:275–302. doi:10.1146/annurev.neuro.28.061604.135651
DeZazzo J, Tully T (1995) Dissection of memory formation: from behavioural pharmacology to molecular genetics. Trends Neurosci 18:212–218. doi:10.1016/0166-2236(95)93905-D
Edwards RL (1954) The host-finding and oviposition behavior of Mormoniella vitripennis, a parasite of muscoid flies. Behavior 7:88–112
Eisenhardt D (2006) Learning and memory formation in the honeybee (Apis mellifera) and its dependency on the cAMP-protein kinase A pathway. Anim Biol 56:259–278. doi:10.1163/157075606777304249
Frieder B, Allweis C (1982) Memory consolidation: further evidence for the four-phase model from the time-courses of diethyldithiocarbamate and ethacrynic acid amnesias. Physiol Behav 29:1071–1075. doi:10.1016/0031-9384(82)90300-6
Gandolfi M, Mattiacci L, Dorn S (2003) Preimaginal learning determines adult response to chemical stimuli in a parasitic wasp. Proc R Soc Lond B 270:2623–2629. doi:10.1098/rspb.2003.2541
Gibbs ME, Ng KT (1977) Psychobiology of memory: towards a model of memory formation. Biobehav Rev 1:113–136
Grassberger M, Frank C (2004) Initial study of arthropod succession on pig carrion in a central European urban habitat. J Med Entomol 41:511–523. doi:10.1603/0022-2585(2004)041[0511:ISOASO]2.0.CO;2
Grillenberger BK, Koevoets T, Burton-Chellew MN, Sykes EM, Shuker DM, van de Zande L, Bijlsma R, Gadau J, Beukeboom LW (2008) Genetic structure of natural Nasonia vitripennis populations: validating assumptions of sex-ratio theory. Mol Ecol 17:2854–2864. doi:10.1111/j.1365-294X.2008.03800.x
Hilker M, McNeil J (2008) Chemical and behavioral ecology in insect parasitoids: how to behave optimally in a complex odorous environment. In: Wajnberg E, Bernstein C, van Alphen J (eds) Behavioral ecology of insect parasitoids. Blackwell, MA, pp 92–112
Isabel G, Pascual A, Preat T (2004) Exclusive consolidated memory phases in Drosophila. Science 304:1024–1027. doi:10.1126/science.1094932
Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038. doi:10.1126/science.1067020
Krogmann L, Vilhelmsen L (2006) Phylogenetic implications of the mesosomal skeleton in Chalcidoidea (Hymenoptera,Apocrita)—tree searches in a jungle of homoplasy. Invertebr Syst 20:615–674. doi:10.1071/IS06012
Lafferty KD, Kuris AM (2002) Trophic strategies, animal diversity and body size. Trends Ecol Evol 17:507–513. doi:10.1016/S0169-5347(02)02615-0
Lewis WJ, Martin WR Jr (1990) Semiochemicals for use with parasitoids: status and future. J Chem Ecol 16:3067–3089. doi:10.1007/BF00979613
Menzel R (1995) Orientierung. In: Gewecke M (ed) Physiologie der Insekten. Gustav Fischer, Stuttgart, pp 387–412
Menzel R (1999) Memory dynamics in the honeybee. J Comp Physiol 185:323–340. doi:10.1007/s003590050392
Menzel R (2001) Searching for the memory trace in a mini-brain, the honeybee. Learn Mem 8:53–62
Menzel R, Giurfa M (2001) Cognitive architecture of a mini-brain: the honeybee. Trends Cogn Sci 5:62–71. doi:10.1016/S1364-6613(00)01601-6
Merwe van der JS (1943) Investigations on the biology and ecology of Mormoniella vitripennis Walk. (Pteromalidae Hym.). J Entomol Soc South Afr 6:48–64
Müller U (2002) Learning in honeybees: from molecules to behaviour. Zoology 105:313–320. doi:10.1078/0944-2006-00075
Müller C, Collatz J, Wieland R, Steidle JLM (2005) Associative learning and memory duration in the parasitic wasp Lariophagus distinguendus. Anim Biol 56:221–232. doi:10.1163/157075606777304195
Oliai SE, King BH (2000) Associative learning in response to color in the parasitoid wasp Nasonia vitripennis (Hymenoptera: Pteromalidae). J Insect Behav 13:55–69. doi:10.1023/A:1007763525685
Papaj DR, Vet LEM (1990) Odor learning and foraging success in the parasitoid, Leptopilina heterotoma. J Chem Ecol 16:3137–3150. doi:10.1007/BF00979616
Peters R (2006) Interaktionen zwischen Wirten und Parasitoiden: Nahrungsnetzstruktur, Wirtsspektren und Wirtsfindung am Beispiel der Arten aus Vogelnestern. Dissertation. Universität Hamburg, Fachbereich Biologie
Petit C, Hossaert-McKey M, Perret P, Blondel J, Lambrechts MM (2002) Blue tits use selected plants and olfaction to maintain an aromatic environment for nestlings. Ecol Lett 5:585–589. doi:10.1046/j.1461-0248.2002.00361.x
Putman RJ (1977) Dynamics of the blowfly, Calliphora erythrocephala, within carrion. J Anim Ecol 46:853–866
Rose SPR (2000) God’s organism? The chick as a model system for memory studies. Learn Mem 7:1–17
Ruther J, Stahl LM, Steiner S, Garbe LA, Tolasch T (2007) A male sex pheromone in a parasitic wasp and control of the behavioral response by the female’s mating status. J Exp Biol 210:2163–2169. doi:10.1242/jeb.02789
Ruther J, Steiner S, Garbe LA (2008) 4-Methylquinazoline is a minor component of the male sex pheromone in Nasonia vitripennis. J Chem Ecol 34:99–102. doi:10.1007/s10886-007-9411-1
Smid HM, Wang G, Bubovinsky T, Steidle JLM, Bleeker MAK, van Loon JJA, Vet LEM (2007) Species-specific variation in acquisition and consolidation of long-term memory. Proc R Soc B 274:1539–1546. doi:10.1098/rspb.2007.0305
Steidle JLM (1998) Learning pays off: influence of experience on host finding and parasitism in Lariophagus distinguendus (Hymenoptera: Pteromalidae). Ecol Ent 23:451–456. doi:10.1046/j.1365-2311.1998.00144.x
Steidle JLM (2000) Host recognition cues of the granary weevil parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). Entomol Exp Appl 95:185–192. doi:10.1046/j.1570-7458.2000.00656.x
Steidle JLM, Schöller M (1997) Olfactory host location and learning in the granary weevil parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). J Ins Behav 10:331–342. doi:10.1007/BF02765601
Steidle JLM, van Loon JJA (2002) Chemoecology of parasitoid and predator oviposition behavior. In: Hilker M, Meiners T (eds) Chemoecology of insect eggs and egg deposition. Blackwell, London, pp 291–317. doi:10.1002/9780470760253.ch11
Steidle JLM, van Loon JJA (2003) Dietary specialization and infochemical use in carnivorous arthropods: testing a concept. Entomol Exp Appl 108:133–148. doi:10.1046/j.1570-7458.2003.00080.x
Steiner S, Hermann N, Ruther J (2006) Characterization of a female-produced courtship pheromone in the parasitoid Nasonia vitripennis. J Chem Ecol 32:1687–1702. doi:10.1007/s10886-006-9102-3
Stephens DW (1993) Learning and behavioural ecology: incomplete information and environmental predictability. In: Papaj DR, Lewis AC (eds) Insect learning: ecological and evolutionary perspectives. Chapman and Hall, New York, pp 195–218
Stough S, Shobe JL, Carew TJ (2006) Intermediate-term processes in memory formation. Curr Opin Neurobiol 16:672–678. doi:10.1016/j.conb.2006.10.009
Sykes EM, Innocent TM, Pen I, Shuker DM, West SA (2007) Asymmetric larval competition in the parasitoid wasp Nasonia vitripennis: a role in sex allocation? Behav Ecol Sociobiol 61:1751–1758. doi:10.1007/s00265-007-0407-1
Turlings TCJ, Wäckers FL, Vet LEM, Lewis WJ, Tumlinson JH (1993) Learning of host-finding cues by hymenopterous parasitoids. In: Papaj DR, Lewis AC (eds) Insect learning: ecological and evolutionary perspectives. Chapman and Hall, New York, pp 51–78
van Emden HF, Sponagl B, Wagner E, Baker T, Ganguly S, Douloumpaka S (1996) Hopkins’ ‘host selection principle’, another nail in its coffin. Physiol Entomol 21:325–328. doi:10.1111/j.1365-3032.1996.tb00873.x
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Werren JH, Gadau J, Beukeboom L, Desplan C, Lynch J, Rivers R, Richards S, van de Zande L (2005) Proposal to sequence the Nasonia genome. http://www.genome.gov/Pages/Research/Sequencing/SeqProposals/NasoniaSeq.pdf
Whiting AR (1967) The biology of the parasitic wasp Mormoniella vitripennis. Q Rev Biol 42:333–406
Wyatt TD (2003) Pheromones and animal behviour. Cambridge University Press, Cambridge
Xia SZ, Feng CH, Guo AK (1998) Multiple-phase model of memory consolidation confirmed by behavioural and pharmacological analyses of operant conditioning in Drosophila. Pharmacol Biochem Behav 60:809–816. doi:10.1016/S0091-3057(98)00060-4
Acknowledgements
We wish to thank Stephanie-Simone Bertenbreiter, Dennis Grimm and Christian König for patiently counting fly pupae and Claudia Hezler and Nadine Timm for performing olfactometer experiments during a student course. Waltraud Laich and Reinhard Mache (Arbeitskreis für Vogelkunde und Vogelschutz e.V.) kindly provided us with old bird nests. Three unknown reviewers helped with their comments to improve an earlier version of the manuscript. The experiments comply with the current law of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schurmann, D., Collatz, J., Hagenbucher, S. et al. Olfactory host finding, intermediate memory and its potential ecological adaptation in Nasonia vitripennis . Naturwissenschaften 96, 383–391 (2009). https://doi.org/10.1007/s00114-008-0490-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-008-0490-9