Abstract
When the frenzied and irregular food-recruitment dances of bumblebees were first discovered, it was thought that they might represent an evolutionary prototype to the honeybee waggle dance. It later emerged that the primary function of the bumblebee dance was the distribution of an alerting pheromone. Here, we identify the chemical compounds of the bumblebee recruitment pheromone and their behaviour effects. The presence of two monoterpenes and one sesquiterpene (eucalyptol, ocimene and farnesol) in the nest airspace and in the tergal glands increases strongly during foraging. Of these, eucalyptol has the strongest recruitment effect when a bee nest is experimentally exposed to it. Since honeybees use terpenes for marking food sources rather than recruiting foragers inside the nest, this suggests independent evolutionary roots of food recruitment in these two groups of bees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bumblebees do not communicate spatial co-ordinates of food sources as honey bees do with the waggle dance, but successful bumblebee foragers do inform nestmates about the general availability and the scent of rewarding food sources (Dornhaus and Chittka 1999, 2001). This helps recruits to avoid searching for food when foraging conditions are unfavourable, as well as aiding in the discovery of rewarding flowers, which bees can recognize by the scent they have learned while in the nest. Successful bumblebee foragers, when returning to the colony, often show a curious behaviour consisting of excited runs with bouts of wing-fanning. The reaction shown by previously unemployed bees in the nest is to become active, i.e. to show increased movement speeds and leave the nest in search for food (Dornhaus and Chittka 2001). Behavioural tests have clearly shown that this communication is mediated by a pheromone (Dornhaus et al. 2003), but the chemical nature of this pheromone had not yet been identified.
Materials and methods
All experiments were performed with lab-reared colonies of Bombus terrestris with 30–50 workers. A total of 10 colonies were used. Each nest was contained in a wooden box (26 cm×14 cm×10 cm), which was connected to a foraging arena (40 cm×60 cm×30 cm) with a Plexiglas tube of 30 cm length. Nest box and foraging arena had transparent Plexiglas covers, so that the behaviour of the bees could be observed. The arena contained a feeder at certain times of the experiment.
Chemicals and reagents
All reference standards and internal standards (IS) were of analytical grade and were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Tokyo Kasei (Nihonbashi, Tokyo, Japan), the internal standard being deuterated p-xylene (p-xylene-d10). A reference standard solution was prepared for each compound using acetone as solvent at 200 μg ml−1 concentration. A multicompound working standard solution (20 μg ml−1 concentration) was prepared from the above by appropriate dilution with acetone and stored under refrigeration (4°C). Organic solvents of chromatographic grade were obtained from Panreac (Barcelona, Spain). The syringe injector of the SPME unit (Supelco, USA), equipped with 65 μm polydimethylsiloxane-divinylbenzene (PDMS-DVB) fibres (Supelco, USA), was used for the extraction procedure. Fibres were conditioned prior to use according to supplier's prescriptions.
Apparatus
GC-MS analysis was performed with a Varian 3800 gas chromatograph with Electronic Flow Control (EFC) and fitted with a Saturn 2000 ion-trap mass spectrometer (Varian Instruments, Sunnyvale, CA, USA). Samples were injected with a Varian 8200 auto sampler with a syringe injector of the SPME unit (Supelco, USA) into an SPI/1079 split/splitless programmed-temperature injector. A Rapid-MS (WCOT fused silica CP-Sil 8 CB low bleed (5% phenyl, 95% dimethylpolysiloxane) of 10 m × 0.53 mm i.d. × 0.25 μm film thickness) analytical column from Varian Instruments (Sunnyvale, CA, USA) was used for high speed analysis. The mass spectrometer was operated in Electron Impact (EI). The controlling computer system had an EI-MS/MS library specially created for the target analytes under our experimental conditions. Other EI-MS libraries were also available. The mass spectrometer was calibrated weekly with perfluorotributylamine. Helium (99.999%) at a flow rate of 1 ml min−1 was used as carrier and collision gas.
Laboratory trial design for pheromone analysis
The insects were not fed during 2 days before the experiments. During tests, bees were fed by placing a dish filled with a feeder with a 73% (volume) sugar solution (sucrose 30%, dextrose 31% and fructose 39% of dry mass) into the arena for 120 min. We sampled the airspace above the colony while foragers were returning from the food source and performing their irregular runs on the comb. This was repeated 10 times with at least 3 h intervals between trials. As controls, the colony airspace was also sampled before each feeding had begun.
Headspace-Solid Phase Microextraction (HS-SPME) conditions were established for validating the analytical method as follows. The performance of the method was assessed calculating linear ranges, recovery rates, precision and lower limits (Mena Granero et al. 2004). For calibration purposes, three empty boxes with the same dimensions as the nest box previously described were spiked with appropriate volumes of the 20 mg l−1 standard mixture containing all the analytes. Linear ranges were assessed in analyte amounts between 100 and 5000 ng; recovery rates and precision were determined at two amount rates, 100 and 500 ng of each analyte. The spiked boxes were sampled during 1, 2 and 3 hours, in order to check whether the equilibrium in the atmosphere of the boxes was reached; precision was obtained checking the standard deviation of 10 replicates of recovery experiments. Finally, lower limits were obtained by sampling boxes spiked with decreasing volumes of the standard solution mixture. This was performed with three replicates, until the recovery rates yielded unacceptably high standard deviations.
Once the compounds were concentrated on the fibre, they were desorbed directly into the injection port of the Varian 3800 gas chromatograph using an autosampler at 250°C. The initial column temperature was set at 35°C during injection, 9 min hold, then increased at 1.5°C min−1 to 55°C, at 3°C min−1 to 65°C, 2 min held, and finally raised to 300°C at 100°C min−1 that was held for 5 min. Earlier tests had identified clusters of gland cells attached to the tergites of bumblebee workers to be the source of the alerting pheromone (Dornhaus et al. 2003). We dissected five foragers that had just returned to the nest from the feeder, and compared the compounds found in extracts of their tergites (containing such glands) with those from five foragers that had not been offered food. Samples of tergites were put in 10 ml headspace vials for analysis, capped with a screw cap and a PTFE/silicone septa, being sampled during 15 min with one fibre inserted through the septum. The identification of pheromones was performed by MS/MS detection mode.
a Effects of pheromone compounds on number of foragers leaving the nest in search for food. Foragers leaving the nest were counted over 5 min intervals in the 30 min preceding the introduction of the compound, and 30 min after its introduction. Black bars: before foraging; white bars: during foraging. b Cumulative number of identified foragers that landed on a feeder in the flight arena before (0–30 min) and after (30–60 min) the insertion of eucalyptol into the nest box. Total number of foragers in the colony was 17
Quantification of behavioural effects
Since three compounds were found to be most likely candidates for being components of the food alerting pheromone, we wished to test the behavioural effects of these compounds (eucalyptol, E,E-farnesol, and Z-ocimene) on forager recruitment. To this end, 10 μl of solution of the individual compounds at 200 mg l−1 acetone were dispensed onto a filter paper placed in a glass tube in the lid of the nest box. This was repeated every 5 min for half an hour, and number of foragers entering the flight arena in search for food were counted in 5 min intervals during the experimental half hour and in the preceding 30 min (control period). This experiment was repeated seven times with each individual compound. In addition, seven control runs were performed with acetone, to exclude the possibility that the solvent itself provoked a behavioural response. Tests were done in random sequence with three bumblebee colonies, with at least 4 h between tests on a single colony. None of the compounds were tested twice on the same day with the same colony.
Finally we were interested to see what percentage of the total forager force of a bumblebee colony could be mobilised by the chemical which turned out to be the most potent, eucalyptol. To this end, we marked all foragers that arrived at an ad libitum sucrose feeder in a flight arena connected to a large nest (with >80 workers), over a period of 2 days. Bees were given individual number plates (Opalith). Then we starved the colony for 1 day, and on the next day, inserted a sucrose feeder into the arena for 1 h. After 30 min, the colony was exposed to eucalyptol as described earlier. Bees were caught immediately upon arrival, so that they could not provide feedback to the colony.
Results
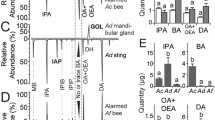
Within the nest box, we found that three substances were not quantifiable while bees did not forage, but increased highly significantly during foraging (Table 1, Fig. 1); these were eucalyptol, ocimene, and farnesol (Wilcoxon test, n=10 pairs, T=0, Z=2.803, p<0.005 in all three cases). Some other volatiles, which were already present before foraging, changed significantly during foraging: these were linalool (n=10 pairs, T=8, Z=1.987, p=0.046), α- and β-pinene (n=10 pairs, T=4, Z=2.395, p=0.016), which were quantified as sum of two isomers, and ethylbenzene, p-xylene and m-xylene (n=10 pairs, T=3, Z=2.497, p=0.012), which were quantified together because they overlapped in one chromatographic peak (Table 1). In the tergal segments, however, we found statistically significant differences only in three compounds, ocimene (one way ANOVA, F=25.54, df=1, p<0.001), eucalyptol (F=107.3, df=1, p<0.001) and farnesol (F=20.62, df=1, p=0.002), which could only be quantified during foraging. Previous studies have identified the tergal glands as the source of the alerting pheromone (Dornhaus et al. 2003). Hence we conjectured that these three volatiles might be especially important in foraging recruitment, and tested their effects on food recruitment directly.
Eucalyptol provoked the strongest response (Wilcoxon test, N=7 pairs, T=0, Z=2.366, df=12, p=0.017; Fig. 2a), showing that this terpene is the most potent component of the bumblebee food alerting pheromone. The test with individually marked foragers revealed that the entire forager force of the colony was activated by eucalyptol. Our pre-test observations had revealed that the colony contained a total of 17 active foragers. In the 30 min prior to the insertion of eucalyptol, four foragers arrived at the feeder. After exposure of the colony to eucalyptol, the remaining 13 foragers arrived within 15 min (Fig. 2b). The effect of ocimene is also significant (Wilcoxon test, N=7 pairs, T=2, Z=2.03, df=12, p=0.042), whereas farnesol produced only a non-significant rise in activity (N=7 pairs, T=5, Z=1.52, df=12, p=0.128), and the solvent acetone yielded none (N=7 pairs, T=10, Z=0.105, df=12, p=0.91; Fig. 2a).
Discussion
Our experiments identify eucalyptol as the main active ingredient of the bumblebee foraging alert pheromone. It is likely that an optimum effect can only be created using a realistic mixture of other components of the tergal gland-produced pheromones, which also include ocimene and farnesol. But our experiment with individually marked bees showed that the entire forager force of the colony can be activated with eucalyptol alone. Thus, it is not possible that a mixture of the identified compounds will produce recruitment of more foragers—but it is conceivable that they might be recruited more speedily. Previous tests showed that the recruitment effect produced by successful bumblebee foragers takes more than 30 min to build up (Dornhaus and Chittka 1999), whereas under the influence of eucalyptol, all foragers had left the colony within 15 min (Fig. 2b). Hence, eucalyptol alone produces at least as strong a response as actual bumblebee recruitment. The function of the other compounds remains unknown at this stage.
One of the compounds found here, farnesol, is known to be present in the honeybee Nasonov gland, which honeybees use to mark food sources (Free et al. 1981). Other compounds from the Nasonov gland (i.e. geraniol, citral, and nerol (Free et al. 1981)) were also found in small quantities on the bumblebee tergites, suggesting homology of the two glands. Terpenes and terpene derivatives from the tergal (or Nasovov) gland are used for marking food sources in honeybees, but to alert nestmates inside the colony in bumblebees. This is evidence against the hypothesis that bumblebees display a behavioural fossil of the honeybee dances. The bumblebee zigzag runs primarily serve the distribution of the alerting pheromone in the nest. If the elaborate dances of honeybees had originally had a similar function, they would be expected to emit pheromones during dances, and to employ similar glands. While it cannot be ruled out that honeybees indeed use pheromones to attract recruits to the dance site (Tautz and Rohrseitz 1998), the Nasonov gland, however, is not typically exposed during dances. Hence we conjecture that even at their evolutionary origins, the motor patterns displayed by successful honeybee and bumblebee foragers had distinct functions.
References
Dornhaus A, Brockman A, Chittka L (2003) Bumblebees alert to food with pheromone from tergal gland. J Comp Physiol A 189:47–51
Dornhaus A, Chittka L (1999) Evolutionary origins of bee dances. Nature 401:38
Dornhaus A, Chittka L (2001) Food alert in bumblebees (Bombus terrestris): possible mechanisms and evolutionary implications. Behav Ecol Sociobiol 50:570–576
Free JB, Ferguson AW, Pickett JA (1981) Evaluation of the various components of the Nasonov pheromone used by clustering honeybees. Physiol Entomol 6:263–268
Mena Granero A, Egea González FJ, Garrido Frenich A, Guerra Sanz JM, Martínez Vidal JL (2004) Single step determination of fragrances in Cucurbita flowers by coupling headspace solid-phase microextraction low-pressure gas chromatography–tandem mass spectrometry. J Chromatogr A 1045:173–179
Tautz J, Rohrseitz K (1998) What attracts honeybees to a waggle dancer? J Comp Physiol A 183:661–667
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Granero, A.M., Sanz, J.M.G., Gonzalez, F.J.E. et al. Chemical compounds of the foraging recruitment pheromone in bumblebees. Naturwissenschaften 92, 371–374 (2005). https://doi.org/10.1007/s00114-005-0002-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-005-0002-0