Abstract
Hepatic lipid metabolism is closely associated with certain diseases, such as obesity, diabetes, fatty liver, and hepatic fibrosis. Hepatic steatosis results from systemic metabolic dysfunction that occurs via multiple processes. The initial process has been characterized as hepatic lipid accumulation that may be caused by increased liver lipid uptake and de novo lipogenesis or decreased lipid oxidation and lipid export; subsequently, multiple additional factors that trigger inflammation and insulin resistance (IR) aggravate the progression of hepatic steatosis. Emerging evidence indicates that inflammation stands at the crossroads of innate immunity and lipid metabolism and links the initial metabolic stress and subsequent metabolic events in lipid metabolism. Therefore, in this review, we summarize the regulatory role of innate immune signaling molecules in maintaining lipid metabolic homeostasis; these revelations can guide the development of potential therapies for nonalcoholic fatty liver disease (NAFLD).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ectopic lipid metabolism leads to multiple liver diseases, ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC). There is a worldwide pandemic of nonalcoholic fatty liver disease (NAFLD), which occurs in parallel with epidemics of obesity, type 2 diabetes (T2D), and metabolic syndrome (MetS) [1]. Patients with NAFLD have high risks of premature cardiovascular- and/or liver-related death [2]. At present, around the world, 23–29% of individuals in the general population are affected by NAFLD. This proportion is even higher among individuals with T2D (60–70%) and individuals who are morbidly obese (75–92%) than in the general population [3]. Currently, lifestyle modification via improving nutrition and exercise is the main treatment for NAFLD [4, 5]. Unfortunately, there have been still no FDA-approved effective therapies for NAFLD so far. Therefore, advanced insights into the pathophysiology of NAFLD have provided potential new therapeutic options.
Accumulating evidence suggests that the liver is not only a metabolic organ but also an innate immune organ. Many types of innate (Kuffer cells, dendritic cells, natural killer cells, and innate lymphoid cells) and adaptive (T cells and B cells) immune cells are enriched within the liver and function for liver physiology and pathology [6]. In addition to nonspecific host defense against invading pathogens, innate immune cell-derived innate immune activation also plays prominent role in driving hepatic metabolic disturbance in NAFLD. In metabolic disorders, the continuing production of metabolites constitutively activate innate immune signaling, leading to excessive inflammatory cytokine release, thereby further promoting hepatic steatosis and even developed to fibrosis [7]. Considering such important driving force of innate immune signaling in NAFLD progression, in-depth recognition of its role in NALFD development is urgently required.
Hepatic lipid metabolism
There has been an extensive viewpoint that NAFLD may result from the accumulation of toxic lipid species (e.g., diacylglycerols, ceramides, and lysophosphatidyl choline species), which are produced from the surplus metabolic energy substrates (e.g., carbohydrates and fatty acids) due to the inadequately metabolic capacity of liver [8]. Hepatic lipid metabolism is closely interrelated with hepatic glucose metabolism, both of which have strong effects on the pathogenesis of liver diseases [9]. Two main processes determine the flux of lipid in the liver, the production of lipid species (cholesterol and fatty acids), and breakdown of them via fatty acid oxidation. Fatty acids from lipolysis of triglyceride in adipose tissue flow to the liver and subsequently be oxidized to production of acetyl-coenzyme A (CoA), which serves as the substrates of gluconeogenesis. In contrast, fatty acids also can be synthesized from glucose by de novo lipogenesis. Glucose from gluconeogenesis and an individual’s diet is metabolized via glycolysis into pyruvic acid, which is then reduced to CoA and used as material for triglyceride synthesis [10] (Fig. 1). The metabolic fate of fatty acids is determined either by mitochondrial β-oxidation or esterification to form triglyceride, which is generally considered as a protective response to a supply of fatty acids exceeding the liver’s metabolic capacity [11]. However, if the transport rates of triglyceride as very low-density lipoprotein (VLDL) are lower than the synthesis rates of triglyceride, an excess of triglyceride accumulates to form lipid droplets in hepatocytes and causes metabolic abnormalities (e.g., endoplasmic reticulum (ER) stress and mitochondrion dysfunction), which are usually coupled with inflammatory cytokine release, microphage infiltration, fibrogenesis, and insulin resistance (IR) [12] (Fig. 2). Fatty acid metabolism is highly influenced by blood glucose. During hypoglycemia, fatty acids from lipolysis are metabolized through β-oxidation and TCA cycle to produce glucose maintaining the blood glucose homeostasis, while hyperglycemia causes insulin resistance. Insulin resistance in adipose tissue dysregulates lipolysis, leading to aberrant release of fatty acids, that further aggravates impaired insulin signaling throughout the body.
Sources and fates of fatty acids in liver. Acetyl-CoA is the central intermediate product in the conversion between hepatic glucose and lipid. Blood glucose is derived from diet, hepatic glycogenolysis, and gluconeogenesis processes. During fasting, the liver is in hepatic glucose output state required to maintain blood glucose homeostasis. FFAs from adipose tissue lipolysis are converted into glucose through β-oxidation and the TCA cycle processes in mitochondria. During feeding, the liver is in hepatic glucose uptake state. Glucose from an individual’s diet is converted into lipid via glycolysis and pyruvate cycling in mitochondria. FFAs from lipolysis or hepatic glucose conversion are esterified into triglycerides (TG), which can be either stored in LDs or packaged into very low density lipoproteins (VLDL) to shuttle lipid to other tissues including adipose tissue. Excessive FFAs in the liver cause ER stress and production of ROS. The excess acetyl-CoA from the hepatic glucose and lipid metabolism can be used to produce ketone bodies for an additional energy shuttle between the liver and other organs and cholesterol for cell membrane and plasma lipoprotein construction
Multiple pathophysiological processes are involved in the progression of NAFLD and NASH. FFA delivery to liver from the adipose or synthesis in liver leads to hepatic lipotoxicity, which triggers inflammatory cytokine release, microphage activation and infiltration, collagen deposition, ER stress, mitochondrion dysfunction, and hepatic stellate cell (HSC) activation, jointly causing hepatic inflammatory injury, insulin resistance, and fibrosis
Innate immune-derived inflammation mediates subsequent metabolic events in lipid metabolism
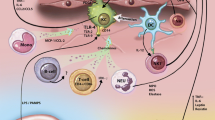
The innate immune system, as the first line of defense against pathogen invasion, recruits immune cells, including macrophages, neutrophils, dendritic cells (DCs), and natural killer (NK) cells, to invaded sites for pathogen recognition and the initiation of a rapid but generic response [13,14,15]. Extracellular pathogens or endogenous injury signaling are first recognized by pattern recognition receptors (PRRs) on cell membranes or endosome membranes; adaptor kinase complexes are then recruited to PRRs to transduct signals to downstream MAPK signal cascades, eventually resulting in the activation of ERK/JNK/p38MAPK/NF-κB signals and leading to the transcription of proinflammatory cytokine genes and type I/II IFNs [16, 17] (Fig. 3). The pathogens induced acute immune inflammation activates the JAK-STAT pathway or other immune receptor-triggered pathways for regulating the activation, differentiation, and/or function of immune cells to defend against antigen invasion. However, the metabolite (e.g., glucose and FAs) driven chronic metabolic inflammation regulates IR-mediated lipid metabolism directly via suppression of the insulin signaling pathway (Fig. 3).
Innate immune-derived immune and metabolic inflammation, respectively, impinges on immune defense and IR. TLR signaling pathways include the MyD88-dependent pathway and the TRIF-dependent pathway. MyD88-dependent pathway: TLR1, TLR2, TLR4, TLR5, TLR6, TLR7, TLR8, and TLR9 initiate signaling by binding to MyD88 and IRAKs and recruiting TRAF6, TAK1, and TAB2, thereby activating p38MAPK, ERK, and JNK; this activation results in AP1 and NF-κB nuclear translocation and the initiation of gene transcription associated with innate immune responses and inflammation. TRIF-dependent pathway: TLR4 and TLR3 recruit TRIF to activate TBK1/IKKε via TRAF3 and TANK, which results in NF-κB nuclear translocation and the activation of IRF-induced inflammatory cytokines. RLR signaling pathway: pathogen association molecular pattern (PAMP) is recognized by RLRs (MDA5 and RIG-I), which are constitutively expressed in the cytoplasm and initiate MAVS-dependent signaling, leading to activation of IRFs and NF-κB and the subsequent production of type I/II IFNs and inflammatory cytokines. NLR signaling pathway: damage-associated molecular pattern (DAMP) triggers NOD1/2 to activate NLRP3/ASC/caspase-1 inflammasome pathway to produce IL-1β and IL-18. P2X7 receptor and pannexin-1 channel: activation of the pannexin channel through binding of ATP to P2X7 receptor leads to activation of NLRP3/ASC/caspase-1 inflammasome pathway and release of IL-1β and IL-18. JAK-STAT pathway: IFNs and cytokines bind to the corresponding receptors and activate JAK kinase, which selectively phosphorylates STATs. Phosphorylated STATs then translocate to the nucleus to regulate the transcription of target genes. Insulin signaling pathway: insulin stimulates insulin receptor to recruit and phosphorylate IRS-1, which subsequently phosphorylates and activates PI3K and AKT, resulting in inhibition of FOXO1 nuclear translocation and activation of PKA and SGK-3β, finally promoting lipolysis and glycogen synthesis, and inhibiting lipogenesis and gluconeogenesis. The release of inflammatory cytokines and activation of p38MAPK, ERK, JNK, and NF-κB pathways in innate immune networks interfere with insulin signaling pathway. Conversely, the inflammatory cytokines bind to specific receptors to activate JAK-STAT pathway. The metabolites from glucose and lipid metabolism and the PAMP from innate immune defense return to trigger innate immune regulatory networks
The metabolites in lipid metabolism serve as a physiological burden to the metabolic system. The physiological response to the metabolites includes ER stress, dysfunctional unfolded protein response (UPR), apoptosis, inflammation, and enhanced injury [18,19,20]. Recent studies in mice have revealed that hepatocyte inflammatory response may be a key link between the initial metabolic stress (e.g., ER stress and UPR response) and subsequent metabolic events (e.g., fibrogenesis and IR) in NASH [21]. The inflammatory signaling derived from stressed or injured hepatocytes and activated macrophages (Kupffer cells in the liver) promotes activation of resident hepatic stellate cells into myofibroblasts to produce excessive extracellular matrix proteins, accumulation of that pushes the occurrence of fibrogenesis [22]. In addition, inflammatory cytokines and phosphorylation of mitogen activated protein kinase (MAPK) in the process of inflammatory activation significantly impair post-receptor insulin signaling [23]. Thus, inflammatory response along with metabolic events drive the pathogenesis of NASH.
Multiple TLR pathway-mediated and inflammasome-mediated inflammatory responses participate in regulation of lipid metabolism. A number of serine kinases, cytokines, and molecular pathways involved in innate immune networks can induce the phosphorylation of serine residues on IRS-1 and then weaken insulin signaling transduction, thereby play an important role in the development of IR in lipid metabolism. The JNK/ERK/p38MAPK pathways phosphorylate FOXO1 to enhance the development of IR and alter lipid metabolism [24, 25]. TNF-α induces the activity of the PKC, SOCS1/3, ERK, JNK, and IKKβ/NF-κB pathways to increase serine phosphorylation of IRS-1. IL-6 activates the JAK-STAT signaling pathway and increases SOCS3 production to reduce the expression of glucose transporter 4 (GLUT4) and IRS-1. MCP-1 regulates IR via the phosphorylation of ERK1/2 and p38MAPK to degrade IRS-1 [26] (Fig. 4). In addition, TNFα and other inflammatory cytokines lead to the induction of ER stress, afterwards downregulating the BCAA/TCA pathway to attenuate lipogenesis in visceral adipose depots.
MAPK signaling molecules push forward IR and fibrogenesis. JNK and p38MAPK activation suppresses PPARα and FGF21, leading to fatty acid oxidation abrogation and fibrosis promotion. The JNK/ERK/p38MAPK pathways phosphorylate IRS-1 or FOXO1 to enhance the development of IR. Activation of JAK-STAT signaling pathway and increase of SOCS3 production reduce the expression of glucose transporter 4 (GLUT4) and IRS-1 to drive IR progression
A variety of innate immune molecules are involved in hepatic lipid metabolism
PRRs participate in regulation of lipid metabolism
Intracellular or surface-expressed PRRs have been revealed to be relevant participants in NAFLD/NASH because of their central roles in recognizing cell damage and pathogen invasion [27]. Mammals have several distinct classes of PRRs, including TLRs, NOD-like receptors (NLRs), RIG-1-like receptors (RLRs), AIM2-like receptors (ALRs), C-type lectin receptors (CLRs), and other nonspecific sensors [28]. The activation of PRR systems, particularly TLR signaling pathways, is a critical response in liver disease.
Blockade of TLR2 signaling prevents high-fat diet (HFD)-induced IR in mice [29]. Hepatocyte TLR4-deficient mice exhibit improved glucose tolerance, decreased inflammation, enhanced insulin sensitivity, and ameliorated hepatic steatosis in the context of a HFD challenge [30]. Another study has revealed that TLR4 deficiency enhances the expression of fatty acid oxidases, including ACOX, CPT-1, MTPa, MTBb, PBE, and 3-ketoacyl-CoA thiolase, and presents lower triglyceride accumulation, which indicates that TLR4 plays an important role in the onset of steatosis [31]. TLR5-deficient mice are susceptible to spontaneous gut inflammation and exhibit elevated neutral lipid level [32]. Dietary short-chain fatty acids (SCFAs) increase liver de novo lipogenesis, further aggravating metabolic syndrome in TLR5-deficient mice [33]. Although increased expression and activity of peripheral and hepatocytic TLR6 in morbidly obese NAFLD/NASH patients has been investigated, the capability of TLR6 regulating NAFLD progression has been unclear [34]. TLR9-deficient mice on a CDAA diet show less steatosis, inflammation, and liver fibrosis than wild-type (wt) mice on the same diet; in addition, the CDAA diet-induced IR and weight gain are suppressed by TLR9-deficiecy [35]. Mitochondrial DNA that serves as a TLR9 ligand drives NASH in mice and patients, whereas blockade of TLR9 reverses NASH [36, 37].
NLRP3 forms a subcellular multiprotein complex that consists of NLRP3, the adaptor protein ASC, and the protease caspase-1. Activation of the inflammasome results in activation of caspase-1 and the proinflammatory cytokines IL-1β and IL-18 and subsequently mediates cell death [38]. NLRP3 inflammasome activation is implicated in the progression of NAFLD from NAFL to NASH. NLRP3(−/−) mice are protected from hepatomegaly, liver injury, and activated macrophage infiltration induced by long-term CDAA feeding [39]. Treatment with the NLRP3 selective inhibitor MCC950 normalizes the expression of hepatic caspase-1, IL-1β, MCP-1, and IL-6 and lowers ALT/AST, which were shown to be increased in vehicle-treated foz/foz mice [40]. The NLRP3 and NLRP6 inflammasomes also negatively regulate NAFLD/NASH progression by modulating the interaction between gut microbiota and the host [41].
Adaptor proteins also regulate hepatic lipid metabolism
TLR4 signaling transduction primarily depends on MyD88 and TRIF adaptor proteins [42]. TRIF(−/−) mice exhibit increased CXCL1 and CCL3 expression and neutrophil and macrophage infiltration, which promotes liver inflammation and injury [43]. Hepatocyte-specific MyD88 deletion predisposes mice to glucose intolerance, inflammation, and hepatic IR, independent of body weight and adiposity [44]. In a study, global deletion of MyD88 drive HFD-fed mice weight gain, proinflammatory CD11c + AT machrophages increase and glucose homeotasis impairment [45]. In addition, genetic deletion of MyD88 in hepatocytes changes the gut microbiota composition and metabolomes of mice in ways that resemble diet-induced obesity [46].
TRAFs are important adaptor proteins in immunity, inflammation, and cell death. The depletion of TRAF1 leads to inhibition of NF-κB activity and reversion of the palmitic acid (PA)-induced attenuation of AKT and GSK-3β phosphorylation. The accumulation of LDs in hepatocytes and the expression of two key gluconeogenesis enzymes, PEPCK and G6Pase, produce a similar tendency, indicating that TRAF1 knockdown blocks the effect of PA on the suppression of glucose uptake [47]. TRAF1 promotes hepatic steatosis by enhancing the activation of apoptosis signal-regulating kinase 1 (ASK1)-mediated JNK/p38MAPK cascades; thus, TRAF1 functions as a positive regulator of IR, inflammation, and hepatic steatosis [48]. TRAF3 also functions as a regulator of hepatic lipid metabolism. TRAF3 deletion enhances the ability of insulin to stimulate the phosphorylation of AKT in hepatocytes, whereas TRAF3 overexpression suppresses insulin signaling. Glucose increases the level of TRAF3 in hepatocytes, which in turn elevates hepatic glucose production to promote hyperglycemia [49]. Specific deletion of TRAF3 in mice myeloid cell significantly attenuates either genetic ob/ob- or HFD-induced IR, hepatic steatosis, glucose intolerance, hyperglycemia and hyperinsulinemia [50]. The CD40-TRAF2/3/5 signaling pathway in MHCII(+) cells protects against adipose tissue inflammation and metabolic complications associated with obesity [51], whereas the CD40-TRAF6 interaction aggravates adipose tissue inflammation and metabolic complications [51]. TRAF5 overexpression significantly improves NASH-like phenotypes in HFD-fed mice and retards NAFLD progression in ob/ob mice [52].
The MAPK family plays important roles in hepatic lipid metabolism
The MAPK family consists of serine and threonine protein kinases that modulate physiological and pathophysiological cell responses, including the ERK1/2, p38MAPK, JNK, and ERK5 signaling pathways. These proteins uniformly play their regulatory roles via the classical MAPKKK-MAPKK-MAPK signaling pathway. Extracellular stimuli phosphorylate various MAPK family members and activate different subtypes of MAPK signal transduction pathways, particularly the ERK1/2, p38MAPK, and JNK signaling pathways, eventually leading to IR, hepatic lipid metabolism abnormalities, and even the development of NAFLD [53, 54].
Research has demonstrated that TAK1, a member of the MAP3K family in innate immune signal transduction, serves as an important regulator of energy homeostasis and involves in the development of metabolic syndrome. TAK1-deficiency greatly decreases the expression of lipid uptake-, synthesis- and storage-related genes (e.g., Cidea and CD36) in mice livers; however, restoration of TAK1 expression produces the opposite effects [55]. In the liver, TAK1-mediated autophagy prevents excessive lipid accumulation by targeting mammalian target of rapamycin complex 1 (mTORC1) [56]. Hepatocyte-specific deletion of TAK1 aggravates hepatosteatosis by suppressing the peroxisome proliferator-activated receptor-alpha (PPARα) target genes and β-oxidation [57]. TAK1 regulates cholesterol and fatty acid biosynthesis by binding to and inhibiting mature forms of SREBPs to maintain lipid homeostasis [58].
Another MAP3K kinase, ASK1 (which is also known as MAP3K5), has been proposed as a novel and effective target for NASH therapies [59]. In a comparison between ASK1-deficient and wt mice, ASK1 deficiency significantly decreased hepatic steatosis in HFD-fed mice [60]. Cellular repressors of E1A-stimulated genes (CREGs) directly interact with ASK1 and inhibit its phosphorylation, thereby blocking the downstream MKK4/7-JNK1 signaling pathway and leading to significantly ameliorated obesity, IR, and hepatic steatosis [61]. DKK3 negatively regulates hepatic IR, inflammation and steatosis; this function depends on DKK3 binding to ASK1 and thereby inhibits activation of the downstream JNK/p38MAPK pathways [62]. Conversely, TRAF1 promotes the activation of ASK1-mediated JNK/p38MAPK cascades and thus enhances hepatic steatosis [48].
JNK is one of the most investigated MAPK signal transducers in obesity and IR. Hepatic JNK signaling activation coincides with the development of steatohepatitis in MCD diet-fed mice. Mice deficient in JNK exhibit decreased inflammation, triglyceride accumulation and lipid peroxidation compared with those in wt and JNK2(-/-) mice [63]. Relative to wt mice, mice injected with the JNK inhibitor XG-102 show significantly lower levels of serum ALT, AST, TC, TG, HOMA-IR, and TNF-α but higher protein expression of p-c-Jun and cleaved caspase-3 [64]. Treatment with the JNK inhibitor SP600125 relieves NAFLD in HFD-fed rats, a finding supported by decreased expression of autophagy-associated genes (Atg3 and Atg5) and microtubule-associated protein 1A/B (MAP1A/B) and phosphorylation of IRS-1 and protein kinase B (PKB) [65]. Feeding stimulates JNK and p38α/βMAPK activation concomitant with downregulation of PPARα and the fibroblast growth factor 21 (FGF21), and ablation of hepatic JNK abrogates this phenomenon and induces fibrosis [23] (Fig. 4).
Mounting evidence has demonstrated that p38MAPK pushes forward the gluconeogenesis. Suppression of hepatic p38MAPK signaling attenuates insulin resistance [66]. In obesity, hepatic p38MAPK promotes the phosphorylation and nuclear tranlocation of the spliced form of X-box binding protein 1 (Xbp1s) so as to reduce the ER stress to maintain euglycemia [67]. Kujiraoka et al. demonstrated that hepatic ERK2 deficiency impairs glucose metabolism and promotes insulin resistance [68]. In addition, ERK1 knockout enhances lipogenesis and triglyceride accumulation, whereas weakens fatty acid oxidation in the development of hepatic steatosis [69].
Transcription factors implicated in innate immunity mediate hepatic lipid metabolism
The interferon regulatory factors (IRFs), as important immune factors, not only perform innate immune function but also exhibit immune-independent function under hepatic pathological conditions (e.g., liver injury and/or metabolic dysfunction) [70, 71]. As mentioned above, TRIF deficiency worsens hepatic steatosis induced by a HFD. This aggravated steatosis in TRIF(−/−) mice is attributable to the direct binding of IRF3 to the stearoyl-CoA desaturase 1 (SCD1) promoter [1, 72]. Global knockout of IRF3 significantly promotes HFD-induced hepatic IR and steatosis; in contrast, overexpression of IRF3 preserves glucose and lipid homeostasis by blocking IKKβ signaling transduction [73]. Interestingly, another study has suggested the opposing view that IRF3 deficiency improves adipose inflammation and insulin sensitivity, which enhances the browning of subcutaneous fat and increases adipose expression of GLUT4 [74]. Research has indicated that IRF7 and IRF9, which have been characterized as major transcription factors in IFN responses, play protective roles against metabolic disorders. IRF7-knockout (KO) mice fed a HFD diet show not only improved insulin sensitivity and glucose and lipid homeostasis but also ameliorated diet-induced hepatic steatosis. Additionally, IRF7 KO decreases macrophage infiltration to protect multiple organs from local and systemic inflammation [75]. Opposite phenotypes are observed for IRF9. IRF9 KO mice fed a HFD diet exhibit higher inflammation, lower insulin sensitivity, and more severe hepatic steatosis, whereas reexpression of IRF9 ameliorates these phenotypes. Importantly, PPARα is the target gene of IRF9, as evidenced by the fact that introduction of PPARα into IRF9 KO mice rescues insulin sensitivity and attenuates hepatic steatosis and inflammation [76].
In summary, most molecules in innate immune networks have a strong impact on liver inflammation, IR, lipid peroxidation, and even hepatic steatosis. The overexpression or knockout of certain genes alleviates or aggravates the development of NAFLD. Thus, an emergent topic for discussion is NAFLD therapies that target innate immune regulatory networks.
Innate immune molecules might be promising targets for metabolic disease therapies
During lipid overload in the liver, the development of hepatic steatosis is determined by types of lipid accumulation and methods of fat consumption. The overaccumulation of lipids causes lipotoxicity, which induces the release of stress signals to trigger the activation of sterile inflammatory pathways; over time, this phenomenon leads to chronic injury and fibrosis [77]. However, importantly, metabolic inflammation is mainly mediated by innate immune signaling pathways. Therefore, our group has been performing a great deal of research to demonstrate that innate immune networks are important in regulating hepatic lipid metabolism and would likely be attractive targets for NAFLD/NASH therapies.
The binding of FFAs and endotoxins to TLR4 enhances the activation of downstream IKK-NF-κB and MAPK signaling pathways, which are important for controlling inflammation, IR, and glucose metabolism [78]. We have demonstrated that transmembrane BAX inhibitor motif-containing 1 (TMBIM1) promotes the lysosomal degradation of TLR4 in cooperation with endosomal sorting complex required for transport (ESCRT), thereby facilitating the formation of multivesicular bodies (MVBs) that ultimately protect against NAFLD in mice and monkeys [79] (Fig. 5). Due to the critical role of TLR4 degradation in TMBIM1-ameliorated NASH, we propose that TLR4 is an attractive target for NASH therapy. However, TLR4 is also crucial for initializing the innate immune response, and the complete deletion of TLR4 may disrupt immunity. Therefore, we believe that maintaining the dynamic balance of TLR4 at an appropriate level rather than severely blocking TLR4 function would be beneficial for NASH therapy.
The most potential drug targets and prototypes for NAFLD therapies. DAMP, PAMP, or metabolites activate TLRs and others (e.g., formyl peptide receptor (FPR) pathway) signaling pathways, leading to activation of p38MAPK, JNK, NF-κB, and AMPK pathways, which blocks insulin signaling and leads to dysfunction of insulin-mediated glucose and lipid metabolism. ASK1 and TAK1, which mainly mediate metabolite-induced TLR signal transduction, may be the most potential targets for NAFLD therapies. More importantly, by targeting ASK1 or TAK1, some molecules, such as CFLAR, A20, CYLD, DKK3, and TMBIM1, are proposed as promising drug prototypes for NAFLD therapies
Research on ACSP8 and FADD-like apoptosis regulator (CFLAR; also known as c-FLIP) has provided strong support for our theory. We have demonstrated that CFLAR attenuates NASH by disrupting ASK1 N-terminal dimerization and autophosphorylation and the subsequent suppression of JNK1 activity [80] (Fig. 5). Similarly, the deubiquitinase tumor necrosis factor alpha-induced protein 3 (TNFAIP3) ameliorates NAFLD and NASH by markedly removing K63, K29, and K11 linkage of ASK1 and inactivation of ASK1-p38-JNK1/2 signaling [81] (Fig. 5). Thus, we propose that with respect to the targeting of ASK1, certain molecules, such as CFLAR and TNFAIP3, are likely to be potential leading molecular targets for NAFLD/NASH treatment.
In addition, ubiquitin-specific protease 18 (USP18) and deubiquitinase cylindromatosis (CYLD), members of the deubiquitinating enzyme family, negatively regulate NAFLD progression by interacting with and deubiquitinating TAK1, leading to the inhibition of TAK1 activation and the subsequent suppression of downstream JNK, p38, and NF-κB signaling pathways [82, 83] (Fig. 5). In contrast, tripartite motif 8 (TRIM8), an E3 ligase, robustly enhances steatohepatitis by binding to TAK1 to promote its phosphorylation and the activation of JNK/p38MAPK/NF-κB signaling [84] (Fig. 5). Another study has shown that the binding of hepatocyte TRAF3 to TAK1 in response to a HFD-induced TAK1 ubiquitination and subsequent autophosphorylation, followed by enhanced activation of downstream IKKβ-NF-κB and MKK-JNK-IRS-1307 signaling cascades, as well as disruption of AKT-GSK-3β/FOXO1 signaling [85] (Fig. 5). Therefore, we believe that TAK1 is responsible for functional homeostasis in the liver. Furthermore, the viable option of maintaining TAK1 expression at an appropriate level rather than completely ablating TAK1 may be helpful for protecting the liver against steatosis.
Conclusions
In conclusion, lipid production and lipid breakdown demonstrate that lipid species positively and negatively regulate inflammation, which in turn significantly alters lipid metabolism in the liver, adipose tissue, and macrophages under conditions involving infection or a HFD. This interconnected network maintains lipid metabolism homeostasis. Both basic and clinical studies have established that innate immune activation directly triggers and amplifies hepatic inflammation and affects the development of hepatic fibrosis in NAFLD/NASH. Given the dearth of effective treatments currently available for these conditions, we must continue to elucidate the mechanisms and pathogenesis underlying the progression of NAFLD, because such knowledge will be important for designing therapeutic interventions. In accordance with this objective, our team has been devoted to identifying key immune molecules, such as TLR4, TAK1, and ASK1, that play important roles in hepatic lipid metabolism and potential drug prototypes (e.g., CFLAR, TMBIM1, TNFAIP3, and CYLD) that target these immune molecules (Fig. 5); this research will provide significant contributions to improving NAFLD therapies.
References
Koliaki C, Roden M (2013) Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol Cell Endocrinol 379(1–2):35–42
Lonardo A, Ballestri S, Guaraldi G, Nascimbeni F, Romagnoli D, Zona S, Targher G (2016) Fatty liver is associated with an increased risk of diabetes and cardiovascular disease—evidence from three different disease models: NAFLD, HCV and HIV. World J Gastroenterol 22(44):9674–9693
Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A (2016) Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol 31(5):936–944
Singh S, Osna NA, Kharbanda KK, Singh S, Osna NA, Kharbanda KK (2017) Treatment options for alcoholic and non-alcoholic fatty liver disease: a review. World J Gastroenterol 23:6549–6570
Ganesh S, Rustgi VK (2016) Current pharmacologic therapy for nonalcoholic fatty liver disease. Clin Liver Dis 20(2):351–364
Byun JS, Yi HS (2017) Hepatic immune microenvironment in alcoholic and nonalcoholic liver disease. Biomed Res Int 2017:6862439
Cai J, Zhang XJ, Li H (2018) Role of innate immune signaling in non-alcoholic fatty liver disease. Trends Endocrinol Metab 29(10):712–722
Neuschwander-Tetri BA (2010) Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52(2):774–788
Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A (2012) The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 56(4):952–964
Jones JG (2016) Hepatic glucose and lipid metabolism. Diabetologia 59(6):1098–1103
Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, Weber MH, Budd JT, Lupi ME, Cusi K (2017) Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 65(4):1132–1144
Yki-Järvinen H (2014) Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2(11):901–910
Sochocka M (2008) Recognition of pathogens by innate immunity. Postepy Hig Med Dosw 62:676–687
Lass-Flörl C, Roilides E, Löffler J, Wilflingseder D, Romani L (2013) Minireview: host defence in invasive aspergillosis. Mycoses 56(4):403–413
Costantini C, Cassatella MA (2011) The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J Leukoc Biol 89(2):221–233
Xie GC, Duan ZJ (2012) Signal transduction of innate immunity to virus infection. Bing Du Xue Bao 28(3):303–310
Arthur JS, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13(9):679–692
Han J, Kaufman RJ (2016) The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res 57(8):1329–1338
Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ (2008) Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134(2):568–576
Szabo G, Petrasek J (2015) Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 12(7):387–400
Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G (2011) Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 54(1):133–144
Koyama Y, Brenner DA (2017) Liver inflammation and fibrosis. J Clin Invest 127(1):55–64
Lawan A, Bennett AM (2017) Mitogen-activated protein kinase regulation in hepatic metabolism. Trends Endocrinol Metab 28(12):868–878
Knebel B, Lehr S, Hartwig S, Haas J, Kaber G, Dicken HD, Susanto F, Bohne L, Jacob S, Nitzgen U, Passlack W, Muller-Wieland D, Kotzka J (2014) Phosphorylation of sterol regulatory element-binding protein (SREBP)-1c by p38 kinases, ERK and JNK influences lipid metabolism and the secretome of human liver cell line HepG2. Arch Physiol Biochem 120(5):216–227
Jiao P, Feng B, Li Y, He Q, Xu H (2013) Hepatic ERK activity plays a role in energy metabolism. Mol Cell Endocrinol 375(1–2):157–166
Khodabandeloo H, Gorganifiruzjaee S, Panahi S, Meshkani R (2016) Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res 167(1):228–256
Bieghs V, Trautwein C (2014) Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr 3(6):377–385
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140(6):805–820
Kuo LH, Tsai PJ, Jiang MJ, Chuang YL, Yu L, Lai KTA, Tsai YS (2011) Toll-like receptor 2 deficiency improves insulin sensitivity and hepatic insulin signalling in the mouse. Diabetologia 54(1):168–179
Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C, Sun K, Liu T, Harper MJ, Lee CE, Lee S, Scherer PE, Elmquist JK (2014) Hepatocyte toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun 5:3878
Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E (2011) Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res 35(8):1509–1518
Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD et al (2012) Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 12(2):139–152
Singh V, Chassaing B, Zhang L, San YB, Xiao X, Kumar M et al (2015) Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab 22(6):983–996
Arias-Loste MT, Iruzubieta P, Puente Á, Ramos D, Santa Cruz C, Estébanez Á, Llerena S, Alonso-Martín C, San Segundo D, Álvarez L, López Useros A, Fábrega E, López-Hoyos M, Crespo J (2016) Increased expression profile and functionality of TLR6 in peripheral blood mononuclear cells and hepatocytes of morbidly obese patients with non-alcoholic fatty liver disease. Int J Mol Sci 17(11). https://doi.org/10.3390/ijms17111878
Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E (2010) Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139(1):323–334
Handa P, Vemulakonda A, Kowdley KV, Uribe M, Méndez-Sánchez N (2016) Mitochondrial DNA from hepatocytes as a ligand for TLR9: drivers of nonalcoholic steatohepatitis? World J Gastroenterol 22(31):6965–6971
Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ (2016) Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest 126(3):859–864
Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H (2017) NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J Cell Physiol 233(3):2116–2132
Wree A, Mcgeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME et al (2014) NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med 92(10):1069–1082
Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM et al (2017) NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 66(5):1037–1046
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA (2012) Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482(7384):179–185
O'Neill LA, Golenbock D, Bowie AG (2013) The history of toll-like receptors [mdash] redefining innate immunity. Nat Rev Immunol 13(6):453–460
Yang L, Miura K, Zhang B, Matsushita H, Yang YM, Liang S, Song J, Roh YS, Seki E (2017) TRIF differentially regulates hepatic steatosis and inflammation/fibrosis in mice. Cell Mol Gastroenterol Hepatol 3(3):469–483
Duparc T, Plovier H, Marrachelli VG, Hul MV, Essaghir A, Ståhlman M et al (2016) Hepatocyte MyD88 affects bile acids, gut microbiota and metabolome contributing to regulate glucose and lipid metabolism. Gut 66(4):620–632
Castoldi A, Andrade-Oliveira V, Aguiar CF, Amano MT, Lee J, Miyagi MT et al (2017) Dectin-1 activation exacerbates obesity and insulin resistance in the absence of MyD88. Cell Rep 19(11):2272–2288
Everard A, Geurts L, Caesar R, Van Hul M, Matamoros S, Duparc T et al (2014) Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun 5:5648
Zhang W, Tang Z, Zhu X, Xia N, Zhao Y, Wang S, Cui S, Wang C (2015) TRAF1 knockdown alleviates palmitate-induced insulin resistance in HepG2 cells through NF-κB pathway. Biochem Biophys Res Commun 467(3):527–533
Xiang M, Wang PX, Wang AB, Zhang XJ, Zhang Y, Zhang P, Mei FH, Chen MH, Li H (2016) Targeting hepatic TRAF1-ASK1 signaling to improve inflammation, insulin resistance, and hepatic steatosis. J Hepatol 64(6):1365–1377
Chen Z, Canet MJ, Liang S, Jiang L, Xiong Y, Yin L, Rui L (2015) Hepatocyte TRAF3 promotes insulin resistance and type 2 diabetes in mice with obesity. Mol Metab 4(12):951–960
Chen Z, Shen H, Sun C, Yin L, Tang F, Zheng P et al (2015) Myeloid cell TRAF3 promotes metabolic inflammation, insulin resistance, and hepatic steatosis in obesity. Am J Physiol Endocrinol Metab 308(6):460–469
Chatzigeorgiou A, Seijkens T, Zarzycka B, Engel D, Poggi M, van den Berg S et al (2014) Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. Proc Natl Acad Sci U S A 111(7):2686–2691
Gao L, Wang PX, Zhang Y, Yu CJ, Ji Y, Wang X, Zhang P, Jiang X, Jin H, Huang Z, Zhang ZR, Li H (2016) Tumor necrosis factor receptor-associated factor 5 (Traf5) acts as an essential negative regulator of hepatic steatosis. J Hepatol 65(1):125–136
Ceppo F, Jager J, Berthou F, Giorgetti-Peraldi S, Cormont M, Bost F, Tanti JF (2014) Implication of MAP kinases in obesity-induced inflammation and insulin resistance. Biol Aujourdhui 208(2):97–107
Kumphune S, Chattipakorn S, Chattipakorn N (2013) Roles of p38-MAPK in insulin resistant heart: evidence from bench to future bedside application. Curr Pharm Des 19(32):5742–5754
Kang HS, Okamoto K, Kim YS, Takeda Y, Bortner CD, Dang H, Wada T, Xie W, Yang XP, Liao G, Jetten AM (2011) Nuclear orphan receptor TAK1/TR4-deficient mice are protected against obesity-linked inflammation, hepatic steatosis, and insulin resistance. Diabetes 60(1):177–188
Seki E (2014) TAK1-dependent autophagy: a suppressor of fatty liver disease and hepatic oncogenesis. Mol Cell Oncol 1(4):e968507
Inokuchi-Shimizu S, Park EJ, Roh YS, Yang L, Zhang B, Song J et al (2014) TAK1-mediated autophagy and fatty acid oxidation prevent hepatosteatosis and tumorigenesis. J Clin Invest 124(8):3566–3578
Morioka S, Sai K, Omori E, Ikeda Y, Matsumoto K, Ninomiya-Tsuji J (2016) TAK1 regulates hepatic lipid homeostasis through SREBP. Oncogene 35(29):3829–3838
Schuster S, Feldstein AE (2017) NASH: novel therapeutic strategies targeting ASK1 in NASH. Nat Rev Gastroenterol Hepatol 14(6):329–330
Yamamoto E, Dong YF, Kataoka K, Yamashita T, Tokutomi Y, Matsuba S, Ichijo H, Ogawa H, Kim-Mitsuyama S (2008) Olmesartan prevents cardiovascular injury and hepatic steatosis in obesity and diabetes, accompanied by apoptosis signal regulating kinase-1 inhibition. Hypertension 52(3):573–580
Zhang QY, Zhao LP, Tian XX, Yan CH, Li Y, Liu YX, Wang PX, Zhang XJ, Han YL (2017) The novel intracellular protein CREG inhibits hepatic steatosis, obesity and insulin resistance. Hepatology 66(3):834–854
Xie L, Wang PX, Zhang P, Zhang XJ, Zhao GN, Wang A, Guo J, Zhu X, Zhang Q, Li H (2016) DKK3 expression in hepatocytes defines susceptibility to liver steatosis and obesity. J Hepatol 65(1):113–124
Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ (2006) JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology 43(1):163–172
Hu Y, Peng N, Lei D, Cheng F, Chen Y (2014) Impact of JNK inhibitor XG-102 in a diet-induced rat model of non-alcoholic steatohepatitis. Zhonghua Gan Zang Bing Za Zhi 22(12):948–952
Yan H, Gao Y, Zhang Y (2017) Inhibition of JNK suppresses autophagy and attenuates insulin resistance in a rat model of nonalcoholic fatty liver disease. Mol Med Rep 15(1):180–186
Pereira S, Yu WQ, Moore J, Mori Y, Tsiani E, Giacca A (2016) Effect of a p38 MAPK inhibitor on FFA-induced hepatic insulin resistance in vivo. Nutr Diabetes 6:e210
Lee J, Sun C, Zhou Y, Lee J, Gokalp D, Herrema H, Park SW, Davis RJ, Ozcan U (2011) p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat Med 17(10):1251–1260
Kujiraoka T, Satoh Y, Ayaori M, Shiraishi Y, Arainakaya Y, Hakuno D et al (2013) Hepatic extracellular signal–regulated kinase 2 suppresses endoplasmic reticulum stress and protects from oxidative stress and endothelial dysfunction. J Am Heart Assoc 2(4):e000361
Khan AS, Subramaniam S, Dramane G, Khelifi D, Khan NA (2017) ERK1 and ERK2 activation modulates diet-induced obesity in mice. Biochimie 137:78–87
Wang PX, Zhang XJ, Li H (2016) Liver capsule: IRFs in hepatocytes: pathophysiology. Hepatology 63(5):1706
Zhao GN, Jiang DS, Li H (2015) Interferon regulatory factors: at the crossroads of immunity, metabolism, and disease. Biochim Biophys Acta 1852(2):365–378
Chen J, Li J, Yiu JHC, Lam JKW, Wong CM, Dorweiler B, Xu A, Woo CW (2017) TRIF-dependent toll-like receptor signaling suppresses Scd1 transcription in hepatocytes and prevents diet-induced hepatic steatosis. Sci Signal 10(491):eaal3336
Wang XA, Zhang R, She ZG, Zhang XF, Jiang DS, Wang T, Gao L, Deng W, Zhang SM, Zhu LH, Guo S, Chen K, Zhang XD, Liu DP, Li H (2014) Interferon regulatory factor 3 constrains IKKβ/NF-κB signaling to alleviate hepatic steatosis and insulin resistance. Hepatology 59(3):870–885
Kumari M, Wang X, Lantier L, Lyubetskaya A, Eguchi J, Kang S, Tenen D, Roh HC, Kong X, Kazak L, Ahmad R, Rosen ED (2016) IRF3 promotes adipose inflammation and insulin resistance and represses browning. J Clin Invest 126(8):2839–2854
Wang XA, Zhang R, Zhang S, Deng S, Jiang D, Zhong J, Yang L, Wang T, Hong S, Guo S, She ZG, Zhang XD, Li H (2013) Interferon regulatory factor 7 deficiency prevents diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab 305(4):E485–E495
Wang XA, Zhang R, Jiang D, Deng W, Zhang S, Deng S, Zhong J, Wang T, Zhu LH, Yang L, Hong S, Guo S, Chen K, Zhang XF, She Z, Chen Y, Yang Q, Zhang XD, Li H (2013) Interferon regulatory factor 9 protects against hepatic insulin resistance and steatosis in male mice. Hepatology 58(2):603–616
Zámbó V, Simon-Szabó L, Szelényi P, Kereszturi E, Bánhegyi G, Csala M (2013) Lipotoxicity in the liver. World J Hepatol 5(10):550–557
Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S (2012) Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med 18(8):1279–1285
Zhao GN, Zhang P, Gong J, Zhang XJ, Wang PX, Yin M, Jiang Z, Shen LJ, Ji YX, Tong J, Wang Y, Wei QF, Wang Y, Zhu XY, Zhang X, Fang J, Xie Q, She ZG, Wang Z, Huang Z, Li H (2017) Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nat Med 23(6):742–752
Wang PX, Ji YX, Zhang XJ, Zhao LP, Yan ZZ, Zhang P, Shen LJ, Yang X, Fang J, Tian S, Zhu XY, Gong J, Zhang X, Wei QF, Wang Y, Li J, Wan L, Xie Q, She ZG, Wang Z, Huang Z, Li H (2017) Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat Med 23(4):439–449
Zhang P, Wang PX, Zhao LP, Zhang X, Ji YX, Zhang XJ, Fang C, Lu YX, Yang X, Gao MM, Zhang Y, Tian S, Zhu XY, Gong J, Ma XL, Li F, Wang Z, Huang Z, She ZG, Li H (2018) The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat Med 24(1):84–94
An S, Zhao LP, Shen LJ, Wang S, Zhang K, Qi Y, Zheng J, Zhang XJ, Zhu XY, Bao R, Yang L, Lu YX, She ZG, Tang YD (2017) USP18 protects against hepatic steatosis and insulin resistance via its DUB activity. Hepatology 66(6):1866–1884
Ji YX, Huang Z, Yang X, Wang X, Zhao LP, Wang PX, Zhang XJ, Alves-Bezerra M, Cai L, Zhang P, Lu YX, Bai L, Gao MM, Zhao H, Tian S, Wang Y, Huang ZX, Zhu XY, Zhang Y, Gong J, She ZG, Li F, Cohen DE, Li H (2018) The deubiquitinating enzyme cylindromatosis mitigates nonalcoholic steatohepatitis. Nat Med 24(2):213–223
Yan FJ, Zhang XJ, Wang WX, Ji YX, Wang PX, Yang Y, Gong J, Shen LJ, Zhu XY, Huang Z, Li H (2017) The E3 ligase tripartite motif 8 targets TAK1 to promote insulin resistance and steatohepatitis. Hepatology 65(5):1492–1511
Wang PX, Zhang XJ, Luo P, Jiang X, Zhang P, Guo J, Zhao GN, Zhu X, Zhang Y, Yang S, Li H (2016) Hepatocyte TRAF3 promotes liver steatosis and systemic insulin resistance through targeting TAK1-dependent signalling. Nat Commun 7:10592
Funding
This work was supported by grants from the National Science Fund for Distinguished Young Scholars (no. 81425005; H.L.), the Key Project of the National Natural Science Foundation (no. 81630011; H.L.), the Major Research Plan of the National Natural Science Foundation of China (no. 91639304, no. 91729303; H. L.), the Creative Groups Project of Hubei Province (no. 2016CFA010; H.L.), and the Hubei Science and Technology Support Project (no. 2018BEC473; H.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bai, L., Li, H. Innate immune regulatory networks in hepatic lipid metabolism. J Mol Med 97, 593–604 (2019). https://doi.org/10.1007/s00109-019-01765-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01765-1