Abstract
Some parasitoids oviposit in nonhosts. Parasitization of nonhosts potentially wastes gametes, risks the death of offspring, and reduces fitness. Associative learning, a strategy for efficient reproduction, has been observed in various parasitoid species. We conducted two types of experiments to reveal whether larval parasitoid wasps, Cotesia kariyai (Hymenoptera: Braconidae), learn associatively by ovipositing in nonhosts. In dissection experiments, we found wasp eggs in both host [Mythimna separata (Lepidoptera: Noctuidae)] and nonhost [Spodoptera litura (Lepidoptera: Noctuidae)] caterpillars. However, the mean number of eggs in nonhosts was significantly smaller than in hosts. In oviposition experiments, most naive C. kariyai females attacked both hosts and nonhosts. On the other hand, wasps that had previously attacked nonhosts tended to avoid them thereafter. We conclude that C. kariyai females may be able to detect and identify nonhost metabolites and/or cuticular hydrocarbons. Negative associative learning enhances C. kariyai reproductive success when hunting in complex host habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the field, insect parasitoids are exposed to various volatile compounds from hosts, nonhosts, healthy plants, and plants infested by hosts and/or nonhosts, and they must locate their hosts in this complicated environment. Recently, a number of studies on host searching behavior of parasitoids in complicated environments have been conducted (reviewed in De Rijk et al. 2013; Takabayashi 2014). In natural habitats, plants are often infested by several herbivore species. Parasitoids sometimes encounter nonhosts on such plants (De Rijk et al. 2013), and mistakenly oviposit in them (Takabayashi and Takahashi 1990; Obonyo et al. 2008). Not only do these wasps waste reproductive effort and energy, but they also risk death due to counterattacks from nonhosts (Potting et al. 1999; Gentry and Dyer 2002).
In order to locate hosts efficiently, parasitoids have developed a variety of tactics, including associative learning. Vet et al. (1990) defined associative learning as “the establishment through experience of an association between two stimuli or between a stimulus and a response.” Various studies have revealed that some parasitoids learn by associating ovipositing experience on their hosts with certain stimuli (Wäckers and Lewis 1994; Takasu and Lewis 2003; Costa et al. 2010; Feng et al. 2015; Giunti et al. 2015).
Some studies have suggested that parasitic wasps recognize host suitability by inserting their ovipositors (Obonyo et al. 2008; McDonald et al. 2015). Therefore, when parasitoids attack nonhosts, they may be able to learn by negative association. However, no studies about associative learning on nonhost by parasitoids have been performed.
Cotesia kariyai (Hymenoptera: Braconidae) is a specialized larval endoparasitoid of the common armyworm, Mythimna separata (Lepidoptera: Noctuidae). When a female C. kariyai finds a common armyworm, she lands on and inserts her ovipositor into it. However, we have observed that female wasps also insert their ovipositors into larvae of Spodoptera litura (Lepidoptera: Noctuidae), a nonhost for C. kariyai. In a preliminary experiment, we dissected S. litura larvae that had been attacked by C. kariyai females and found wasp eggs in their hemolymph. C. kariyai eggs likely cannot develop properly due to an immune response (Hayakawa and Yazaki 1997), since S. litura is a nonhost. Therefore, it is unclear whether C. kariyai females recognize S. litura as a nonhost and reduce the number of eggs deposited. Both M. separata and S. litura are distributed in Southeast and East Asia, and they share some host plant species, such as grasses (Poaceae) (Zaidi et al. 2009; Koyama and Matsumura 2019). Therefore, it is possible that C. kariyai encounters S. litura in the field.
In this study, we dissected larvae attacked by C. kariyai females and observed their ‘attack’ behavior. Herein, we discuss how C. kariyai females can recognize nonhosts using associative learning, so as to minimize waste of reproductive output by reducing inappropriate oviposition or by avoiding nonhosts.
Materials and Methods
Insects
Cotesia kariyai, Mythimna separata, and Spodoptera litura were from colonies that have been maintained for many years in the Laboratory of Applied Entomology and Zoology, University of Tsukuba, Japan. Mythimna separata and S. litura were reared on an artificial diet (Silkmate® 2 M, Nosan Corporation) in the rearing room of the laboratory (25 ± 1 °C, 16 L: 8D photoperiod, 700 lx., and 60 ± 10% RH). Third and fourth instars were used for experiments.
Cotesia kariyai was reared under the same conditions as M. separata. Third to early 6th instar larvae of M. separata were offered to C. kariyai females for parasitization. After wasp larvae spun cocoons, they were put into plastic cages (15 × 17 × 24 cm). Honey and wet cotton were provided for food and water. When adult wasps emerged from their cocoons, they mated immediately in the plastic cages. Mated females were kept in the cages and 3- to 4-day-old females were used for experiments.
Dissection
In this study, we define “attack behavior” as riding on the dorsum of a larva, bending the abdomen, inserting the ovipositor into the larva, and remaining stationary for more than 0.5 s. Duration of oviposition insertion was recorded using a stopwatch.
To determine whether C. kariyai females oviposit into S. litura, we dissected larvae that had been attacked by C. kariyai. In Petri dishes (9 cm × 1.5 cm), one 3rd instar larva of M. separata or S. litura was exposed to a 3–4-day-old C. kariyai female for a single attack. We measured the time that a wasp spent attacking a caterpillar. Soon after oviposition behavior, we transferred larvae to dissection plates and dissected them with micro-scissors and fine forceps, adding a small amount of distilled water in order to separate wasp eggs from body fluid contaminants. Under a stereomicroscope (SZH, Olympus, Tokyo, Japan), we counted the number of wasp eggs.
Attacking Experiment
In order to clarify whether C. kariyai females use associative learning to distinguish hosts and nonhosts, we exposed nonhost S. litura to C. kariyai that had attacked nonhosts previously and observed wasp behavior in a Petri dish, as before. We put a single 4-day-old C. kariyai female and a single 4th instar S. litura larva into a Petri dish together and the wasp was allowed to attack the larva once. Next, the wasp was transferred to an empty Petri dish and kept with honey and wet cotton for 3 h. Then, the wasp was allowed to contact a 4th instar M. separata or S. litura larva once. We observed wasp behavior and recorded whether wasps attacked the larvae. The same experiments with host-experienced C. kariyai were also performed using the method described above.
Statistics
An exact Wilcoxon rank sum test was used to analyze egg numbers and attack time of wasps. We used Fisher’s exact test to analyze the attack rate by wasps in the attacking experiment. All data were analyzed with R version 3.0.2 (R Development Core Team 2013).
Results
Dissection
Parasitoid eggs were readily removed from S. litura larvae and counted. The mean number of wasp eggs found in nonhost, S. litura (9.2 ± 2.0, mean ± S.E., n = 27) was significantly smaller than counts from host, M. separata (17.8 ± 2.2, n = 24) (Exact Wilcoxon rank sum test, p < 0.01) (Fig. 1a, Supplementary Material 1). The mean attack duration of wasps on nonhost, S. litura (2.08 ± 0.23 s, mean ± S.E., n = 27) was significantly shorter than that on host, M. separata (2.91 ± 0.16 s, n = 24) (Exact Wilcoxon rank sum test, p < 0.01) (Fig. 1b).
Attack of C. kariyai females on M. separata (host) and S. litura (nonhost) larvae. a Numbers of wasp eggs found by dissecting M. separata and S. litura larvae that had been attacked once by a single female C. kariyai and b attack duration of C. kariyai females on M. separata and S. litura larvae. Whiskers show maximum and the minimum values. The bold dark line shows the median, and box top and bottom edges represent the first and third quartiles (**: p < 0.01 by Exact Wilcoxon rank sum test)
Attacking Experiment
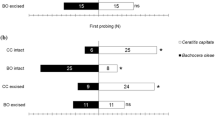
The attack rate on nonhost S. litura by C. kariyai females that had previously attacked S. litura (6.9%, n = 29) was significantly lower than that on M. separata (30%, n = 30) (Fisher’s exact test, p < 0.05, Fig. 2). On the other hand, the attack rates on M. separata and on nonhost, S. litura by naive C. kariyai females were 95% (n = 20) and 85% (n = 20) respectively. There was no significant difference between these rates (Fisher’s exact test, p < 0.05, Fig. 2). Host-experienced C. kariyai females showed low (10.0–13.3%) attack rates and there was no significant difference between host and nonhost (Fisher’s exact test, p < 0.05, Fig. 2). C. kariyai females that did not attack an S. litura larva immediately retreated from the larva after the first contact and avoided it thereafter.
Discussion
From the dissection experiment, C. kariyai females clearly oviposited in S. litura larvae, despite the fact that these are nonhosts for the wasp. However, the number of eggs deposited in nonhosts was significantly smaller than in M. separata, the normal host (Fig. 1a). In addition, we also found that C. kariyai females spent less time when they attacked nonhost caterpillars than when they attacked appropriate hosts (Fig. 1b). From these results, we conclude that C. kariyai females recognize nonhost S. litura and that they reduce the number of eggs laid. These results suggest that C. kariyai females that commence oviposition in S. litura recognize the inappropriate choice after inserting the ovipositor and stop oviposition. C. kariyai females may recognize nonhosts by detecting metabolites and/or cuticular hydrocarbons in the larva with the ovipositor. In fact, some hymenopteran parasitoids have chemosensory sensilla on their ovipositors (van Lenteren et al. 2007; Quicke 2015). According to the results of rearing experiments, Cotesia kariyai females normally lay 27–56 eggs in 3rd instar larvae of M. separata (Sato and Tanaka 1984). The number of eggs that we found in 3rd instars was smaller than in the previous study. Therefore, we hesitate to claim that using a stereomicroscope, we were able to find all eggs laid by the wasps; however, we are confident that our dissection technique was at least consistent, and that numbers of eggs laid in the two caterpillar species were significantly different.
There was a tendency for C. kariyai females to avoid S. litura when they had previously attempted to oviposit in them. In this study, 85% and 95% of naive wasps attacked nonhost and host wasps, respectively (Fig. 2). On the other hand, the attack rate on hosts decreased from 95% to 30% if C. kariyai females had previously attacked nonhosts and to 13.3% if they had attacked hosts previously. This decrease may be caused by lower oviposition activity of wasps due to a previous attack, as parasitoids need time after oviposition to produce additional mature eggs (Ueno 1999; Islam and Copland 2000). Therefore, attack rate on nonhosts by host-experienced females was also low (10.0%), even when the females had never experienced the nonhost before. However, even taking that into consideration, experienced wasps had a tendency to avoid nonhosts more than hosts. It appears that female wasps that had attacked nonhosts learned to recognize certain cue(s), enabling them to avoid nonhosts in subsequent encounters. It is probable that C. kariyai females recognize S. litura larvae as nonhosts by inserting their ovipositors. Because C. kariyai larvae cannot complete development in S. litura larvae (Hayakawa and Yazaki 1997), this behavior minimizes the cost of inappropriate oviposition. Thus, early experience with S. litura inhibits C. kariyai females subsequent attempts to parasitize this nonhost species. Since we put only one C. kariyai female and one S. litura larva together in a Petri dish, it is clear that C. kariyai females were responding only to compounds from S. litura, and that C. kariyai females had learned from their prior experience with nonhosts.
From these results, we still do not know how C. kariyai females recognize S. litura as a nonhost. Previous studies revealed that some parasitic wasps have sensilla on their ovipositors that detect gustatory stimuli (van Lenteren et al. 2007; Goubault et al. 2011; Ruschioni et al. 2015). Cotesia kariyai females may also have ovipositor sensilla to detect metabolites specific to S. litura or the absence of compound(s) from M. separata when they insert their ovipositors. SEM evaluation of C. kariyai ovipositors may reveal useful morphological information, and electro-physiological techniques may also be applied.
When C. kariyai females contact host or nonhost larvae, they drum on the larvae with their antennae. We observed that many wasps that declined to oviposit in nonhost larvae first drummed on them briefly before aborting an attack. Studies on other parasitic wasps suggest that wasps can detect compounds on host body surfaces (Obonyo et al. 2010). Ohara et al. (1996) showed that when presented with a brown glass rod coated with host body wax, C. kariyai females examined the rod using their antennae and tried to insert their ovipositors into the rod. C. kariyai females may likewise detect body wax of nonhost caterpillars. It is important to emphasize that we did not put any other materials, e.g., host feces or diet, in the Petri dishes beside a single wasp and a larva, so wasps were exposed only to compounds from larvae in these experiments. Behavioral observations and/or experiments on C. kariyai responses to isolated chemical(s) from body wax and fluids of nonhosts are needed.
These results show that C. kariyai females detect chemical(s) in S. litura larvae and lay fewer eggs, recognizing them as nonhosts. They also show that C. kariyai females avoid laying eggs in S. litura larvae after prior experience attacking them. Thanikkul et al. (2017) suggested that C. kariyai females can distinguish host-related and nonhost-related herbivore-induced plant volatiles (HIPVs). From these results, C. kariyai females apparently minimize egg waste caused by oviposition into nonhost species. They accomplish this by recognizing host habitat using HIPVs and by associative learning from experience with nonhosts.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Costa A, Ricard I, Davison AC, Turlings TCJ (2010) Effects of rewarding and unrewarding experiences on the response to host-induced plant odors of the generalist parasitoid Cotesia marginiventris (Hymenoptera: Braconidae). J Insect Behav 23:303–318
De Rijk M, Dicke M, Poelman EH (2013) Foraging behaviour by parasitoids in multiherbivore communities. Anim Behav 85:1517–1528
Feng Y, Wratten S, Sandhu H, Keller M (2015) Host plants affect the foraging success of two parasitoids that attack light brown apple moth Epiphyas postvittana (Walker) (Lepidoptera: Tortricidae). PLoS One 10:e0124773
Gentry GL, Dyer LA (2002) On the conditional nature of neotropical caterpillar defenses against their natural enemies. Ecology 83:3108–3119
Giunti G, Canale A, Messing RH, Donati E, Stefanini C, Michaud JP, Benelli G (2015) Parasitoid learning: current knowledge and implications for biological control. Biol Control 90:208–219
Goubault M, Cortesero AM, Paty C, Fourrier J, Dourlot S, Ralec AL (2011) Abdominal sensory equipment involved in external host discrimination in a solitary parasitoid wasp. Micros Res Techniq 74:1145–1153
Hayakawa Y, Yazaki K (1997) Envelope protein of parasitic wasp symbiont virus, polydnavirus, protects the wasp eggs from cellular immune reactions by the host insect. Eur J Biochem 246:820–826
Islam KS, Copland MJW (2000) Influence of egg load and oviposition time interval on the host discrimination and offspring survival of Anagyrus pseudococci (Hymenoptera: Encyrtidae), a solitary endoparasitoid of citrus mealybug, Planococcus citri (Hemiptera: Pseudococcidae). Bull Entomol Res 90:69–75
Koyama J, Matsumura M (2019) Ecology and control of armyworm, Mythimna separata (Lepidoptera: Noctuidae) in Japan, with special reference to outbreak and migration. Jpn J Appl Entomol Zool 63:39–56
McDonald H, Reed DA, Ahmadian S, Paine TD (2015) Parasitoid discrimination between suitable and unsuitable congener hosts. J Insect Behav 28:417–425
Obonyo M, Schulthess F, Gerald J, Wanyama O, Rü BL, Calatayud P-A (2008) Location, acceptance and suitability of lepidopteran stemborers feeding on a cultivated and wild host-plant to the endoparasitoid Cotesia flavipes Cameron (Hymenoptera: Braconidae). Biol Control 45:36–47
Obonyo M, Schulthess F, Ru BL, Van Den Berg J, Silvain J-F, Calatayud P-A (2010) Importance of contact chemical cues in host recognition and acceptance by the braconid larval endoparasitoids Cotesia sesamiae and Cotesia flavipes. Biol Control 54:270–275
Ohara Y, Takabayashi J, Takahashi S (1996) Oviposition kairomones in the cuticular wax of host larvae, Pseudaletia separata, toward its parasitic wasp, Cotesia kariyai. Appl Entomol Zool 31:271–277
Potting RPJ, Vermeulen NE, Conlong DE (1999) Active defence of herbivorous hosts against parasitism: adult parasitoid mortality risk involved in attacking a concealed stemboring host. Entomol Exp Appl 91:143–148
Quicke DLJ (2015) The ovipositor and ovipositor sheaths. In: Quicke DLJ (ed) The braconid and ichneumonid parasitoid wasps: biology, systematics, evolution and ecology. WileyChichester, pp. 35–56
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ruschioni S, van Loon JJA, Smid HM, van Lenteren JC (2015) Insects can count: sensory basis of host discrimination in parasitoid wasps revealed. PLoS One 10:e0138045
Sato Y, Tanaka T (1984) Effect of the number of parasitoid (Apanteles kariyai) eggs [Hym. : Braconidae] on the growth of host (Leucania separata) larvae [Lep. : Noctuidae]. Entomophaga 29:21–28
Takabayashi J (2014) Infochemical webs and tritrophic interactions. In : eLS 2014, John Wiley & Sons Ltd : Chichester http://www.els.net/
Takabayashi J, Takahashi S (1990) An allelochemical elicits arrestment in Apanteles kariyai in feces of nonhost larvae Acantholeucania loreyi. J Chem Ecol 16:2009–2017
Takasu K, Lewis WJ (2003) Learning of host searching cues by the larval parasitoid Microplitis croceipes. Entomol Exp Appl 108:77–86
Thanikkul P, Piyasaengthong N, Menezes-Netto AC, Taylor D, Kainoh Y (2017) Effects of quantitative and qualitative differences in volatiles from host- and non-host-infested maize on the attraction of the larval parasitoid Cotesia kariyai. Entomol Exp Appl 163:60–69
Ueno T (1999) Host-feeding and acceptance by a parasitic wasp (Hymenoptera: Ichneumonidae) as influenced by egg load and experience in a patch. Evol Ecol 13:33–44
van Lenteren JC, Ruschioni S, Romani R, van Loon JJA, Qiu YT, Smid HM, Isidoro N, Bin F (2007) Structure and electrophysiological responses of gustatory organs on the ovipositor of the parasitoid Leptopilina heterotoma. Arthropod Struct Dev 36:271–276
Vet LEM, Lewis WJ, Papaj DR, van Lenteren JC (1990) A variable-response model for parasitoid foraging behavior. J Insect Behav 3:471–490
Wäckers FL, Lewis WJ (1994) Olfactory and visual learning and their combined influence on host site location by the parasitoid Microplitis croceipes (Cresson). Biol Control 4:105–112
Zaidi MA, Ye G, Yao H, You TH, Loit E, Dean DH, Riazuddin S, Altosaar I (2009) Transgenic rice plants expressing a modified cry1Ca1 gene are resistant to Spodoptera litura and Chilo suppressalis. Mol Biotechnol 43:232–242
Acknowledgements
We thank Profs. Hiroshi Honda and DeMar Taylor, and Drs. Seiichi Furukawa, Shigeru Matsuyama, and Natsuko Kinoshita for giving us valuable counsel. We are grateful to all members of the Laboratory of Applied Entomology and Zoology, University of Tsukuba for helping with this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest to declare.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Material
Female parasitoid wasps (Cotesia kariyai) consistently oviposit more eggs per attack upon host (Mythimna separata) larvae than upon nonhost (Spodoptera litura) larvae, and attacks in which few eggs (0–2) were deposited occurred nearly 3 times more often in S. litura. The frequency distribution of C. kariyai eggs found by dissecting (a) M. separata and (b) S. litura larvae that had been attacked once. (PPTX 351 kb)
Rights and permissions
About this article
Cite this article
Aikawa, F.K., Kuramitsu, K. & Kainoh, Y. Oviposition Experience by the Larval Parasitoid, Cotesia kariyai, on Nonhost, Spodoptera litura, Can Deter Subsequent Attacks. J Insect Behav 33, 91–96 (2020). https://doi.org/10.1007/s10905-020-09749-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-020-09749-7