Abstract
Workers of polydomous colonies of social insects must recognize not only colony-mates residing in the same nest but also those living in other nests. We investigated the impact of a decentralized colony structure on colony- and nestmate recognition in the polydomous Australian meat ant (Iridomyrmex purpureus). Field experiments showed that ants of colonies with many nests were less aggressive toward alien conspecifics than those of colonies with few nests. In addition, while meat ants were almost never aggressive toward nestmates, they were frequently aggressive when confronted with an individual from a different nest within the same colony. Our chemical analysis of the cuticular hydrocarbons of workers using a novel comprehensive two-dimensional gas chromatography technique that increases the number of quantifiable compounds revealed both colony- and nest-specific patterns. Combined, these data indicate an incomplete transfer of colony odor between the nests of polydomous meat ant colonies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term nestmate recognition is typically used in preference to colony-mate recognition for social insects, perhaps because colonies of most social insects comprise a single nest and colony members are also nestmates (Bourke and Franks 1995; Crozier and Pamilo 1996; Greaves and Hughes 1974). However, the colonies of many species comprise multiple, discrete nesting units and the nests of these polydomous colonies are connected by the interchange of workers, food, and sometimes brood. The level of cooperation among individuals from different nests of a polydomous colony can vary and the different nests within a colony often have some degree of separate identity (Crozier and Pamilo 1996; Rosengren and Pamilo 1983).
To maintain colony integrity, social insects discriminate between colony-mates and alien conspecifics on the basis of a common colony odor. Colony odor can be derived from the environment, genes, or both (Silverman and Liang 2001; Vander Meer and Morel 1998). According to the Gestalt model, workers compare an internal representation of the colony odor, a template with the odor of the other individual, and discriminate on the basis of overlap (Crozier and Dix 1979) (for review, see Breed and Bennett 1987; Vander Meer and Morel 1998). Behavioral assays indicate that the cues responsible for colony-mate recognition are cuticular hydrocarbons (Lahav et al. 1999; Thomas et al. 1999; Wagner et al. 2000) and colonies often exhibit colony-specific cuticular hydrocarbon profiles (Nielsen et al. 1999; Nowbahari et al. 1990).
The variation between workers’ hydrocarbon profiles at the colony level is presumably higher in colonies consisting of several nests because nests within polydomous colonies may differ in their microenvironment. Polydomy may also lead to a decreased or nonrandom transfer of hydrocarbons within a colony if, for example, nestmates interact more with each other than with colony-mates from distant nests. Theoretically, a higher variation of cues between nests within a colony leads to a broader template and makes ants of polydomous colonies more tolerant toward ants of alien colonies.
The polydomous Australian meat ant (Iridomyrmex purpureus) is an ideal model species to test such a prediction because their multiple nests of colonies are connected by well-defined paths enabling clear identification of both the number of nests per colony and colony boundaries. Meat ants use cuticular hydrocarbons for colony-mate recognition (Thomas et al. 1999).
Here, we investigate colony- and nestmate recognition in I. purpureus using field-based behavioral aggression assays and chemical analysis of cuticular hydrocarbons using comprehensive two-dimensional gas chromatography (GC×GC). This novel technique allows the improved separation of compounds that may otherwise coelute under normal gas chromatography (GC) and increases the peak capacity and the signal to noise ratios (Dimandja 2004). Field-based experiments tested whether ants of colonies with many nests are less aggressive toward noncolony members than those of colonies with small numbers of nests. We also assessed whether aggression occurred between ants of different nests within the same colony.
Materials and methods
Recognition experiment
Colonies
Field experiments took place during April and May 2003 at a sheep station near Hillston, NSW, Australia. This population of I. purpureus is mainly monogynous (E. van Wilgenburg, unpublished data). We conducted a series of bioassays to test whether ants of colonies with many nests are less aggressive toward noncolony members than those of colonies with small numbers of nests. We randomly selected 25 alien donor colonies. From each donor colony, we introduced one ant in a polydomous recipient colony and one ant in a colony of a single or a few nests (referred to as an oligodomous colony). Both recipient colonies were situated 250 m from the donor colony. In total, we conducted 50 intercolony assays and 50 intranest controls. In addition, we tested whether ants are sometimes aggressive toward colony-mates from a different nest than their own by conducting a third assay in which the intruder ant was from a different nest of the same colony (25 assays). The third assay was only conducted within the 25 polydomous colonies because the oligodomous colonies often consisted of just a single nest.
Assay
We tested the level of aggression resident ants show toward an intruder by placing a plastic enclosure (10×15 cm) lined with fluon over an arbitrarily chosen ant, located 1 m from a nest. We then introduced an intruder ant, which was marked by a dot of nontoxic paint on the abdomen, in the enclosure. The observer was blind to whether the intruder ant was collected from the same, or another colony. Observations of the behavior of the resident ant were made every 15 s for 3 min following the first contact of the ants. An aggression level was determined by summing the number of times the ant showed antagonistic behavior, either ritualized or actual fighting over the 3 min. Ritualized fighting is a form of “polite” fighting in which none of the contestants are hurt (Ettershank and Ettershank 1982; van Wilgenburg et al. 2005). Physical fighting includes grabbing, biting, or any action that might harm the other ant.
Chemical analysis
Insect collection and hydrocarbon extraction
Meat ants of four colonies were collected for the hydrocarbon extraction in May 2003 from the same population described above. Two of these colonies consisted of a single nest and two were polydomous (with 13 and 19 nests per colony). For the polydomous colonies, we collected ants from two nests of the same colony. Collections were carried out early in the morning while the first ants were emerging from their nest. Cuticular lipids of 22 ants per nest were extracted by immersing each ant in 1 ml of pesticide-grade hexane for 10 min. The samples were blown dry under a stream of nitrogen after which they were reconstituted in 100 μl of hexane.
Hydrocarbon analysis
Samples were analyzed using comprehensive GC×GC, which differs from conventional GC in that a cryogenic modulator traps small portions of the eluate of the first column and is subsequently released to a second column, allowing a sample to be separated on two different stationary phase columns.
To detect quantitative differences in the hydrocarbon patterns of our samples, we injected 1 μl of each sample into a Hewlett-Packard 6890 GC instrument fitted with a longitudinal cryogenic modulator system equipped with a nonpolar BPX5 first dimension column (30 m, 0.25 mm, and 0.25 μm film thickness) and a moderately polar BPX50 second dimension column (1 m, 0.1 mm, and 0.2 μm film thickness) connected to a flame ionization detector. The split/splitless injector was operated in a splitless mode and hydrogen was used as the carrier gas. The modulation period was 5.0 s. The oven program was as follows: 50°C for 0.5 min, then 20°C/min to 200°C followed by 5°C/min to 340°C, after which the temperature was held constant for 5 min. Data output response was recorded by HP Chemstation software and processed to generate quantitative peak data used to present the data as a 2−D contour plot.
Identification of the cuticular compounds from a pool of 15 ants from four colonies was achieved on a GC×GC system as above (Agilent 6890) with a time-of-flight mass spectrometer (Pegasus 3 TOF MS, Leco) (GC×GC-TOFMS) using the same columns as those described above. The split/splitless injector was operated in splitless mode and helium was used as a carrier gas. The oven temperature program commenced at 50°C, which was held for 1 min. The temperature was then increased to 340°C at 3°C/min. We used a different, slower temperature program for the GC×GC-TOFMS run than that used for the GC×GC runs to ensure we achieve the maximum separation of compounds. We confirmed the consistency in elution pattern between the two different methods by comparing runs of the same individual sample on the GC×GC-TOFMS and the GC×GC.
Injection of known n-alkane standards was used to confirm the consistency of elution times (Liu et al. 2001) and calculate temperature-programmed indices (I) by I=100n+100x[(t′Rx−t′RCn)/(t′RCn+1−t′RCn)]. Calculated Is of branched alkanes were compared to published data (Carlson et al. 1998) to confirm assignment of the branching positions.
Statistical analysis
We used Mann–Whitney U tests to examine statistical differences in the level of aggression between the trials, followed by a sequential Bonferroni test to correct for multiple tests using the same variable (Rice 1989). We evaluated the profiles of individual ants with GC image software version 1.4b. Unfortunately, 25 of the 132 runs failed and we eliminated those samples for further analysis. Thirty-five peaks reoccurred in all of the samples and we calculated the relative proportions of each peak height to that of the total sample. The data are compositional and thus the heights were transformed following Reyment (1989): Z=ln[Ap/g(Ap)] in which Ap is the peak height and g(Ap) is the geometric mean of all the peak volumes in the sample. We used SPSS version 12 for our statistical analysis and conducted a principal components analysis (PCA) with the 35 transformed peaks to reduce the number of describing variables for a discriminant function analysis (DA). We extracted eight of the resulting components with eigenvalues greater than 1, which together explained 85% of the total variance. We performed a DA with the scores produced by the PCA to investigate whether colonies and/or nests within colonies could be separated on the basis of their hydrocarbon profiles. To investigate differences between colonies, we only took data from one nest of each polydomous colony, thereby ensuring similar sample sizes. We report Wilks’ lambda significances and the percentage of correct assignment to assess how well the discriminant functions separate the groups. In addition, we give the assignment percentages when the functions are derived from all cases other than that case (cross-validating).
Results
Level of aggression
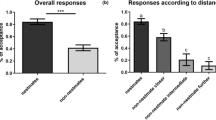
The level of aggression was higher toward noncolony-mates than toward nestmates in both poly- and oligodomous colonies (Mann–Whitney test: U=191, df=1, p=0.003 and U=87, df=1, p<0.001, respectively) (see Fig. 1). Ants of polydomous colonies were more aggressive toward nonnest- colony-mates than toward nestmates (Mann–Whitney test: U=216, df=1, p=0.015). Ants from polydomous colonies (12–27 nests per colony, mean 15.60, SD=4.03) were less aggressive toward ants from alien colonies than ants from oligodomous colonies (1–4 nests per colony, mean 2.56, SD=0.96) (Mann–Whitney test: U=211, df=1, p=0.041). All P values remain significant after a sequential Bonferroni adjustment. Actual fighting was scored in five of the 19 occasions in which ants of oligodomous colonies displayed aggression toward ants from alien colonies and in two out of the 12 occasions in which ants of polydomous colonies acted aggressively toward noncolony-mates.
Chemical analysis: colony and nest membership
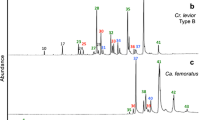
We identified, using GC×GC-TOFMS, 31 of the 35 cuticular hydrocarbons that were present in all samples (see Fig. 2). The four colonies could be significantly discriminated on the basis of the 35 hydrocarbons (Fig. 3a; Function 1 explains 57.1% of the variation, canonical correlation=0.843, Wilks’ λ=0.083, χ 2=161.91, df=24, and p<0.001; Function 2 explains 32.2% of the variation, canonical correlation=0.763, Wilks’ λ=0.287, χ 2=81.169, df=14, and p<0.001; and Function 3 explains 10.6% of the variation, canonical correlation=0.561, Wilks’ λ=0.686, χ 2=24.534, df=6, and p<0.001). The model correctly classifies 83.3% of the workers and 79.2% of the workers are classified correctly in cross validation. The four nests within two colonies could be significantly discriminated on the basis of the hydrocarbons (see Fig. 3b; Function 1 explains 83.9% of the variation, canonical correlation=0.868, Wilks’ λ=0.150, χ 2=123.399, df=24, and p<0.001; Function 2 explains 12.3% of the variation, canonical correlation=0.557, Wilks’ λ=0.607, χ 2=32.466, df=14, and p=0.003; and Function 3 explains 3.8% of the variation, canonical correlation=0.348, Wilks’ λ=0.879, χ 2=8.371, df=6, and p=0.212). The model correctly classifies 73.6% of the individuals and 65.3% of the individuals are classified correctly in cross validation.
GC×GC-FID chromatogram of the hydrocarbons obtained from a single ant of I. purpureus. 1 C21; 2 9- + 10- + 11-MeC21 + 10,14-diMeC21; 3 C22; 4 C23; 5 9,13- + 10,14-diMeC23; 6 C24; 7 C25; 8 11- + 13-MeC25; 9 11,15- + 10,14-diMeC25 10 C26; 11 C27; 12 11- + 13-meC27; 13 7,11- + 7,15-diMeC27; 14 C28; 15 12- + 14- + 16- + 18-MeC28; 16 ?; 17 C29; 18 11- + 13- + 15-MeC29; 19 11,15- + 13,17-diMeC29; 20 7,15-diMeC29; 21 C30; 22 11- + 12- + 13- + 14- + 15-MeC30; 23 11,15- + 13,17-diMe30; 24 C31 25 11- + 13- + 15-MeC31; 26 11,15- + 13,17-diMeC31; 27 C32 28 12- + 13- + 14- + 15- +16-MeC32; 29 11- + 13- + 15- + 17-MeC33; 30 11,15- + 13,17-diMeC33; 31 11- + 13- + 15- + 17-MeC35; 32 ?; 33 11- + 13MeC37; 34?; and 35?. Because all alkanes are nonpolar, members of the series will elute at similar retention times in the second dimension and thus appear as a single band clustered along a slight diagonal
Discriminant analysis based on proportion of cuticular hydrocarbons extracted from ants from a four different colonies (colony 1 n=21, colony 2 n=16, colony 3 n=16, and colony 4 n=19) and b four different nests of two different colonies (1 and 2) (colony 1: nest a n=21, nest b n=18 and colony 2: nest a n=16, nest b: n=17). The percentages of variance explained by each of the functions are given in parentheses
Discussion
Our behavioral data shows that workers of colonies with many nests are less aggressive toward alien conspecifics than those of colonies with few nests. In addition, workers are significantly more aggressive toward colony-mates of other nests within their colony than they are toward nestmates. Our chemical data is in accord with the behavioral data; colonies of I. purpureus have both colony-specific hydrocarbon profiles and nest-specific hydrocarbon profiles within polydomous colonies.
To our knowledge, different within colony, nest-specific hydrocarbon profiles were not previously reported. There are several reasons why we may be the first to do so. First, the few polydomous species in which the hydrocarbon profiles of individual nests were investigated are characterized by small monogynous colonies, comprising hundreds of workers that inhabit only a few nests (Dahbi and Lenoir 1998; Debout et al. 2003). Hydrocarbon sources in such species are thus likely to be more homogenous than in I. purpureus. Second, enhanced separation afforded by GC×GC analyses (compared to traditional GC analyses) may be responsible for the improved characterization of hydrocarbon profiles.
In colony-mate recognition systems, cues are matched against a recognition template (Crozier and Pamilo 1996). Our observation of nest-specific hydrocarbon patterns indicates that cue transfer within colonies is incomplete and that individuals within a colony differ in their cue profile. A greater diversity of cues within colonies should lead to a broader template and make individuals more tolerant toward alien ants. This hypothesis was supported by observations of reduced aggression in polygynous species (Keller and Passera 1989; Vander Meer and Morel 1998) and mixed species groups (Errard et al. 2006). The negative relationship we found between nest number and aggression suggests that polydomy is another factor influencing template breadth. With incomplete cue transfer within a colony, workers should have a broader template than when cue transfer is complete because they have to accept workers with a variety of cue profiles. The efficacy of any such adjustment is apparently challenged by the relatively high level of aggression directed by meat ants toward colony-mates from other nests. It is likely, however, that colony-mates of nests that are far apart are never directly in contact and that their template need not allow for this. For our experiments, ants were sampled from nests that were at opposite extremes of the colony; ants from nests closer together may be more amicable.
References
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton Univ. Press, Princeton
Breed MD, Bennett B (1987) Kin recognition in highly eusocial insects. In: Fletcher DJC, Michener CD (ed) Kin recognition in animals. Wiley, Chichester, pp 243–285
Carlson DA, Bernier UR, Sutton BD (1998) Elution patterns from capillary GC for methyl-branched alkanes. J Chem Ecol 24:1845–1865
Crozier RH, Dix MW (1979) Analysis of two genetic models for the innate components of colony odor in social. Hymenoptera. Behav Ecol Sociobiol 4:217–224
Crozier RH, Pamilo P (1996) Evolution of social insect colonies. Oxford Univ. Press, Oxford
Dahbi A, Lenoir A (1998) Nest separation and the dynamics of the Gestalt odor in the polydomous ant Cataglyphis iberica (Hymenoptera, Formicidae). Behav Ecol Sociobiol 42:349–355
Debout G, Provost E, Renucci M, Tirard A, Schatz B, McKey D (2003) Colony structure in a plant-ant: behavioural, chemical and genetic study of polydomy in Cataulacus mckeyi (Myrmicinae). Oecologia 137:195–204
Dimandja J (2004) Comprehensive 2-D GC provides high-performance separations in terms of selectivity, sensitivity, speed and structure. Anal Chem 76:167A–174A
Errard C, Hefetz A, Jaisson P (2006) Social discrimination tuning in ants: template formation and chemical similarity. Behav Ecol Sociobiol 59:353–363
Ettershank G, Ettershank JA (1982) Ritualised fighting in the meat ant Iridomyrmex purpureus (Smith) (Hymenoptera: formicidae). J Aust Entomol Soc 21:97–102
Greaves T, Hughes RD (1974) The population biology of the meat ant. J Aust Entomol Soc 13:329–351
Keller L, Passera L (1989) Influence of the number of queens on nestmate recognition and attractiveness of queens to workers in the Argentine ant, Iridomyrmex humilis (Mayr). Anim Behav 37:733–740
Lahav S, Soroker V, Hefetz A, Vander Meer RK (1999) Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86:246–249
Liu ZB, Bagneres AG, Yamane S, Wang QC, Kojima J (2001) Intra-colony, inter colony and seasonal variations of cuticular hydrocarbon profiles in Formica japonica (Hymenoptera, Formicidae). Insectes Soc 48:342–346
Nielsen J, Boomsma JJ, Oldham NJ, Petersen HC, Morgan ED (1999) Colony-level and season-specific variation in cuticular hydrocarbon profiles of individual workers in the ant Formica truncorum. Insect Soc 46:58–65
Nowbahari E, Lenoir A, Clément JL, Lange C, Bagneres AG, Joulie C (1990) Individual, geographical and experimental variation of cuticular hydrocarbons of the ant Cataglyphis cursor (Hymenoptera: formicidae): their use in nest and subspecies recognition. Biochem Syst Ecol 18:63–74
Reyment RA (1989) Compositional data analysis. Terra Rev 1:29–34
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rosengren R, Pamilo P (1983) The evolution of polygyny and polydomy in mound-building Formica ants. Acta Entomol Fenn 42:65–77
Silverman J, Liang D (2001) Colony disassociation following diet partitioning in a unicolonial ant. Naturwissenschaften 88:73–77
Thomas ML, Parry LJ, Allan RA, Elgar MA (1999) Geographic affinity, cuticular hydrocarbons and colony recognition in the Australian meat ant Iridomyrmex purpureus. Naturwissenschaften 86:87–92
van Wilgenburg E, van Lieshout L, Elgar MA (2005) Conflict resolution strategies in meat ants Iridomyrmex purpureus: ritualised displays versus lethal fighting. Behaviour 142:701–716
Vander Meer RK, Morel L (1998) Nestmate recognition in ants. In: Vander Meer RK et al (eds) Pheromone communication in social insects. Westview, Boulder, CO, pp 79–103
Wagner D, Tissot M, Cuevas W, Gordon DM (2000) Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J Chem Ecol 26:2245–2257
Acknowledgements
This research was supported by an International Postgraduate Research Scholarship awarded by the Australian government, a Melbourne International Research Scholarship awarded by the University of Melbourne, the Holsworth Wildlife Research Fund, and the Joyce W. Vickery Scientific Research Fund of the Linnean Society of New South Wales. We would like to thank Ian and Linda Mclean for their generosity in permitting fieldwork on their property.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Wilgenburg, E., Ryan, D., Morrison, P. et al. Nest- and colony-mate recognition in polydomous colonies of meat ants (Iridomyrmex purpureus). Naturwissenschaften 93, 309–314 (2006). https://doi.org/10.1007/s00114-006-0109-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-006-0109-y