Abstract
Galectin-3, a member of the β-galactoside-binding lectin family, expresses in many different immune cells and modulates broad biological functions including cell adhesion, cell activation, cell growth, apoptosis, and inflammation. However, the role of galectin-3 in mucosal immunity or inflammatory bowel diseases is still not clear. We demonstrate here that galectin-3 knockout mice have more severe disease activity in the dextran sulfate sodium (DSS)-induced colitis model, indicating that galectin-3 may protect from inflammation in DSS-induced colitis. Furthermore, treating with galectin-3 reduced body weight loss, shortened colonic length, and ameliorated mucosal inflammation in mice having DSS-induced colitis. However, the protective effects of galectin-3 were eliminated by the administration of anti-CD25 mAb. In addition, primary T cells treated with galectin-3 ex vivo induced the expression of FOXP3, ICOS, and PD-1 with a Treg cell phenotype having a suppression function. Moreover, adoptive transfer of galectin-3-treated T cells reduced bowel inflammation and colitis in the T cell transfer colitis model. In conclusion, our results indicate that galectin-3 inhibited colonic mucosa inflammation and reduced disease severity by inducing regulatory T cells, suggesting that it is a potential therapeutic approach in inflammatory bowel disease.
Key messages
-
Galectin-3 offers protection from inflammation in experimental colitis.
-
Galectin-3 knockout mice have more severe disease activity in DSS-induced colitis.
-
Adoptive transfer of galectin-3-treated T cells reduced bowel inflammation.
-
Galectin-3 inhibited colonic mucosa inflammation by inducing regulatory T cells.
-
Galectin-3 is a potential therapeutic approach in inflammatory bowel disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Galectins are lectins that bind to beta-galactosides in free form or to glycolipids and are expressed in many different immune cells. Galectins interact with cell-surface and extracellular matrix glycoconjugates (glycoproteins and glycolipids) through lectin-carbohydrate interactions. Through this action, they can promote cell growth, affect cell survival, modulate cell adhesion, and induce cell migration. Galectin-3 is the sole member of the chimera type of galectin, which contains a single C-terminal carbohydrate recognition domain (CRD) and an extra-long, flexible N-terminal domain responsible for its intracellular function. Galectin-3 modulates a variety of biological functions, including cell adhesion, cell activation, cell growth, and apoptosis [1]. It also participates in different stages of inflammation and plays multiple other roles, such as being a mediator of phagocytosis by macrophages, T cell apoptosis inductor, and TCR signal down-regulator [2–4]. Galectin-3 is generally viewed as pro-inflammatory in many experimental animal models, such as experimental autoimmune encephalitis (EAE) [5] and collagen-induced arthritis (CIA) [6], while it is immune-modulatory when T cell apoptosis induction or TCR signal regulation is considered. In addition, galectin-3 is critical for developing the allergic inflammatory response in a mouse model of atopic dermatitis [7]. It has also been recently revealed that galectin-3 suppresses Th17 responses by regulating DC cytokine production in fungal infection [8].
The roles of galectin-3 in the experimental colitis model are still unclear. Immune cells, mucosal epithelia, and intestinal microbiota all contribute to the pathogenesis of inflammatory bowel diseases [9, 10]. Serum galectin-3 levels are elevated in patients with ulcerative colitis and Crohn’s disease [11]. Conversely, galectin-3 expression is reduced in the inflamed intestinal epithelium in inflammatory bowel disease (IBD) and is suppressed by TNF-α [12, 13]. However, the differential expression of galectin-3 as a cause or result of bowel inflammation is still not clear. Galectins can modify T cell functions, which are pivotal in the immune system. T cells densely infiltrate the mucosa in IBD, and the presence of T cells in various animal colitis models implicates that they have an essential role in disease pathogenesis [14–18]. Recent IBD studies demonstrate that Th17 and Treg cells are crucial in the pathogenesis of IBD [15, 17, 18], either directly or through their influence on other T cell subsets [19, 20]. However, the effects of galectins on the differentiation of T cell subsets and their interactions in IBD are still unknown. Deeper insights into the interactions between glycoproteins in inflammatory conditions will contribute toward advancing our understanding of the mechanisms that regulate mucosal inflammation in the gut, promoting the design of novel therapeutic approaches for IBD.
In this study, we investigate the effects of galectin-3 in gut mucosa inflammation to further explore its therapeutic potential in IBD. We demonstrate that galectin-3 suppressed mucosa inflammation in dextran sulfate sodium (DSS)-induced colitis and the severe combined immunodeficiency (SCID) T cell transfer model of colitis, both of which have many features in common with human IBDs [21–23]. Our results indicate that galectin-3 inhibited colonic mucosa inflammation and reduced disease severity by inducing regulatory T cells, implying that it has therapeutic potential in human IBDs.
Materials and methods
Mice
C57BL/6 mice were purchased from the Animal Center of National Taiwan University and National Laboratory Animal Center (Taiwan). C.B-17 SCID mice were purchased from the Animal Center of National Taiwan University. Galectin-3−/− mice (in C57BL/6 background ) were provided by Dr. Fu-Tong Liu, which were generated as previously described [24]. All mice were maintained in a specifically pathogen-free environment in the animal facility at the National Taiwan University. All experimental conductions were approved by the Animal Study Committee of the College of Medicine, National Taiwan University. Female mice aged 5 to10 weeks were used in all experiments.
Preparation and purification of recombinant human galectin-3
The human galectin-3 expression vector was obtained from Dr. Fu-Tong Liu (Institute of Biomedical Sciences, Academia Sinica, Taiwan). For a detailed description of pEF1-Gal3 (pGal3) preparation, see [25]. Briefly, galectin-3 cDNA was excised using EcoRI from clone 2.2 [26] and cloned into pEF1-neo, which was prepared by replacing the CMV promoter in the pIRES1-neo bicistronic vector (Clontech, Palo Alto, CA). Truncated galectin-3 clones were prepared using PCR to amplify full-length galectin-3 (nt 1–750) and its N1 (nt 1–336), N2 (nt 1–399), and C fragments (nt 333–750) and then cloning these into pEGFP-N1 (Clontech, GenBank accession no.U55762). The pEF1-Gal3 (pGal3) plasmid was then transformed into Escherichia coli BL21. When E. coli were grown to an absorbance of 0.6~0.8, 1 mM isopropyl 1-thio-β-D-galactopyranoside (IPTG) (Biosynth, Staad, Switzerland) was used to induce galectin-3 expression. Pellets of E. coli were collected and lysed in 20 ml wash buffer containing 1 mg/ml lysozyme (Sigma, St. Louis, MO, USA), 5 μg/ml DNase, 5 μg/ml RNase, and 1 % Triton X-100 (USB Corporation, Cleveland, OH, USA). Recombinant human galectin-3 was produced in E. coli and purified by affinity chromatography on lactosyl-agarose as described previously [27], followed by extensive dialysis against phosphate-buffered saline (PBS) pH 7.4. Galectin-3 was then concentrated by ultradiafiltration using Amicon Ultra-15 centrifugal filters (Millipore, Bedford, MA). Galectin-3 concentration was determined by using the Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Hercules, CA), separated into aliquots and stored at −20 °C until use.
Isolation of human and mouse T cells
Human peripheral venous blood was obtained from study subjects, and peripheral blood mononuclear cells (PBMC) were isolated with Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden). Human T cells or CD4+ T cells were separated in a RosetteSep® Human T Cell Enrichment Cocktail (STEMCELL Technologies, Vancouver, BC, Canada) or RosetteSep® Human CD4+ T Cell Enrichment Cocktail (STEMCELL Technologies, Vancouver, BC, Canada). Furthermore, human CD4+CD25+ regulatory T cells were purified from enriched CD4+ T cells using an EasySep® Human CD25 Positive Selection Kit (STEMCELL Technologies, Vancouver, BC, Canada).
Single-cell suspensions of splenocytes from donor mice were subjected to negative selection of CD4+ T cells using an EasySep® Mouse CD4+ T Cell Enrichment Kit (Vancouver, BC, Canada), and CD4+CD25− T cells were then separated with an EasySep® Mouse CD25 Positive Enrichment Kit (STEMCELL Technologies, Vancouver, BC, Canada).
T cell proliferation suppression assay
At first, human primary CD4+CD25+ T cells or CD4+CD25− T cells were isolated and cultured at 105 cells/well (100 μl/well) with 5 μM recombinant galectin-3 in 96-well plates for 2 days. Purified CD4+CD25− T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen, Paisley, UK) as responders and then co-cultured with 106 cells/well irradiated PBMCs and galectin-3-treated CD4+CD25+ T cells or CD4+CD25− T cells for 5 days in a 96-well round-bottom culture cluster precoated with 8 μg/ml of anti-CD3 (eBioscience, San Diego, CA) and 3 μg/ml of anti-CD28 (Beckman Coulter, Fullerton, CA, USA). The suppressive function of different T cell preparations was analyzed according to CFSE dilution patterns by flow cytometry.
Flow cytometric analysis of surface marker staining
Human CD4+ T cells treated with various concentrations of recombinant galectin-3 for 2 days were resuspended in FACS staining buffer (2 × 105 cells/100 μl) and stained with the following antibodies at 4 °C for 30 min: fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD4 (BD Pharmingen, San Diego, CA), PE-conjugated anti-CD25, and FITC-conjugated anti-ICOS (eBioscience, San Diego, CA). After washing with FACS staining buffer, cells were resuspended in 500 μl of FACS staining buffer and analyzed by FACSCanto (BD Biosciences, San Diego, CA) and FACS Diva software.
For PD-1 and ICOS staining, human primary CD4+ T cells were cultured for 2 days with plate-bound 5 μg/ml of anti-CD3 (eBioscience, San Diego, CA) and 2 μg/ml of anti-CD28 (Beckman Coulter, Brea, CA). PE-conjugated anti-ICOS and PE-conjugated anti-PD-1 (eBioscience, San Diego, CA) antibodies were used to analyze the expression of PD-1 and ICOS following the method described above.
Mouse primary cells (2 × 105 cells/100 μl) isolated from the spleen or mesenteric lymph nodes were stained at 4 °C for 30 min with the following antibodies: PE- or FITC-conjugated anti-CD4, PerCP-Cy5-conjugated anti-CD25, and FITC-conjugated anti-PD-1 (eBioscience, San Diego, CA), followed by washing with 1 ml FACS staining buffer and resuspended in 500 μl FACS staining buffer. All cells were analyzed by FACSCanto (BD Biosciences, San Diego, CA) and FACS Diva software.
Flow cytometric analysis-intracellular staining
Human primary T cells treated with or without recombinant galectin-3 and mouse primary cells extracted from spleens or mesenteric lymph nodes were stained with FITC- or PE-conjugated anti-human FOXP3 and APC-conjugated anti-mouse FOXP3 (eBioscience, San Diego, CA). A prior membrane permeabilization step was performed by using a cell fixation/cell permeabilization kit (eBioscience, San Diego, CA) according to manufacturer protocols.
DSS-induced colitis model
To induce acute experimental colitis, C57BL/6 mice (5–8 weeks old) were treated with 2.5 % DSS (36–50 kDa; MP Biomedicals, Aurora, OH) in filter-purified and sterilized drinking water for 5 days, followed by 2 days of water. The amount of DSS water consumed was recorded for all treatment groups to ensure that all are consuming the same amount. Control mice were given normal drinking water. Mice were monitored for weight loss and clinical manifestations (rectal bleeding and diarrhea) every day. On the seventh day, mice were humanely euthanized and their colons removed followed by weight and length measurement and histological examination. Colonic tissues were fixed in 10 % (w/v) paraformaldehyde (Merck, Darmstadt, Germany), and serial paraffin sections were stained with hematoxylin and eosin (H&E). Histological features, including cellular infiltrates, goblet cell loss, and increased epithelial proliferation, were evaluated. H&E-stained sections were scored according to a previously described scoring system [28] by a blinded observer. A cumulative scale with a maximum score of 10 was used. Three parameters were assessed: (1) severity of inflammation (0, none; 1, slight; 2, moderate; and 3, severe); (2) depth of injury (0, none; 1, mucosal; 2, mucosal and submucosal; and 3, transmural); and (3) crypt damage (0, none; 1, basal one-third damaged; 2, basal two-thirds damaged; 3, only surface epithelium intact; and 4, complete loss of crypt and epithelium).To investigate the effects of galectin-3 treatment in vivo, mice were given DSS solution instead of drinking water and daily intraperitoneal injections of 20 μg of galectin-3.
T cell transfer colitis model
Mouse CD4+CD25− T cells were purified from spleens and cultured with or without recombinant galectin-3 (2.5 μM) for 2 days. Then, 3–5 × 105 cells (in 200 μl PBS) were adoptively transferred into C.B-17 SCID mice following a previously described method [29]. Body weight loss and clinical symptoms were recorded until the weight loss of any one of the experimental groups reached 20 %. At the end point, mice were humanely euthanized and their colons removed. Serial paraffin sections were prepared for histological analysis, followed by H&E staining.
Statistical analysis
All data are expressed as the mean ± SD. Statistical significance was determined by the Student t test for unpaired samples. For the analysis of knockout mice data, Mann-Whitney U test was performed to assess differences between wild-type and knockout mice group. P values are expressed as *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Enhanced mucosal inflammation and disease severity in DSS-induced colitis in galectin-3 knockout mice
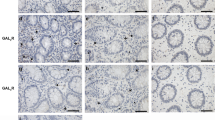
In order to explore the possible role of galectin-3 in IBD, we first studied experimental colitis in galectin-3 knockout mice. Acute experimental colitis was induced by 2.5 % DSS oral solution in wild-type C57BL/6 and galectin-3−/− [GAL-3 knockout (KO)] mice. This demonstrated that body weights in wild-type and galectin-3−/− mice were similar to control groups (Fig. 1); however, the body weights in DSS-treated galectin-3−/− mice were significantly lower than in DSS-treated wild-type mice (Fig. 1). Furthermore, the colon lengths of DSS-treated galectin-3−/− mice were also significantly shorter than in DSS-treated wild-type mice. The histology of wild-type and galectin-3−/− mice treated with DSS showed epithelial damage, goblet cell loss, and dense cellular infiltrates, and more cellular infiltration and inflammation was present in galectin-3−/− mice when compared to wild-type mice. According to body weight, colon length, and histological changes, this demonstrated more severe intestinal inflammation and disease severity in galectin-3 KO mice treated with DSS.
Increased mucosal inflammation and disease severity in DSS-induced colitis in galectin-3 knockout mice. a Body weight change (% original body weight) in galectin-3 knockout (GAL-3 KO) and wild-type (WT) mice treated with DSS (mean ± SD; n = 10 for each group; *p < 0.05 when compared to the body weight changes in WT and GAL-3 KO mice treated with DSS). b Comparison of colon length in WT and GAL-3 KO mice treated with DSS. Data represent mean ± SD; n = 10 for each group; *p < 0.05. c Comparison of colon weight/length ratio in WT and GAL-3 KO mice treated with DSS. Data represent mean ± SD; *p < 0.05. d Histopathology of colon mucosa in DSS-induced colitis (distal part of colon). Colonic tissues were fixed and serial paraffin sections were stained with H&E (X100). Epithelial damage, goblet cell loss, and cell infiltration were detected in WT and GAL-3 KO mice after DSS treatment. e Histological injury score (see “Materials and methods” section) for sections of colons isolated from WT and GAL-3 KO mice treated with DSS. Data represent mean ± SD; n = 10 for each group; *p < 0.05
Recombinant galectin-3 reduces the inflammation and disease severity of DSS-induced colitis
To investigate the therapeutic potential of galectin-3 in IBD, 20 μg of soluble recombinant galectin-3 proteins were injected intraperitoneally every day in mice treated with DSS. The results in Fig. 2 demonstrated that treatment with galectin-3 protein resulted in less weight loss and longer colon length in galectin-3-treated mice. Histological analysis by H&E staining showed much less inflammatory cellular infiltration and mucosa damage in galectin-3-treated mice when compared to PBS-treated controls. This indicated that recombinant galectin-3 protein reduced colonic inflammation and ameliorated disease severity in DSS-induced colitis. Taken together, our results indicate that treatment with galectin-3 reduced the inflammation and severity of DSS-induced colitis.
Treatment with galectin-3 reduced DSS-induced colitis inflammation and disease severity. Mice were administered with DSS in the drinking water to induce colitis. Twenty micrograms of soluble recombinant galectin-3 proteins or PBS was injected intraperitoneally every day in mice treated with DSS. a Body weights (% of original body weight) of mice treated with DSS were measured (mean ± SD; n = 16 for each group). Significant weight differences were found on the sixth and seventh days between the galectin-3-treated and PBS group (*p < 0.05; **p < 0.01). b Comparison of colon length in mice treated with DSS in the presence or absence of galectin-3. Data represent mean ± SD; n = 16 for each group; **p < 0.01. c Comparison of colon weight/length ratio in mice treated with DSS in the presence or absence of galectin-3. Data represent mean ± SD; *p < 0.05. d Histopathology of colon mucosa in DSS-induced colitis (distal part of colon). Colonic tissues were fixed and serial paraffin sections were stained with H&E (X100). e Histological injury score for sections of colons isolated from mice. Data represent mean ± SD; n = 16 for each group; **p < 0.01
Galectin-3 induces FOXP3, ICOS, and PD-1 expression in primary human T cells ex vivo
In IBDs, there is an excessive and uncontrolled immune response through the activation of CD4+ T cells. Dense T cells infiltrate the mucosa in IBD, and the presence of T cells in various animal colitis models implies an essential role for T cells in disease pathogenesis [14–18]. To test the possible effects of galectin-3 on human T cells, primary human CD4+ T cells (2 × 105/well) were cultured with 1.25, 2.5, and 5 μM of exogenous galecin-3 in the presence or absence of lactose for 2 days. This significantly increased the expression of FOXP3, ICOS, and PD-1 on CD4+ T cells in a dose-dependent manner (Fig. 3), and this effect was eliminated in the presence of lactose, which interrupted the interaction between galectin-3 and its ligands. These results indicate that treatment with galectin-3 induced primary T cells with markers of regulatory T cells (Treg).
Galectin-3 induced FOXP3, ICOS, and PD-1 expression in primary human T cells. a Human primary CD4+ T cells (2 × 105/well) were cultured with 1.25, 2.5, and 5 μM of recombinant galectin-3 in the presence or absence of lactose for 2 days. PE-conjugated anti-ICOS and PE-conjugated anti-PD-1 (eBioscience, San Diego, CA, USA) antibodies were used to analyze the expression of PD-1 and ICOS. FOXP3 was intracellularly stained with fluorescence-conjugated anti-FOXP3 mAb and analyzed by flow cytometry. Results are representative of three experiments. b Cell surface marker expression percentages of PD-1, ICOS, and FOXP3 in primary human T cells after treatment with galectin-3 in the presence or absence of lactose (mean ± SD; n = 5; *p < 0.05; **p < 0.01)
To further investigate whether these galectin-3-induced FOXP3+ T cells have T cell suppression activity, primary human CD4+CD25− T cells were isolated from PBMCs and treated with galectin-3 for 2 days and co-cultured with CFSE-labeled CD4+CD25− responder T cells, which were activated by anti-CD3 and anti-CD28 mAb. The CFSE dilutions in responder T cells were further analyzed by flow cytometry. The results in Fig. 4 demonstrated that galectin-3-treated CD4+CD25− cells suppressed proliferation of responder T cells in a dose-dependent manner (Fig. 4). In contrast, primary human CD4+CD25− T cells without galectin-3 treatment were not able to suppress T cell proliferation, indicating that galectin-3 induces Treg cells in primary T cells.
Galectin-3 treated CD4+CD25− primary T cells suppressed the T cell proliferation response induced by anti-CD3/anti-CD28 stimulation. a CD4+CD25− T cells were isolated from PBMCs and treated with galectin-3 (5 μM) for 2 days. Purified CD4+CD25− T cells were labeled with CFSE as responders and then co-cultured with 106 cells/well of irradiated PBMCs and galectin-3-treated CD4+CD25+ T cells or untreated CD4+CD25− T cells for 5 days in 96-well round-bottom culture clusters precoated with 8 μg/ml of anti-CD3 and 3 μg/ml of anti-CD28. The suppressive function of different T cell preparations was analyzed according to CFSE dilution patterns by flow cytometry. Results are representative of three independent experiments. b, c Percentage of proliferative responder T cells cultured with galectin-3-treated CD4+CD25+ T cells or untreated CD4+CD25− T cells (mean ± SD; *p < 0.05; **p < 0.01)
Depletion of CD25+ T cells by anti-CD25 mAb neutralizes the protective effect of galectin-3 in DSS-induced colitis
Our results indicate that treatment with galectin-3 reduces the inflammation and disease severity of DSS-induced colitis, suggesting that this effect could be due to the induction of Treg cells, leading to the suppression of colonic mucosa inflammation. We further investigated whether the protective effects of galectin-3 on DSS-induced colitis were due to the induction of Treg cells by depleting Treg cells with anti-CD25 mAb. DSS solution was given to C57BL/6 mice to induce colitis, and 20 μg of soluble recombinant galectin-3 was given intraperitoneally every day. Anti-CD25 mAb (eBioscience, San Diego, CA) was administered intraperitoneally on the second day of colitis induction in the presence or absence of galectin-3. The mouse Ig serves as a control for nonspecific Ab. The results in Fig. 5 demonstrated that treatment with anti-CD25 mAb eliminated the protective effect of galectin-3 on body weight loss in the DSS colitis model. Furthermore, after colitis was induced, colon length was significantly shorter in mice treated with galectin-3 and anti-CD25 than in mice treated with galectin-3 and mouse Ig. A histological examination of DSS-induced colitis mice revealed that more inflammatory cellular infiltration and histological injury score occurred in the anti-CD25 mAb treated group when compared to controls and the galectin-3-treated group. Taken together, these results indicate that the protective effect of galectin-3 on DSS-induced colitis was dependent on CD25+ T cells.
Depletion of CD25+ T cells by anti-CD25 mAb eliminates the protective effect of galectin-3 in DSS-induced colitis. Mice were administered with DSS in the drinking water to induce colitis. Soluble recombinant galectin-3 (20 μg) was given intraperitoneally every day. Anti-CD25 mAb (eBioscience, San Diego, CA) or mouse Ig (Southern Biotech, Birmingham, AL) was administered intraperitoneally on the second day of colitis induction in the presence or absence of galectin-3. a Body weight (%) over time in the DSS-induced colitis model (mean ± SD; n = 15 for each group; *p < 0.05; **p < 0.01 when compared to the body weight changes in mice treated with recombinant galectin-3 in the presence or absence of anti-CD25 mAb). b Comparison of colon length in DSS-induced mice colitis treated with recombinant galectin-3 in the presence of anti-CD25 mAb or mouse Ig. Mice were sacrificed and the colon lengths and weights were measured. Data represent mean ± SD; n = 15 for each group; **p < 0.01. c Comparison of colon weight/length ratio in DSS-induced mice colitis treated with recombinant galectin-3 in the presence of anti-CD25 mAb or mouse Ig. Data represent mean ± SD; *p < 0.05. d Histological injury score for sections of colons isolated from mice (distal part of colon). Data represent mean ± SD; n = 15 for each group; **p < 0.01
Galectin-3-treated T cells reverse the pathogenic effects induced by the transfer of CD4+CD25− T cells in the T cell transfer colitis model
To further confirm the immune modulation effects of galectin-3 on T cells and colitis, we used the T cell transfer colitis model to evaluate the protective effects of galectin-3 on the development of colitis. Colitis was induced by the transfer of CD4+CD25− T cells (5 × 105 cells) into SCID mice (6–8 weeks old). SCID mice receiving CD4+CD25− T cells with pretreatment by galectin-3 (5 μM) ex vivo suffered lower body weight losses in comparison to controls (Fig. 6). Colon shortening by the sixth week was also lower in the galectin-3 treatment group. Histology examination revealed less inflammation and histological injury score in the galectin-3 treatment group when compared to mice with CD4+CD25− T cells transfers without galectin-3 pretreatment. We further investigated the effects of galectin-3-treated CD4+CD25− T cells on the SCID T cell transfer colitis model, finding that T cells from spleens and mesenteric lymph nodes had a significantly higher expression of FOXP3 and upregulation of PD-1 on the cell surface in mesenteric lymph node T cells (Fig. 6). Taken together, our results indicate that galectin-3 induced Treg-like cells, which suppressed colon mucosal inflammation and reduced disease severity.
Galectin-3-treated T cells reverse the pathogenic effects induced by the T cell transfer colitis model. Colitis was induced by reconstitution of SCID mice with CD4+CD25− T cells or galectin-3-treated CD4+CD25− T cells (5 × 105 cells). In the experimental group, CD4+CD25− T cells were treated with galectin-3 (5 μM) in vitro for 2 days before transfer. PBS (200 μl) was administrated as the control. a Body weight (%) over time (6 weeks) is expressed as a percentage of the original weight at the beginning of the experiment (mean ± SD; n = 12 in each group; *p < 0.05 when compared to the body weight changes in mice administrated with CD4+CD25− T cells and galectin-3-treated CD4+CD25− T cells). b Mice were sacrificed and the colon lengths were measured. Data represent the mean value ± SD; n = 12 for each group; *p < 0.05. c Histological injury score for sections of colons isolated from mice (distal part of colon). Data represent the mean value ± SD; n = 12 for each group; *p < 0.05. d PD-1 and FOXP3 expression in CD4+ T cells from spleen and mesentery lymph nodes (mLNs) were examined by flow cytometry. Results are representative of three independent experiments
Discussion
In this study, we demonstrated that galectin-3 KO mice had more severe disease activity in the DSS-induced colitis model, indicating that galectin-3 may offer protection from inflammation in this form of colitis. Furthermore, treating with recombinant galectin-3 reduced body weight loss and colon length shortening and ameliorated mucosal inflammation in mice suffering DSS-induced colitis and SCID T cell transfer colitis, implying a therapeutic potential. The complexity of autoimmune diseases and immune reactions in different physiological compartments makes it difficult to determine the role of galectin-3 in different autoimmune diseases. For example, galectin-3 KO mice have less severe disease in EAE and CIA [5, 6]. Conversely, a galectin-3 deficiency is associated with more severe damage in a hepatitis animal model [30, 31] and a diabetic glomerulonephritis model [32] and in our colitis model herein. The role of galectin-3 in IBDs has been confirmed in the DSS-induced colitis model and T cell transfer colitis model in this study. This indicates that galectin-3 might protect gut mucosa from inflammation in experimental colitis and implies that it could have a therapeutic potential in IBD. Our results are supported by a previous study where galectin-3 was shown to modulate T cell functioning in IBD [13]. These imply that galectin-3 may play a regulatory role in autoimmune responses.
An important characteristic of colonic mucosa is its microbiota. Bacterial antigens are pro-inflammatory at most body sites, but their presence is crucial for the generation and/or expansion of regulatory T cells in the mouse intestine [33]. Recently, experimental models have helped to establish a crucial role for microbial flora in the induction and perpetuation of colitis and suggest that gut inflammation results from a dysregulated immune response toward bacterial antigens [34, 35]. Galectins are conserved glycan-binding proteins that play various roles critical to innate and adaptive immunity. Galectin-3 modulates broad biological functions and participates in different stages of inflammation where it plays multiple roles [1]. During inflammation, the production of galectin-3 in gut mucosa may generate a microenvironment that limits effector T cell responses.
Although galectin-3 is generally viewed as pro-inflammatory in many experimental animal models such as EAE [5] and CIA [6], it is immune-modulatory when T cell apoptosis induction or TCR signal regulation is considered. Previous studies suggest that galectin-3 induces apoptosis in T cells [4], and it may result in immune suppression and reduced initiation of inflammation in gut mucosa during IBD. Moreover, there are several different phenotypes observed in the galectin-3 KO mice [36–40], suggesting that in addition to the local tissue microenvironment and immune regulation, the phenotype of many galectin KO mice often reflects a variety of different galectin-regulated pathways converging on the overall phenotype. It has also been recently revealed that galectin-3 suppresses Th17 responses by regulating DC cytokine production during fungal infection [8]. In addition, it has recently been demonstrated that galectin-9 inhibits autoimmunity and induces tolerance by multiple mechanisms, including the inhibition of effector T cells by interacting with Tim-3 and inducing iTreg cells via CD44-TGF-bRI [41]. We have demonstrated herein that galectin-3 protects gut mucosa from inflammation in experimental colitis, suggesting that galectin-3 potentially acts as an effector molecule that delivers immunosuppressive effects.
Our results demonstrate that treating with galectin-3 resulted in protecting gut mucosa from inflammation in DSS-induced colitis. However, the protective effects of galectin-3 were eliminated by the administration of anti-CD25 mAb. Moreover, an adoptive transfer of galectin-3-treated T cells reduced bowel inflammation and colitis in the T cell transfer colitis model, indicating that galectin-3 modulated T cell function during mucosa inflammation and experimental colitis. Galectins can modify T cell functions, which are pivotal in the immune system. Our results implicate a possible role for CD25+ T cells, particularly in Treg cells, in mediating the protective effects of galectin-3 in colonic inflammation. Recent studies of IBD demonstrate that Th17 and Treg cells are crucial in the pathogenesis of IBD [15, 17, 18], either directly or by its influence on other T cell subsets [19, 20]. However, the effects of galectin-3 on the differentiation of T cell subsets and their interactions in IBD are still unknown. Our results also demonstrated that primary T cells treated with galectin-3 ex vivo induced the expression of FOXP3, ICOS, and PD-1 with a Treg cell phenotype having a suppression function. Furthermore, the adoptive transfer of galectin-3-induced CD25+ T cells suppressed mucosa inflammation and colitis in the T cell transfer colitis animal model. These results all imply that galectin-3 protected the gut from inflammation during experimental colitis via induction of regulatory T cells. Regulatory T cells are important in IBDs [42–44]. FOXP3 and ICOS positive T cells represent a subset of functional, active regulatory T cells that modulate DCs and T cells [45]. Recent studies also reveal that other galectins modulate the functions of regulatory T cells; i.e., galectin-1 is a suppressive effector of Treg and galectin-9 is an inducer of Treg [41, 46, 47]. Considering the critical role of Treg cells in autoimmunity and colonic inflammation, identifying how galectin-3 induces Treg might offer potential targets for inhibiting pathogenic immune responses and mucosa inflammation.
In summary, we have demonstrated herein that the treatment of galectin-3 inhibited colonic mucosa inflammation and suppressed disease severity by inducing regulatory T cells. Galectin-3 induces regulatory T cell functions that protect from colitis after transfer of lymphocytes into immunodeficient hosts. Our study revealed the importance of the direct effects on T cells by galectin-3 on experimental colitis but do not exclude the possible roles of other cell types. Some translational studies show that immune cells, mucosal epithelia, and intestinal microbiota all contribute to the pathogenesis of IBDs, which makes experimental colitis more complex [9, 10]. Taken together, our results indicate that galectin-3 protects gut mucosa from inflammation in experimental colitis and imply that it may have therapeutic potential in IBD.
References
Rabinovich GA, Toscano MA (2009) Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 9:338–352
Chen HY, Fermin A, Vardhana S, Weng IC, Lo KF, Chang EY, Maverakis E, Yang RY, Hsu DK, Dustin ML et al (2009) Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci U S A 106:14496–14501
Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A (2003) CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res 63:8302–8311
Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG (2006) Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 176:778–789
Jiang HR, Al Rasebi Z, Mensah-Brown E, Shahin A, Xu D, Goodyear CS, Fukada SY, Liu FT, Liew FY, Lukic ML (2009) Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol 182:1167–1173
Forsman H, Islander U, Andreasson E, Andersson A, Onnheim K, Karlstrom A, Savman K, Magnusson M, Brown KL, Karlsson A (2011) Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum 63:445–454
Saegusa J, Hsu DK, Chen HY, Yu L, Fermin A, Fung MA, Liu FT (2009) Galectin-3 is critical for the development of the allergic inflammatory response in a mouse model of atopic dermatitis. Am J Pathol 174:922–931
Fermin Lee A, Chen HY, Wan L, Wu SY, Yu JS, Huang AC, Miaw SC, Hsu DK, Wu-Hsieh BA, Liu FT (2013) Galectin-3 modulates Th17 responses by regulating dendritic cell cytokines. Am J Pathol 183:1209–1222
Kamada N, Seo SU, Chen GY, Nunez G (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13:321–335
Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153
Frol'ova L, Smetana K Jr, Borovska D, Kitanovicova A, Klimesova K, Janatkova I, Malickova K, Lukas M, Drastich P, Benes Z et al (2009) Detection of galectin-3 in patients with inflammatory bowel diseases: new serum marker of active forms of IBD? Inflamm Res 58:503–512
Jensen-Jarolim E, Gscheidlinger R, Oberhuber G, Neuchrist C, Lucas T, Bises G, Radauer C, Willheim M, Scheiner O, Liu FT et al (2002) The constitutive expression of galectin-3 is downregulated in the intestinal epithelia of Crohn's disease patients, and tumour necrosis factor alpha decreases the level of galectin-3-specific mRNA in HCT-8 cells. Eur J Gastroenterol Hepatol 14:145–152
Muller S, Schaffer T, Flogerzi B, Fleetwood A, Weimann R, Schoepfer AM, Seibold F (2006) Galectin-3 modulates T cell activity and is reduced in the inflamed intestinal epithelium in IBD. Inflamm Bowel Dis 12:588–597
Bregenholt S, Claesson MH (1998) Splenic T helper cell type 1 cytokine profile and extramedullary haematopoiesis in severe combined immunodeficient (scid) mice with inflammatory bowel disease (IBD). Clin Exp Immunol 111:166–172
Hundorfean G, Neurath MF, Mudter J (2012) Functional relevance of T helper 17 (Th17) cells and the IL-17 cytokine family in inflammatory bowel disease. Inflamm Bowel Dis 18:180–186
Iijima H, Takahashi I, Kishi D, Kim JK, Kawano S, Hori M, Kiyono H (1999) Alteration of interleukin 4 production results in the inhibition of T helper type 2 cell-dominated inflammatory bowel disease in T cell receptor alpha chain-deficient mice. J Exp Med 190:607–615
Monteleone I, Sarra M, Pallone F, Monteleone G (2012) Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr Mol Med 12:592–597
Tanner SM, Staley EM, Lorenz RG (2013) Altered generation of induced regulatory T cells in the FVB.mdr1a-/- mouse model of colitis. Mucosal Immunol 6:309–323
Okamura M, Yoh K, Ojima M, Morito N, Takahashi S (2014) Overexpression of GATA-3 in T cells accelerates dextran sulfate sodium-induced colitis. Exp Anim 63:133–140
Sujino T, Kanai T, Ono Y, Mikami Y, Hayashi A, Doi T, Matsuoka K, Hisamatsu T, Takaishi H, Ogata H et al (2011) Regulatory T cells suppress development of colitis, blocking differentiation of T-helper 17 into alternative T-helper 1 cells. Gastroenterology 141:1014–1023
Lindebo Holm T, Poulsen SS, Markholst H, Reedtz-Runge S (2012) Pharmacological evaluation of the SCID T cell transfer model of colitis: as a model of Crohn’s disease. Int J Inflam 2012:412178
Rose WA 2nd, Sakamoto K, Leifer CA (2012) Multifunctional role of dextran sulfate sodium for in vivo modeling of intestinal diseases. BMC Immunol 13:41
Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, Merlin D (2009) Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 4, e6073
Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT (2000) Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol 156:1073–1083
Yang RY, Hsu DK, Liu FT (1996) Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A 93:6737–6742
Robertson MW, Albrandt K, Keller D, Liu FT (1990) Human IgE-binding protein: a soluble lectin exhibiting a highly conserved interspecies sequence and differential recognition of IgE glycoforms. Biochemistry 29:8093–8100
Hsu DK, Zuberi RI, Liu FT (1992) Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem 267:14167–14174
Schepp-Berglind J, Atkinson C, Elvington M, Qiao F, Mannon P, Tomlinson S (2012) Complement-dependent injury and protection in a murine model of acute dextran sulfate sodium-induced colitis. J Immunol 188:6309–6318
Maloy KJ (2007) Induction and regulation of inflammatory bowel disease in immunodeficient mice by distinct CD4+ T-cell subsets. Methods Mol Biol 380:327–335
Nakanishi Y, Tsuneyama K, Nomoto K, Fujimoto M, Salunga TL, Nakajima T, Miwa S, Murai Y, Hayashi S, Kato I et al (2008) Nonalcoholic steatohepatitis and hepatocellular carcinoma in galectin-3 knockout mice. Hepatol Res 38:1241–1251
Nomoto K, Tsuneyama K, Abdel Aziz HO, Takahashi H, Murai Y, Cui ZG, Fujimoto M, Kato I, Hiraga K, Hsu DK et al (2006) Disrupted galectin-3 causes non-alcoholic fatty liver disease in male mice. J Pathol 210:469–477
Pugliese G, Pricci F, Iacobini C, Leto G, Amadio L, Barsotti P, Frigeri L, Hsu DK, Vlassara H, Liu FT et al (2001) Accelerated diabetic glomerulopathy in galectin-3/AGE receptor 3 knockout mice. FASEB J 15:2471–2479
Strauch UG, Obermeier F, Grunwald N, Gurster S, Dunger N, Schultz M, Griese DP, Mahler M, Scholmerich J, Rath HC (2005) Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut 54:1546–1552
Elson CO, Cong Y, Iqbal N, Weaver CT (2001) Immuno-bacterial homeostasis in the gut: new insights into an old enigma. Semin Immunol 13:187–194
Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB (1998) Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 66(66):5224–5231
Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG (2006) Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol 177:4718–4726
Quattroni P, Li Y, Lucchesi D, Lucas S, Hood DW, Herrmann M, Gabius HJ, Tang CM, Exley RM (2012) Galectin-3 binds Neisseria meningitidis and increases interaction with phagocytic cells. Cell Microbiol 14:1657–1675
Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT (2003) Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest 112:389–397
Sato S, St-Pierre C, Bhaumik P, Nieminen J (2009) Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol Rev 230:172–187
Stowell SR, Arthur CM, McBride R, Berger O, Razi N, Heimburg-Molinaro J, Rodrigues LC, Gourdine JP, Noll AJ, von Gunten S et al (2014) Microbial glycan microarrays define key features of host-microbial interactions. Nat Chem Biol 10:470–476
Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, Hirashima M, Anderson AC, Kuchroo VK (2014) Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 41:270–282
Boehm F, Martin M, Kesselring R, Schiechl G, Geissler EK, Schlitt HJ, Fichtner-Feigl S (2012) Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol 12:97
Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, Neurath MF (2006) Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut 55:671–680
Himmel ME, Yao Y, Orban PC, Steiner TS, Levings MK (2012) Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology 136:115–122
Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ (2008) Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 28:870–880
Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI (2007) Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood 109:2058–2065
Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, Ito K, Takeshita K, Niki T, Saita N et al (2008) Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol 127:78–88
Acknowledgements
We thank Dr. Fu-Tong Liu (Academia Sinica, Taiwan) for kindly providing the galectin-3 knockout mice. We also thank the Department of Medical Research and core laboratory of National Taiwan University Hospital for facility support. This work was supported by grants from the National Science Council, Taiwan (NSC 98-2320-B-002-049-MY3, 100-2320-B-038-026, and 101-2321-B-002-008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare that they have no competing financial interests.
Additional information
Hwei-Fang Tsai and Chien-Sheng Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tsai, HF., Wu, CS., Chen, YL. et al. Galectin-3 suppresses mucosal inflammation and reduces disease severity in experimental colitis. J Mol Med 94, 545–556 (2016). https://doi.org/10.1007/s00109-015-1368-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1368-x