Abstract
Lung cancer remains the leading cause of cancer-related death worldwide. Paclitaxel, either as monotherapy or combined with other agents, is the standard treatment for advanced non-small cell lung cancer (NSCLC), the most common type of lung cancer. However, both de novo and acquired resistance against paclitaxel frequently occurs and represents a huge clinical problem. The underlying mechanisms remain poorly characterized. Here, by comparing microRNA (miRNA) expression levels using miRNA arrays, we observed differential expression of miR-30a-5p in two independent lung cancer cell pairs (paclitaxel-resistant vs paclitaxel-sensitive A549 cell lines). Overexpression of miR-30a-5p sensitizes NSCLC cells to paclitaxel both in vitro and in vivo. In addition, miR-30a-5p increases paclitaxel sensitivity by promoting chemotherapy-induced apoptosis via downregulating BCL-2, a key apoptosis regulator. High miR-30a-5p expression is positively correlated with enhanced responsiveness to paclitaxel and predicts a more favorable clinical outcome in NSCLC patients. Moreover, miR-30a-5p expression is negatively correlated with BCL-2 expression in NSCLC tissues. These data indicate that miR-30a-5p may be useful to treat paclitaxel-resistant lung cancer and may also provide a biomarker to predict paclitaxel responsiveness in lung cancer.
Key messages
-
BCL-2 is a novel direct target of miR-30a-5p.
-
miR-30a-5p enhances NSCLC paclitaxel sensitivity in vitro and in vivo.
-
miR-30a-5p sensitizes NSCLC cells to paclitaxel by inducing apoptosis through BCL-2 inhibition.
-
miR-30a-5p negatively correlates with BCL-2 and predicts a favorable clinical outcome in NSCLC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. Approximately 70–80% of lung cancers are non-small cell lung cancer (NSCLC), including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma [1, 2]. In NSCLC, the leading causes of death are chemotherapy resistance and metastasis. Chemotherapy resistance frequently drives tumor progression. Paclitaxel, either as monotherapy or combined with other agents, is the standard treatment for advanced NSCLC. However, both de novo and acquired resistance to paclitaxel frequently occur and represent a huge clinical problem, with response rates between 21 and 24% due to chemoresistance [3, 4]. Resistance against paclitaxel resulted from multiple mechanisms, including P-glycoprotein overexpression or other drug efflux pumps [5, 6], variations in microtubules in drug-binding or aberrant expression of tubulin isotypes and microtubule-associated proteins [7,8,9], dysregulated cell cycle and cell survival pathways [10,11,12], and the induction of treatment-related autophagy [13,14,15]. However, the molecular mechanisms of paclitaxel resistance are still needed to be investigated.
MicroRNAs (miRNAs) are small non-coding RNA molecules (about 22 nucleotides in length), which frequently inhibits gene expression at the post-transcriptional level. miRNAs play important roles in many biological processes, such as cell proliferation, invasion, metastasis, and apoptosis [16]. Aberrant expression of miRNAs has been shown to associate with tumor chemotherapy resistance, including resistance to paclitaxel [17]. However, how miRNAs regulate paclitaxel resistance is poorly understood. Previous findings indicate the suppressive role of miR-30a-5p in tumorigenesis in multiple cancers [18,19,20]. However, whether miR-30a-5p regulates paclitaxel sensitivity of NSCLC is unknown.
In our present work, we demonstrated that miR-30a-5p was significantly decreased in paclitaxel-resistant NSCLC cells. Overexpression of miR-30a-5p enhanced the NSCLC cell sensitivity to paclitaxel both in vitro and in vivo by inducing apoptosis via inhibition of the BCL-2 expression, a key apoptosis regulator. miR-30a-5p abundance in NSCLC patients was positively correlated with a favorable response to paclitaxel therapy and predicted good clinical prognosis. Therefore, upregulation of miR-30a-5p may be a promising way to treat paclitaxel-resistant NSCLC, and miR-30a-5p may be a useful biomarker to predict the paclitaxel responsiveness in NSCLC patients.
Materials and methods
Patients and tumor tissues
A total of 94 human NSCLC samples were obtained from the Chinese PLA General Hospital with the informed consent of patients and approval of the appropriate ethics committee from the Chinese PLA General Hospital. Diagnoses were based on pathological evidence. Patients had not undergone immunotherapy, chemotherapy, or radiotherapy before specimen collection. Tissue samples were snap-frozen in liquid nitrogen and stored at −80 °C until RNA extraction. Among the 94 patients, 63 patients underwent intravenous paclitaxel chemotherapy (paclitaxel 200 mg/m2 at 3-week intervals) after surgery, while the rest 31 patients did not receive paclitaxel treatment. The effect of chemotherapy was evaluated by response rate (RR) and disease-free survival (DFS) according to the Response Evaluation Criteria in Solid Tumors.
Cell culture and transfection
Human lung cancer cell lines, A549 (human lung adenocarcinoma epithelial cell line) and H460 (human large cell lung cancer), were obtained from the American Type Culture Collection (Manassas, VA, USA) and tested for mycoplasma contamination. Paclitaxel-resistant A549/PR cells and H460/PR cells were developed by treatment with gradually increasing concentrations of paclitaxel in cell culture medium. Briefly, cells were seeded in six-well plates and reached about 80% confluency in fresh medium before treating with paclitaxel. The doses of paclitaxel ranged from 0.1 to 20 nM with a dose gradient of 25–50% of the previous dose. The next dose was given until the cells were stable in proliferation without significant death.
Stable cell lines overexpressing miR-30a were established by lentiviral transduction using a pCDH plasmid (System Biosciences, Mountain View, CA, USA) carrying miR-30a. Lentiviruses were produced by transfection of the 293T producer cell line with the lentiviral vector and packing vector mix (System Biosciences). Lentiviruses were collected 48 h later and then infected, indicating lung cancer cells. Stable cell lines expressing miR-30a were selected with 1 μg/mL puromycin 48 h after infection. Pooled clones (more than 40 individual clones) or individual clones (15 clones) were screened by testing the level of miR-30a using qPCR. Out of the 15 individual clones, three clones expressing different levels of miR-30a were tested for its target gene expression as well as cell proliferation. Similar results were obtained with individual clones or pooled clones. All cells were cultured at 37 °C in a humidified atmosphere with 5% CO2 in DMEM or RPMI-1640 medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, USA) and 1% penicillin/streptomycin (Life Technologies). For transfection [21], cells were seeded in 24-well or six-well plates and then transfected with the indicated plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol.
Plasmid construction and reagents
Wild-type and mutated BCL-2 3′-UTRs were cloned into a dual-luciferase reporter vector (Promega, Madison, WI, USA) as described previously [22]. Briefly, about 541 bp of the 3′UTR of human BCL-2 gene was obtained by genomic PCR using the following primers: 5′-CGGAATTCATCCAGATGGCAAATGACCA-3′ (forward) and 5′-CCGCTCGAGTTAGCCCCCGTGACCTCTT-3′ (reverse). To introduce mutations into the seed sequences of the predicted miR-30a-5p target sites within the BCL-2 3′UTR, recombinant PCR was performed using the above-mentioned primers and the following primers: 5′-TTATTTTATGAAAGTGGGCACTTGTCAAAGTGATG-3′ (forward) and 5′-CATCACTTTGACAAGTGCCCACTTTCATAAAATAA-3′ (reverse). The miRNA-30a-5p mimics or antisense of miR-30a-5p (anti-miR-30a-5p) and their corresponding miRNA controls were synthesized from Gene Pharma (Shanghai, China). The negative controls are scrambled and non-targeted irrelevant RNA sequences.
Anti-BCL2 (sc-492) and anti-GAPDH (sc-25778) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Paclitaxel was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Luciferase reporter assay
Cells were seeded in 24-well plates at a density of 1 × 105 cells per well. The cells were co-transfected with luciferase reporters, either wild-type or mutant BCL-2 3′-UTR, in combination with anti-miR-30a-5p or a scramble using Lipofectamine 2000. Forty-eight hours later, cells were harvested and analyzed for luciferase activity using a luciferase assay kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
miRNA microarray analysis
Total RNA was extracted from A549 cells and their paclitaxel resistant counterparts using a Qiagen miRNeasy mini kit according to the manufacturer’s protocol. Total RNA was sent to CapitalBio Corporation (Beijing, China) for miRNA labeling, quality control, chip hybridization, and microarray analysis. Briefly, total RNA was labeled with Cy3 and Cy5 fluorescent dyes. Pairs of labeled samples were hybridized to miRCURY LNA™ microRNA array slides with 2549 human miRNAs. Normalization was performed using a LOWESS filter (Locally Weighted Regression) method to remove system-related variations. An analysis of variance was first applied to produce a miRNA expression profile overview across all samples, and then t tests were performed to identify significantly differentiated miRNA expression among all interested combinations of paired groups.
miRNA extraction and quantitative RT-PCR
Total RNA, including miRNA, was extracted from cultured cells or tissues samples with a miRNeasy Mini kit (Qiagen). Target miRNA was reverse transcribed to cDNA using a specific miRNA primer and miScript Reverse Transcription Kit (Qiagen). miRNA expression was measured with a miScript SYBR Green PCR Kit (Qiagen). Primers for miRNAs and the endogenous control, U6 gene, were displayed in Supplementary Table 1. The relative fold expression of the target was calculated by the comparative Ct method and was normalized to control.
Cell viability assay
Cell viability was measured using a CCK-8 Kit (Dojindo, Kumamoto, Japan) according to the manufacturer’s protocol. To analyze the effects of miR-30a-5p on paclitaxel sensitivity, cells transfected with either miR-30a-5p or anti-miR-30a-5p were treated at concentrations of 0, 2.5, 5, 10, 20, 40, and 80 nM paclitaxel for 24 h. The IC50 value was calculated as the concentration of paclitaxel that reduced cell viability by 50%.
Apoptosis and flow cytometry analysis
miR-30a-5p-overexpressing and miR-30a-5p plus BCL-2-overexpressing or control cells (1 × 106 cells) were cultured in 60-mm dishes and treated with paclitaxel (20 nM) for 24 h before harvesting. The cells were labeled with propidium iodide and annexin V according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA). A minimum of 10, 000 events for each sample were collected and analyzed using a FACScalibur Flow Cytometer (Becton Dickinson, BD Biosciences).
In vivo lung tumor xenograft model
All animal experimentation conforms to protocols approved by the animal care committee of Beijing Institute of Biotechnology, Beijing. Six-week-old BALB/c nu/nu male mice were purchased from Vital River Inc. (Beijing, China). For the tumor growth model, A549 cells (1 × 107 cells) stably infected with the pCDH control vector or pCDH-miR-30a were injected subcutaneously into the backs of BALB/c nu/nu mice, which were divided into 4 groups (n = 7 based on minimal 30% decrease from 1 g tumors with 250 μg standard deviation, α error of 0.05, and β error of 0.8) using random number method. After 3 weeks, all the seven animals in each group had tumors greater than 5 mm in diameter, and either paclitaxel (15 mg/kg−1) or saline was injected intraperitoneally once a week for 6 weeks with no blinding. Tumor sizes were measured at the indicated times using calipers. Tumor volumes were estimated according to the following formula: volume = (longest diameter × shortest diameter2)/2.
Statistical analysis
All in vitro experiments were performed in triplicate and repeated three times. Differences between variables were assessed by two-tailed Student’s t test, when sample size is small, with overall standard deviation unknown and data normal distributed. The survival rates in relation to miR-30a-5p expression were estimated using the Kaplan-Meier method, and the difference in survival curves was analyzed with a log-rank test. The relationship between miR-30a-5p and the IC50 of paclitaxel was examined using the Spearman’s rank correlation. The SPSS 17.0 statistical software package was used to perform all statistical analyses. Data are presented as the means ± standard deviation (SD). P < 0.05 was considered statistically significant.
Results
miRNA profiles in paclitaxel-sensitive and paclitaxel-resistant lung cancer cells
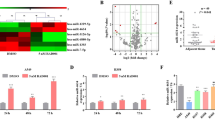
To investigate critical miRNAs that are potentially associated with paclitaxel resistance in NSCLC, we performed miRNA microarray analysis of the NSCLC cell line A549 and its paclitaxel-resistant counterpart A549/PR (Supplementary Fig. 1a) The total number of miRNAs detected was 2549, of which 208 miRNAs were differentially expressed between A549 and A549/PR cells (109 miRNAs upregulated and 99 miRNAs downregulated) (Fig. 1a and Supplementary Table 2). Quantitative RT-PCR confirmed the paclitaxel-mediated expression of 20 miRNAs, with about 90% consistency with the miRNA microarray results, including previously reported miRNAs (e.g., miR-125a and miR-375) [27, 28] (Fig. 1b). Among them, miR-30a-5p was the most differentially expressed miRNA detected, with more than fivefold lower expression in A549/PR cells when compared with A549 cells. Therefore, we hypothesized that miR-30a-5p may play a role in regulating paclitaxel resistance of NSCLC patients.
Differential expression of miRNAs in paclitaxel-sensitive and paclitaxel-resistant lung cancer cells. a A heat map showing the miRNA expression profiles and supervised hierarchical clustering analysis for paclitaxel-sensitive (A549) and paclitaxel-resistant (A549/PR) lung cancer cell lines. Significantly, differentially expressed miRNAs matching the threshold (difference ≥twofold and chip signal value >500) and statistical analysis standard (P < 0.05) were selected. Each column represents a cell line, and each row shows the relative expression level for individual miRNAs. The yellow and blue colors indicate high or low expression, respectively. b Twenty miRNAs differentially expressed in the lung cancer cell lines from a was validated by RT-qPCR. U6 small nuclear RNA was used as an internal control. All values are the mean ± SD of triplicate measurements, and the experiments were repeated three times. The P values were generated using two-tailed Student’s t test. *P < 0.05, **P < 0.01 versus matched lung cancer cells
miR-30a-5p enhances NSCLC paclitaxel sensitivity in vitro
To investigate the biological function of miR-30a-5p in paclitaxel sensitivity of NSCLC cells, we studied the relationship between miR-30a-5p and the IC50 of paclitaxel. As expected, miR-30a-5p expression was negatively correlated with the IC50 values of paclitaxel in two NSCLC cell lines (P = 0.0482, r = −0.876; Supplementary Fig. 1b). To further verify the association between miR-30a-5p expression and paclitaxel resistance, A549 and H460 lung cancer cells was transfected with miR-30a-5p and then treated with increasing doses of paclitaxel. Viability assay demonstrated that overexpression of miR-30a-5p increased the sensitivity of A549 and H460 cells to paclitaxel (Fig. 2a, b), whereas inhibition of miR-30a-5p with specific anti-miR-30a-5p in A549 cells and H460 cells reduced paclitaxel sensitivity (Fig. 2c, d). Taken together, these results collectively indicate that miR-30a-5p expression levels are positively correlated with the NSCLC cell sensitivity to paclitaxel.
miR-30a-5p modulates sensitivity to paclitaxel in vitro. a and b miR-30a-5p-overexpressing A549 (a) or H460 (b) lung cancer cells were treated with increasing concentrations of paclitaxel as indicated. After 72 h, cell viability assays were performed using CCK-8 kit. The group not treated with paclitaxel was presented as 100% viable cells and was used as an internal control for comparison. Histograms on the right show the level of miR-30a-5p overexpression. Scramble, negative control for miRNAs. c and d Cell viability assay of A549 (c) or H460 (d) cells transfected with anti-miR30a-5p and treated and analyzed as in a. Scramble, negative control for miRNA inhibitors. All values shown are the mean ± SD of triplicate samples. Experiments were repeated three times. The P values were generated using two-tailed Student’s t test. **P < 0.01 versus NC or scramble
miR-30a-5p represses BCL-2 expression by targeting its 3′-UTR
To explore the mechanisms responsible for the functions of miR-30a-5p in NSCLC, we searched for the potential target genes of miR-30a-5p using publicly available databases (TargetScan and miRanda). Multiple genes were predicted as the potential targets of miR-30a, from which we picked out those reported to play a role in NSCLC (Supplementary Fig. 2a). Next, we carried out the Western blot analysis to confirm the potential targets in 293T cells. As previously reported, miR-30a-5p inhibited the EYA2 oncogene expression (Supplementary Fig. 2b). The house-keeping gene GAPDH was not regulated in response to miR-30a-5p expression. Importantly, miR-30a-5p repressed the expression of BCL-2, a key negative regulator of apoptosis. Thus, we chose BCL-2 as the target gene and GAPDH as the internal control for further study (Supplementary Fig. 2b).
Consistent with the results in 293T cells, miR-30a-5p suppressed BCL-2 expression in A549 and H460 cells (Fig. 3a). In contrast, anti-miR-30a-5p increased BCL-2 expression in the above-mentioned cell lines (Fig. 3b). To investigate whether BCL-2 is a direct and specific target of miR-30a-5p, we predicted the free energy of the binding between the miR-30a-5p and the target 3′UTR (−6.03 kcal/mol) using the software at http://RNA.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAcofold.cgi and then performed luciferase reporter assays with wild-type (WT) or mutated BCL-2 3′-UTR. miR-30a-5p reduced the WT BCL-2 3′-UTR reporter activity in A549 and H460 cells (Fig. 3c). However, miR-30a-5p did not affect the luciferase activity of the mutant reporter in which the binding sites for miR-30a-5p were mutated. These results indicate that miR-30a-5p inhibits BCL-2 expression by directly binding to its 3′-UTR in NSCLC cells.
miR-30a suppresses BCL-2 expression by directly targeting its 3′-UTR. a and b Immunoblot analysis of human lung cancer cells transfected with miR-30a-5p mimics (a) or anti-miR-30a-5p (b) as well as their corresponding negative controls. The histograms under the immunoblot graphs demonstrate corresponding miRNA expression levels determined by RT-qPCR. c miRNA luciferase reporter assays of A549 and H460 cells transfected with wild-type or mutated BCL-2 reporters plus miR-30a-5p. A schematic diagram of the BCL-2 3′-UTR luciferase reporter constructs is shown. Italicized fonts indicate the putative miR-30a-5p-binding site in the human BCL-2 3′-UTR. Underline indicates mutations introduced into the BCL-2 3′-UTR. All values shown are the mean ± SD of triplicate samples. Experiments were repeated three times. The P values were generated using two-tailed Student’s t test. *P < 0.05, **P < 0.01 versus corresponding control
miR-30a-5p enhances sensitivity of NSCLC cells to paclitaxel by promoting apoptosis through downregulating BCL-2
BCL-2 has been shown to inhibit apoptosis, which contributes to paclitaxel resistance [23,24,25]. Thus, we analyzed the expression of the apoptosis inhibitor BCL-2 and the apoptosis inducer BAX using Western blot assays in paclitaxel-sensitive and paclitaxel-resistant NSCLC cell lines. The results indicated that the expression of BCL-2 was increased and the expression of BAX was decreased in paclitaxel-resistant cells (A549/PR and H460/PR) compared with paclitaxel-sensitive cells (A549 and H460) (Supplementary Fig. 3).
Since miR-30a-5p directly targets BCL-2 and enhances paclitaxel sensitivity, we further investigated if miR-30a-5p increases paclitaxel sensitivity in NSCLC via inducing apoptosis through downregulating BCL-2 expression. To verify the hypothesis, we examined the effects of miR-30a-5p on the apoptosis of A549 cells by flow cytometry. The result showed that overexpression of miR-30a-5p alone or paclitaxel treatment increased the proportion of apoptotic A549 cells to 4.97 and 8.93%, respectively, compared with that of the control cells (1.76%) (Fig. 4a). Importantly, A549 cells transfected with miR-30a-5p and treated with paclitaxel had a significantly higher proportion of apoptotic cells (61.51%) than those transfected with miR-30a-5p alone (4.97%) or treated with paclitaxel alone (8.93%), suggesting that miR-30a-5p and paclitaxel synergistically induce apoptosis. Treatment with paclitaxel or miR-30a-5p overexpression suppressed the expression of BCL-2 and increased the expression of BAX. Paclitaxel combined with miR-30a-5p overexpression caused the greatest inhibition of BCL-2 and upregulation of BAX and cleaved PARP, a marker for apoptosis (Fig. 4b). Re-expression of BCL-2 in A549 cells transfected with miR-30a-5p abolished the synergistic effects of miR-30a-5p and paclitaxel (Fig. 4a, b). Similar results were observed in H460 cells (Supplementary Fig. 4). These data indicates that miR-30a-5p promotes NSCLC cell paclitaxel sensitivity by activating apoptotic pathway via downregulation of BCL-2.
miR-30a-5p enhances paclitaxel sensitivity by downregulating BCL-2. a Representative flow cytometry analysis of Annexin V (1:1000) and propidium iodide (1:1000) staining in A549 cells transfected with miR-30a-5p mimics or miR-30a-5p mimics plus Myc-tagged BCL-2 without its 3′-UTR and treated with or without paclitaxel (20 nM) for 24 h. The proportion of apoptotic cells is shown as the mean ± SD from three independent experiments. The P values were generated using two-tailed Student’s t test (*P < 0.05, **P < 0.01). Histograms show the miR-30a expression levels. b Representative Western blots using the indicated antibodies in A549 cells transfected and treated as in a
miR-30a modulates NSCLC paclitaxel resistance in vivo
Based on the fact that miR-30a-5p mediates paclitaxel sensitivity in NSCLC cells in vitro, we investigated the phenotype of cells with miR-30a overexpression in vivo. To this end, we established A549 cells stably expressing miR-30a. We first tested pooled clones (more than 40 individual clones) and three individual clones expressing different levels of miR-30a for its target gene expression as well as cell viability. The trends among the pooled clones and the three individual clones were similar (Supplementary Fig. 5). Thus, we chose pooled clones for animal study. miR-30a-overexpressing A549 cells or control A549 cells were subcutaneously injected into the backs of BALB/c nude mice. Once the mice developed palpable tumors (greater than 5 mm in diameter within 3 weeks), they were randomly assigned into paclitaxel or saline treatment groups. There was no significant difference in initial tumor volumes between the four groups (Fig. 5a). Paclitaxel (15 mg/kg−1) or the same volumes of saline were intraperitoneally injected once a week for 6 weeks. Like paclitaxel, miR-30a overexpression in A549 cells induced an inhibition of tumor growth (Fig. 5a). The combination of miR-30a overexpression and paclitaxel treatment induced a significantly synergistic reduction of tumor growth, accompanied by increased expression of miR-30a-5p, BAX, and cleaved PARP and decreased BCL-2 (Fig. 5b, c). These findings suggest that miR-30a-5p upregulation enhances paclitaxel sensitivity of NSCLC cells in vivo.
miR-30a-5p mediates paclitaxel sensitivity in vivo. a A549 cells overexpressing miR-30a or control cells were injected into nude mice respectively. After 3 weeks, paclitaxel (15 mg/kg−1) or saline was intraperitoneally injected once a week for 6 weeks. At the indicated times, tumors were measured with Vernier Calipers (mean ± SD; n = 7). Arrow represents the beginning treatment with paclitaxel or saline. b RT-qPCR analysis of miR-30a-5p expression of representative excised tumors from a. c Immunoblot analysis of representative excised tumors from a. The P values were generated using two-tailed Student’s t test (*P < 0.05, **P < 0.01 versus corresponding control)
miR-30a-5p expression positively correlates with DFS and RR in lung cancer patients who received paclitaxel-based chemotherapy
To study the relationship between miR-30a-5p and paclitaxel sensitivity in NSCLC, we first selected 63 patients who had received paclitaxel chemotherapy. We stratified patients into high miR-30a-5p expression and low miR-30a-5p expression according to miR-30a-5p expression levels of NSCLC cells. The effect of paclitaxel chemotherapy on the patient’s disease-free survival (DFS) was evaluated according to the Response Evaluation Criteria in Solid Tumors. The patients with low miR-30a-5p expression had poorer DFS (P = 3.12 × 10−5) than those with high miR-30a-5p expression (Fig. 6a). It should be noted that the other 31 patients, who were not treated with paclitaxel, had no significant differences in their DFS regardless of the miR-30a amounts in the tumors (P = 0.303) (Fig. 6b). Consistent with the results of the DFS, for the patients treated with paclitaxel, the patients who had no response rate (RR) expressed lower levels of miR-30a-5p than those who had RR (P = 0.003) (Fig. 6c). These data suggest the important role for miR-30a-5p in regulation of NSCLC paclitaxel sensitivity.
Expression of miR-30a-5p correlates with DFS and RR in paclitaxel-treated lung cancer patients. a Lung cancer patients who received paclitaxel-based chemotherapy were divided into two groups based on low or high miR-30a-5p expression levels. Kaplan-Meier survival curves and log-rank tests were used to compare the DFS between the two groups. b Patients who did not receive paclitaxel-based chemotherapy were analyzed as in a. c Expression of miR-30a-5p in patients responding to paclitaxel (n = 27) and non-responding (n = 36) to paclitaxel was compared using the two-tailed Student’s t test. U6 small nuclear RNA was used as an internal control. d Correlation between miR-30a-5p and BCL-2 expression in NSCLC. The patients were divided into three groups based on BCL-2 expression scores in the tumors, representing low (scores 0–3), medium (scores 4–8), and high (scores 9–12) expression of BCL-2. Data were analyzed by one-way analysis of variance (ANOVA) test with Games-Howell’s correction
miR-30a-5p expression negatively correlates with expression of BCL-2 in lung cancer patients
To understand the correlation of miR-30a-5p expression with BCL2, we divided NSCLC samples into three groups on the basis of BCL2 amounts defined by their expression scores and examined the differences in miR-30a-5p expression among the three groups. The expression levels of miR-30a-5p in tumors with high BCL-2 expression were lower than those in tumors with low BCL-2 expression (Fig. 6d), indicating that miR-30a-5p expression negatively correlates with BCL-2 expression in NSCLC.
Discussion
Tumor chemoresistance is the major cause of treatment failure in clinic [26]. Although recent data indicate that aberrant miRNA expression is closely linked to chemoresistance by targeting genes related to chemosensitivity [27] or chemoresistance [28, 29], the specific chemoresistance-related miRNAs are largely unknown. More research is needed to identify miRNAs associated with chemoresistance and the mechanisms of how they cause chemoresistance. In our present work, we utilized miRNA microarray assay to analyze the miRNA expression in paclitaxel-resistant and paclitaxel-sensitive NSCLC cells. In total, 208 miRNAs had altered expression between the cell lines; several of which (e.g., miR-125a and miR-375) have been reported to associate with paclitaxel resistance in various cancers [27, 28]. Importantly, we showed that miR-30a-5p was the most significantly downregulated miRNA in the resistant cells. To the best of our knowledge, miR-30a-5p is firstly reported to associate with paclitaxel resistance in NSCLC.
In solid tumors, miR-30a-5p has been identified as a tumor suppressor that inhibits tumorigenesis and cancer progression. In lung cancer, miR-30a-5p has been demonstrated to be downregulated in tumor tissues and downregulation of miR-30a-5p is associated with poor prognosis. miR-30a-5p inhibits lung cancer cell growth and migration by suppressing EYA2 [30]. miR-30a-5p suppresses NSCLC progression through AKT signaling pathway by targeting IGF1R [31]. The anti-tumor functions were also confirmed in other types of tumors, such as breast cancer [18], pancreatic cancer [19], and liver cancer [32]. In addition, miR-30a-5p has been associated with many diseases, including osteoarthritis [33], neuron-related disorders [34], mycobacteria tuberculosis [35], pulmonary vascular hyperpermeability [36], and autoimmune diseases [37]. Recent data indicate that miR-30a-5p upregulation sensitizes cisplatin sensitivity in cultured gastric cancer cells, although its clinical significance remains unknown [38]. We demonstrated that miR-30a-5p overexpression increases the sensitivity of NSCLC cells to paclitaxel and knockdown of miR-30a-5p in NSCLC cells results in resistance to paclitaxel. Moreover, we showed that high miR-30a-5p predicts good paclitaxel response in NSCLC patients. It should also be noted that tumor samples were collected before treatment with paclitaxel started. Patient chemotherapy strictly followed the NCCN (National Comprehensive Cancer Network®) Guideline. Briefly, among the NSCLC patients, those intolerant with cisplatin therapy were selected to undergo paclitaxel treatment. The paclitaxel therapy continued 4–6 periods. Therefore, the analysis of the patient’s data might have some limitations since the way of patient selection might be a possible confounding factor.
BCL-2, the founding member of the Bcl-2 family that regulates cell death by inhibiting apoptosis, has been demonstrated to contribute to tumorigenesis and chemoresistance [39]. In this study, we show for the first time that miR-30a-5p can directly bind to the BCL-2 3′-UTR and suppress its expression. Overexpression of miR-30a-5p enhances apoptosis through regulation of apoptosis-related proteins such as BCL-2. Furthermore, re-expression of BCL-2 reverses the function of miR-30a-5p. Overexpression of miR-30a-5p and treatment with paclitaxel in NSCLC cells synergistically induce apoptosis. These data suggest that miR-30a-5p contributes to chemosensitivity by inhibiting BCL-2 expression in NSCLC. Therefore, re-expression of miR-30a-5p or BCL-2 inhibition may be beneficial for the treatment of paclitaxel-resistant NSCLC.
In conclusion, paclitaxel-resistant NSCLC cells have reduced miR-30a-5p expression. miR-30a-5p negatively regulates paclitaxel resistance in NSCLC by reducing BCL-2 expression, thus promoting apoptosis. miR-30a-5p upregulation or BCL-2 inhibition in combination with paclitaxel may be useful for treating chemoresistant NSCLC.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29
Chang AY, Kim K, Glick J, Anderson T, Karp D, Johnson D (1993) Phase II study of taxol, merbarone, and piroxantrone in stage IV non-small-cell lung cancer: the eastern cooperative oncology group results. J Natl Cancer Inst 85:388–394
Murphy WK, Fossella FV, Winn RJ, Shin DM, Hynes HE, Gross HM, Davilla E, Leimert J, Dhingra H, Raber MN et al (1993) Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer. J Natl Cancer Inst 85:384–388
Zheng H, Liu Z, Liu T, Cai Y, Wang Y, Lin S, Chen J, Wang J, Wang Z, Jiang B (2014) Fas signaling promotes chemoresistance in gastrointestinal cancer by up-regulating P-glycoprotein. Oncotarget 5:10763–10777
Vargas JR, Stanzl EG, Teng NN, Wender PA (2014) Cell-penetrating, guanidinium-rich molecular transporters for overcoming efflux-mediated multidrug resistance. Mol Pharm 11:2553–2565
Goncalves A, Braguer D, Kamath K, Martello L, Briand C, Horwitz S, Wilson L, Jordan MA (2001) Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc Natl Acad Sci U S A 98:11737–11742
He W, Zhang D, Jiang J, Liu P, Wu C (2014) The relationships between the chemosensitivity of human gastric cancer to paclitaxel and the expressions of class III β-tubulin, MAPT, and survivin. Med Oncol 31:950
Kavallaris M (2010) Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer 10:194–204
Ingemarsdotter CK, Tookman LA, Browne A, Pirlo K, Cutts R, Chelela C, Khurrum KF, Leung EY, Dowson S, Webber L et al (2015) Paclitaxel resistance increases oncolytic adenovirus efficacy via upregulated CAR expression and dysfunctional cell cycle control. Mol Oncol 9:791–805
Choi JH, Sheu JJ, Guan B, Jinawath N, Markowski P, Wang TL, Shih IM (2009) Functional analysis of 11q13.5 amplicon identifies Rsf-1 (HBXAP) as a gene involved in paclitaxelresistance in ovarian cancer. Cancer Res 69:1407–1415
Le XF, Bast RC Jr (2011) Src family kinases and paclitaxel sensitivity. Cancer Biol Ther 12:260–269
Peng X, Gong F, Chen Y, Jiang Y, Liu J, Yu M, Zhang S, Wang M, Xiao G, Liao H (2014) Autophagy promotes paclitaxel resistance of lung cancer cells: involvement of Warburg effect activated hypoxia-induced factor 1-α-mediated signaling. Cell Death Dis 5:e1367
Chatterjee A, Chattopadhyay D, Chakrabarti G (2015) MiR-16 targets Bcl-2 in paclitaxel-resistant lung cancer cells and overexpression of miR-16 along with miR-17 causes unprecedented sensitivity by simultaneously modulating autophagy and apoptosis. Cell Signal 27:189–203
Chatterjee A, Chattopadhyay D (2014) Chakrabarti G (2014) miR-17-5p downregulation contributes to paclitaxel resistance of lung cancer cells through altering beclin1 expression. PLoS One 9:e95716
Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ (2006) miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34(Database issue):D140–D144
Kanakkanthara A, Miller JH (2013) MicroRNAs: novel mediators of resistance to microtubule-targeting agents. Cancer Treat Rev 39:161–170
Fu J, Xu X, Kang L, Zhou L, Wang S, Lu J, Cheng L, Fan Z, Yuan B, Tian P et al (2014) miR-30a suppresses lung cancer cell proliferation and migration by targeting Eya2. Biochem Biophys Res Commun 445:314–319
Tsukasa K, Ding Q, Miyazaki Y, Matsubara S, Natsugoe S, Takao S (2016) miR-30 family promotes migratory and invasive abilities in CD133(+) pancreatic cancer stem-like cells. Hum Cell 29:130–137
Sestito R, Cianfrocca R, Rosanò L, Tocci P, Semprucci E, Di Castro V, Caprara V, Ferrandina G, Sacconi A, Blandino G, Bagnato A (2016) miR-30a inhibits endothelin A receptor and chemoresistance in ovarian carcinoma. Oncotarget 7:4009–4023
Xu X, Fan Z, Kang L, Han J, Jiang C, Zheng X, Zhu Z, Jiao H, Lin J, Jiang K et al (2013) Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis. J Clin Invest 123:630–645
Ji Q, Xu X, Xu Y, Fan Z, Kang L, Li L, Liang Y, Guo J, Hong T, Li Z et al (2016) miR-105/Runx2 axis mediates FGF2-induced ADAMTS expression in osteoarthritis cartilage. J Mol Med 94:681–694
Lee JH, Kim C, Sethi G, Ahn KS (2015) Brassinin inhibits BCL-2 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 6:6386–6405
Al-Dimassi S, Abou-Antoun T, El-Sibai M (2014) Cancer cell resistance mechanisms: a mini review. Clin Transl Oncol 16:511–516
Shajahan AN, Dobbin ZC, Hickman FE, Dakshanamurthy S, Clarke R (2012) Tyrosine-phosphorylated caveolin-1 (Tyr-14) increases sensitivity to paclitaxel by inhibiting BCL2 and BCLxL proteins via c-Jun N-terminal kinase (JNK). J Biol Chem 287:17682–17692
Periti P, Mini E (1989) Drug resistance in cancer: an overview of the clinical aspects. J Chemother 1:5–9
Fan Z, Cui H, Yu H, Ji Q, Kang L, Han B, Wang J, Dong Q, Li Y, Yan Z et al (2016) MiR-125a promotes paclitaxel sensitivity in cervical cancer through altering STAT3 expression. Oncogene 5:e223
Shen Y, Wang P, Li Y, Ye F, Wang F, Wan X, Cheng X, Lu W, Xie X (2013) miR-375 is upregulated in acquired paclitaxel resistance in lung cancer. Br J Cancer 109:92–99
Li B, Ren S, Li X, Wang Y, Garfield D, Zhou S, Chen X, Su C, Chen M, Kuang P et al (2014) MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer 83:146–153
Yuan Y, Zheng S, Li Q, Xiang X, Gao T, Ran P, Sun L, Huang Q, Xie F, Du J, Xiao C (2016) Overexpression of miR-30a in lung adenocarcinoma A549 cell line inhibits migration and invasion via targeting EYA2. Acta Biochim Biophys Sin 48:220–228
Wen XP, Ma HL, Zhao LY, Zhang W, Dang CX (2015) MiR-30a suppresses non-small cell lung cancer progression through AKT signaling pathway by targeting IGF1R. Cell Mol Biol (Noisy-le-grand) 61:78–85
Li WF, Dai H, Ou Q, Zuo GQ, Liu CA (2016) Overexpression of microRNA-30a-5p inhibits liver cancer cell proliferation and induces apoptosis by targeting MTDH/PTEN/AKT pathway. Tumour Biol 37:5885–5895
Ji Q, Xu X, Zhang Q, Kang L, Xu Y, Zhang K, Li L, Liang Y, Hong T, Ye Q, Wang Y (2016) The IL-1β/AP-1/miR-30a/ADAMTS-5 axis regulates cartilage matrix degradation in human osteoarthritis. J Mol Med 94:771–785
Wang P, Zhang N, Liang J, Li J, Han S, Li J (2015) Micro-RNA-30a regulates ischemia-induced cell death by targeting heat shock protein HSPA5 in primary cultured cortical neurons and mouse brain after stroke. J Neurosci Res 93:1756–1768
Chen Z, Wang T, Liu Z, Zhang G, Wang J, Feng S, Liang J (2015) Inhibition of autophagy by MiR-30A induced by mycobacteria tuberculosis as a possible mechanism of immune escape in human macrophages. Jpn J Infect Dis 68:420–424
Qi F, He T, Jia L, Song N, Guo L, Ma X, Wang C, Xu M, Fu Y, Li L, Luo Y (2015) The miR-30 family inhibits pulmonary vascular hyperpermeability in the premetastatic phase by direct targeting of Skp2. Clin Cancer Res 21:3071–3080
Wan Q, Zhou Z, Ding S, He J (2015) The miR-30a negatively regulates IL-17-mediated signal transduction by targeting Traf3ip2. J Interf Cytokine Res 35:917–923
Wang LL, Zhang XH, Zhang X, Chu JK (2016) MiR-30a increases cisplatin sensitivity of gastric cancer cells through suppressing epithelial-to-mesenchymal transition (EMT). Eur Rev Med Pharmacol Sci 20:1733–1739
Hata AN, Engelman JA, Faber AC (2015) The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov 5:475–487
Acknowledgements
This work was supported by the National Natural Science Foundation (81330053, 81372161, 81472589, 81630067, 81672602, 81502264, and 81572597) and Beijing Nova Program (Z141102001814055). Beijing Institute of Biotechnology and PLA General Hospital contribute to this work equally.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Electronic supplementary material
ESM 1
(PDF 1023 kb).
Rights and permissions
About this article
Cite this article
Xu, X., Jin, S., Ma, Y. et al. miR-30a-5p enhances paclitaxel sensitivity in non-small cell lung cancer through targeting BCL-2 expression. J Mol Med 95, 861–871 (2017). https://doi.org/10.1007/s00109-017-1539-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1539-z