Abstract
The liver is recognized as the target organ of metastases of almost all prominent malignancies. Its unique biology renders this organ particularly susceptible to circulating disseminated tumour cells (DTCs), and it can be assumed that very early metastasis occurs in the liver. The premetastatic niche concept may explain very early metastasis, as it defines priming of a future target organ of metastasis by factors that may already be secreted from premalignant lesions. This review shows that comprehensive knowledge on mechanisms of premetastatic niche formation in the liver is based on pre-clinical models only and still rather rare, mostly due to the scarcity of mouse liver metastasis models displaying a tumour cell-free period in the liver or lack of liver-tropic syngeneic tumour cells to probe for the niche. Attentive re-assessment of previous studies and reviews was undertaken revealing only two clearly identified tumour-derived secreted factors (TDSFs), both inducing infiltration of the liver by bone marrow-derived cells and increased liver metastasis, namely tissue inhibitor of metalloproteinases-1 (TIMP-1) and macrophage-inducing factor (MIF). Future directions of this research area will comprise elucidation of the impact of TDSFs on regulation and activity of myeloid-derived suppressor cells and/or the specific architecture and homeostasis of the liver, as well as development of prognostic TDSF detection in patients at risk of liver metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis is still the major problem in the management of malignant tumour diseases. The greatest challenge is that tumours can disseminate cells already at a very early stage [1–3] so that synchronous metastases or undetected micrometastases are already present at the time of primary tumour diagnosis. The mechanisms of very early metastasis are still unknown. Creation of a ‘premetastatic niche’ is one currently proposed concept helping to explain mechanisms of very early metastasis formation [4]. The premetastatic niche is defined as a tumour cell-free microenvironment in a target organ of metastasis, which has been primed by factors secreted from the primary tumour to the effect that it exhibits increased susceptibility to disseminated tumour cells [4, 5]. It is hypothesized that premetastatic niches allow even non-malignant cells (disseminated tumour cells (DTCs)) to colonize to organs [2, 6], i.e. before they have accumulated all mutations which are generally considered to be necessary for the full-blown autonomous metastatic potential [7].
The focus of this review is on the current state of knowledge on premetastatic niche formation in the liver. Identification of tumour-derived secreted factors (called TDSFs [8]) exhibiting the ability to create a premetastatic niche in the liver will be discussed as well as the cellular and molecular changes that occur in the liver through their action. Cellular changes comprise the TDSF-induced infiltration of the liver by bone marrow-derived cells (BMDCs) including myeloid-derived suppressor cells (MDSCs), macrophages and neutrophils, which were all already described to be a component of the premetastatic niche in the lung [4]. Molecular changes comprise alterations in hepatic gene expression, which may induce the creation of an altered pattern of growth factors, cytokines, and extracellular matrix molecules, which favour BMDC and tumour cell colonization. It is quite striking that, for the liver, this information is still relatively limited. In spite of the fact that the liver is the most afflicted target organ of metastases from all kinds of malignancies and despite the wealth of knowledge on the liver’s intriguing biological complexity in respect to tumour-host interactions during metastasis [9], there are many loose ends of knowledge for this particular organ.
Intrinsic metastasis-supporting features of the liver
In the clinic, the liver is recognized as the target organ of metastases of almost all prominent malignancies including carcinomas of pancreas, colorectum, colon, oesophagus, stomach, breast, and liver [10], as well as sarcomas [11, 12] and melanomas [13]. In addition, the liver can be colonized by haematogenous tumours such as myelomas and leukemias [14]. The liver is often not the first site where overt large metastases of the above-mentioned tumours are diagnosed, but it is likely that it harbours micrometastases from early on. These micrometastases become clinically apparent in late stages of these tumours by inducing jaundice, abdominal pain, or ascites and are also considered as direct cause of death by destroying the vital function of this organ in colorectal carcinoma [15] or breast carcinoma [16, 17].
Its unique biology already renders the liver particularly susceptible to circulating DTCs (Fig. 1). For one, the liver is connected via the blood circulation to those sites where many malignant tumours arise [18]. In addition, the healthy liver actually displays intrinsic architectural and functional features, whose balance can easily be tipped towards favouring metastasis to this organ [9, 19, 20], even without priming by factors secreted by the original tumour site, as it would be the case in classic premetastatic niche formation. They comprise the following: (1) the liver-specific microcirculation with its unique sinusoidal cell population, (2) the perivascular mesenchymal cells including hepatic stellate cells (HSCs), (3) the morphologically and metabolically heterogeneous parenchymal cell compartment, and (4) the hepatic regional immunity (Fig. 1). Each of these four features can, under certain conditions, favour metastasis formation. The unique and complex microcirculation in the hepatic sinusoids can positively impact on trafficking and retention of DTCs in the liver [9, 21, 22]. The diverse cell types in the liver, namely the hepatic sinusoidal endothelial cells (HSEC), Kupffer cells (KC), HSCs, pit cells, and hepatocytes all contribute to the fine balance between failure or success of liver metastasis, as they closely interact in that sequence with incoming DTCs and impact on BMDCs, MDSCs and lymphocytes [9] (Fig. 1). HSECs, activated HSCs, and hepatocytes can provide the stromal support for metastatic growth and angiogenesis of metastatic colonies [9]. In a complex concerted action together with KCs, neutrophils, MDSCs, and lymphocytes, HSECs, activated HSCs and hepatocytes are able to provide prometastatic conditions by expression of a specific set of autocrine growth factors and cytokines, eventually allowing immune escape of tumour cells in the liver-specific immune-suppressive environment [9, 23, 24] (Fig. 1). Whether this latter pattern of activity can be triggered and/or promoted by TDSFs during premetastatic niche formation is unknown and should be one goal of research in this area.
Intrinsic metastasis-supporting features of the sinusoidal compartment of the liver (red arrows). The details of these features have been reviewed extensively elsewhere [9, 19–24]. (a) Connection of liver sinusoids to many possible primary tumour sites via the blood circulation and entrance of TCs via the portal triad. (b) Slow microcirculation promoting attachment of DTCs to HSECs. Provision of autocrine prometastatic factors by (c) perivascular portal tract fibroblasts and (d) HSCs. (e) Metabolically heterogenous parenchymal cells and oxygen gradient. (f) Hepatic regional immune suppression evolving from interactions (black arrows) of MDSCs with other immune cells and activated HSC. Dotted arrow: invasion of mature neutrophils. Fibr portal tract fibroblasts, Hep hepatocyte, HSC hepatic stellate cells, actHSC activated hepatic stellate cells, KC Kupffer cells, MDSC myeloid-derived suppressor cells, NK natural killer cells, NΦ neutrophil granulocytes, O 2 conc oxygen concentration, SoD space of Disse, TC tumour cell, T T lymphocyte
Experimental considerations towards studying the premetastatic niche in the liver
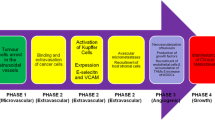
Limited information on molecular and cellular events of premetastatic niche formation in the liver, not to speak of mechanisms and their spatio-temporal context, is, at least in part, a consequence of the shortage of suitable liver metastasis models and their employment in current research activities in the field, with one very recent exception [6]. A definite elucidation of mechanisms of premetastatic niche formation, i.e. modulation of the tumour cell-free future target organ by TDSFs, requires a clear experimental setup, which complies with this definition of a premetastatic niche. Most importantly, premetastatic niche formation has to occur in absence of tumour cells in the target organ. A niche in presence of tumour cells would be called ‘prometastatic’ and would be aimed at the clinical management or therapy of already existing metastases. Prometastatic niches are generated, in part, through crosstalk mechanisms already well established including tumour cell-intrinsic and liver homeostasis-intrinsic mechanisms favouring attachment, invasion, survival, and growth of tumour cells in the liver [9, 25]. Very recently, a detailed analysis of sequential steps involved in premetastatic niche formation in the liver was presented [6]. From this conclusive investigation in a defined tumour model, one can deduce five essentials towards achieving a totally comprehensive picture of premetastatic niche formation (Fig. 2), namely a defined source of TDSFs, confirmed absence of tumour cells in the premetastatic organ, immunocompetence, demonstration of the niche with tagged tumour cells, and experimental manipulation of TDSFs and niche-creating factors allowing conclusions on definite mechanisms (Fig. 2).
As we will see below, the studies on the premetastatic niche in the liver were not always as clearly defined. In respect to liver metastasis, two of the essentials seem to be particularly difficult to fulfil. Firstly, to show conclusively a tumour cell-free period in the liver and, secondly, to challenge the niche with liver-tropic tumour cells. Both are the crucial proofs for the existence of a premetastatic niche. Only a few syngeneic tumour cell lines experimentally metastasize to the liver upon straightforward intravenous injection into the tail vein of mice, including the chemically induced murine DBA/2 lymphoma ESb [26] and its lacZ-tagged variant L-CI.5s [27–29], the recently selected murine colon carcinoma cell lines CT26-Fl3 [30], and, to a much lesser extent and with higher variability, murine LLC Lewis lung carcinoma [5, 31] and B16 melanoma [5] cell lines. Experimental liver metastasis can be achieved alternatively by the relatively complicated intrasplenic/portal inoculation of tagged tumour cells [6, 32]. It is currently most accepted to employ transgenic mice that spontaneously develop tumours, which spontaneously metastasize to the liver. For example, different spontaneous pancreas carcinoma models were shown to generate liver metastasis [6, 33, 34]. However, the kinetics of metastasis formation, even in these defined genetically engineered mouse models, is unknown or metastases arise so early that these models as such are not suitable for the study of premetastatic niche formation [2], unless further experiments according to the above-mentioned five essentials are performed.
Creators and elements of premetastatic niche formation in the liver
From a previous review, one was led to erroneously assume that quite a few mechanisms of premetastatic niche creation in the liver are already known from the various employed mouse models at the time [4]. For three studies mentioned there [5, 31, 35], the review lists TDSFs, BMDCs, and the mechanisms of premetastatic niche formation suggesting that they create a premetastatic niche not only in the lung but also in the liver [4]. However, careful examination of the original literature and its supplementary material revealed that the seemingly conclusive scenarios for the lung do not hold true for premetastatic niche-creation in the liver. In fact, this was actually not specifically claimed in the original papers either.
The study by Kaplan et al. presented clear evidence for the mechanism of premetastatic niche formation in the lung [5] identifying vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) as TDSFs. They mobilize BMDCs (VEGFR1+ haematopoietic precursor cells) and upregulate fibronectin (FN) in the liver, to which the BMDCs also home. BMDCs produce MMP-9 and SDF-1 attracting CXCR4+ tumour cells to the lungs [5]. It was not shown that the liver was premetastatic in the spontaneous metastasis assays. Injection of conditioned media from these tumour cell lines had not been performed to examine premetastatic niche formation in the liver [5]. Also, neither the identity of the BMDC nor the expression of MMP-9 and SDF-1 were confirmed in the liver [5]. Furthermore, expression of FN in resident fibroblasts in the niche, which is thought to attract BMDCs, was shown for the lung, while neither an association of the BMDC clusters with FN nor the source of FN was demonstrated in the liver [5]. Last but not the least, the correlation of the findings to human disease revealed no such clusters in the liver parenchyma but only in hepatic lymph nodes [5]. Altogether, this study only demonstrates upregulation of FN in yet unidentified liver cells as a possible factor for premetastatic niche formation in the liver. VEGF and PlGF do not seem to be crucial TDSFs for the premetastatic niche in the liver, as inhibition of these factors in breast carcinoma cells does not interfere with efficacy of liver metastasis [35]. Similarly, the second study [31] listed in that review provided conclusive data only for the premetastatic niche in the lungs. In that study, it was shown that the TDSF Versican mobilized BMDCs (characterized as CD11b+/Gr-1+ myeloid cells and M2 macrophages) creating a prometastatic inflammatory microenvironment via TLR2-induced TNFα secretion by the myeloid cells in the lungs [31]. Liver metastasis assays were performed without demonstrating a clear tumour-free period in this organ. While Versican clearly affects liver metastasis, the authors themselves do not claim that they have found a Versican-dependent mechanism for premetastatic niche formation in the liver [31]. Finally, the third study listed in Sceneay et al. in context with the liver is the first publication revealing a mechanism of BMDC mobilization in the liver by a defined TDSF [35]. The authors of that study ensured a clear tumour-free premetastatic phase in the liver by administration of recombinant granulocyte-colony stimulating factor (G-CSF) to tumour-free mice [35]. G-CSF induces mobilization of Bv8-expressing BMDCs (the Ly6G+/Ly6C+ granulocyte subset of CD11b+/Gr-1+ myeloid cells) to the liver leading to expression of MMP-9, S100A8, and S100A9 in this organ. However, the established niche was not challenged with liver-tropic tumour cell lines in order to check whether they more efficiently colonized the liver [35]. Therefore, it is still unclear whether the described niche in the liver is more susceptible to metastasis or not. In summary, the three studies [5, 31, 35] neither claimed nor could they hold up to the mechanisms assigned to them in the review [4]. Although the studies quoted in that review provided important first hints, there are other publications adding to the information on premetastatic niche formation in the liver in the past few years.
The importance of MDSCs in premetastatic niche formation in the liver was shown conclusively in a study on the regulation of the permissiveness of organs for tumour metastasis during mouse gestation [8]. The advantage of this study was that niche-creation in the liver definitely occurred in absence of tumour cells in that organ. The experimental abstraction in this study was that the niche-creating factors were pregnancy-associated [8]. However, these factors can be characterized in the future and may turn out to be growth factors or cytokines that also act as TDSFs. It was shown previously that primary tumours [36, 37] cause infiltration of the liver by MDSCs in the premetastatic phase leading to immunosuppression by upregulation of the negative T cell costimulatory molecule PD-L1 in KCs [36] or suppression of T cell proliferation [38]. MDSCs can also induce a mild liver damage, which may lead to the release of factors [39] or induce apoptosis of hepatocytes [40], both able to promote liver metastasis. Mauti et al. showed that MDSCs can also repress antitumour immunity by inhibiting perforin-dependent NK cell cytotoxicity [8] leading to the re-direction of intravenously inoculated genetically tagged tumour cells from the lungs (which the employed B16F10 murine melanoma cells would normally metastasize to) to the liver [8]. The potency of the NK cell-dependent premetastatic niche in the liver was more recently confirmed, when it was shown that more mature NK cells protect against lung metastasis, while the CD27 + CD11b− subset protects against liver metastasis [41]. In addition, NK-depleted SCID mouse models are known as multiple-organ metastasis models [42]. Absence of NK cells promoted liver metastasis, when tumour cells secrete macrophage-stimulating protein (MSP) [42]. It is important to note that the interrelationship between MDSCs and NK cells is still not completely understood and likely not uni-directional in the context of tumours [43, 44]. This circumstance has to be explored further in the context of premetastatic niche formation. Nevertheless, local hepatic immunosuppression is an important parameter of the premetastatic liver [8], which could be targeted therapeutically. Towards this end, it was shown that liver metastasis was inhibited by preventing the infiltration of CD11b+ cells, which include MDSCs and many other cells of the myeloid lineage, with the CXCR4 antagonist AMD3465 [45] or preventing neutrophil infiltration by the CXCR4 antagonist AMD3100 [46]. Also, inhibition of immunosuppressive CCR2+ macrophages appearing in livers of pancreas carcinoma-bearing mice led to suppression of liver metastasis [47]. These studies reveal potential strategies for the prevention of premetastatic niche formation. In two of the studies, the treatments in the mouse models were initiated without excluding that tumour cells were already present in the niche [45, 47].
The first study making an effort to ensure a clearly defined premetastatic phase in livers of a mouse model with growing primary tumours employed genetically tagged tumour cells (CT26-FL3, a RFP-tagged CT26 colon carcinoma cell variant of Balb/c origin selected for efficient metastasis to the liver) and genetically tagged BMDCs (from eGFP-transgenic C57/Bl6 mice backcrossed for five generations into Balb/c) [30]. These cell lines were investigated in syngeneic recipient mice (Balb/ccByJ) [30]. eGFP-tagged bone marrow was transplanted prior to orthotopic (cecum) implantation of liver-metastasizing RFP-tagged CT26 cells (CT26-FL3) [30]. In contrast to the parental control cell line (CT26), which metastasized to the liver only at low efficiency, CT26-FL3 cells express several candidate TDSFs, namely mRNAs of Hgf, Il6, Tnf, Ifn, Csf2, Csf3, Cxcl1, Cxcl4 and Cxcl11. The primary tumours systemically induced serum levels of OPN, MMP-9, S100A8, S100A9, SAA3 and VEGFA and infiltration of BMDCs within 2 to 3 weeks after tumour cell inoculation [30]. RFP-tagged metastases could not be detected in the liver within this time frame [30]. The BMDCs expressed VEGFR1, S100A8, and S100A9, while hepatocytes were found to express high levels of LOX [30]. The comparison with the control cell line revealed these factors as components of the premetastatic niche in the liver [30]. Which of the suggested TDSFs are mainly responsible for this niche in the liver and which of the factors in the niche are crucial for increased liver metastasis in this experimental model was not shown [30].
The first individual TDSF, which was characterized as creator of a premetastatic niche in the liver, is the tissue inhibitor of metalloproteinases (TIMP)-1 [46]. TIMP-1 is of special interest, not only because elevated levels correlate with poor prognosis of many tumour diseases including breast [48], colorectal [49], lung [50] carcinomas, and haematopoietic tumours [51], but also because plasma levels are a prognostic factors of patients with liver metastases [52]. TIMP-1 is a pluripotent molecule with anti-proteolytic as well as cytokine-like functions [53]. Due to these functions, TIMP-1 impacts on a wide range of effects on tissue homeostasis by inhibiting metalloproteinases, as well as on cell behaviour including inhibition of apoptosis [54], promotion of cell proliferation [55–57], and the metastatic potential of tumour cells [58]. In addition, elevation of systemic TIMP-1 levels in mice by adenoviral gene transfer promoted liver metastasis mediated by HGF signaling [59] and uPA activity [60] in the host tissue. Recently, TIMP-1 was identified to create a premetastatic niche in the liver [46]. The part of this study which led to this conclusion allowed for a clearly defined premetastatic period in which recombinant TIMP-1 or TIMP-1 expressed by a non-metastatic primary tumour could prime the metastatic site without interference by tumour cells in the liver [46]. The characteristics of the TIMP-1-induced premetastatic niche comprised a liver-specific upregulation of SDF-1 and S100A8, and an SDF-1/CXCR4-dependent accumulation of Ly6G+ neutrophils in the liver tissue [46] (Fig. 3). The mechanism of SDF-1-mediated recruitment of neutrophils to the liver still has to be elucidated further. So far, it is known that elevated systemic TIMP-1 levels induce neutrophilia in mice not by prolonging survival or direct mobilization of neutrophils but by inducing granulopoiesis in the bone marrow in a TIMP-1/CD63 signaling-dependent manner [61]. Challenge of the TIMP-1-induced premetastatic niche in the liver by intravenous inoculation of lacZ-tagged tumour cells revealed its functionality as permissive target site for metastasis for murine T lymphoma cells and breast carcinoma cells [46]. Altogether, these findings are one explanation for the correlation between elevated systemic levels of TIMP-1 and poor prognosis of many tumour diseases.
Candidate creators and elements of the premetastatic niche in the liver. Confirmed creators and effects on important cellular and molecular factors in the premetastatic liver are shown. Effects of Tissue inhibitor of metalloproteinases-1 (TIMP-1) (red arrows, factors in red) and exosomes containing migration inhibitory factor (MIF) (orange arrows, factors in orange). Promising but yet inconclusive candidate creators and factors are depicted in grey. References rendering cells and factors likely to play a role in the premetastatic liver are associated with the respective entity (numbers in brackets)
A whole sequence of events of premetastatic niche formation in the liver including the characterization of the relevant players was published only very recently [6]. Exosomes containing migration inhibitory factor (MIF) were identified as TDSFs in pancreatic ductal adenocarcinomas (PDACs) mouse models. Uptake of these exosomes by KCs induces TGFβ secretion, which triggers FN production by HSCs. FN deposits are one component of the KC-induced pro-inflammatory milieu, which attracts bone marrow-derived CD11b+/F480+ macrophages and CD11b+ Gr-1+ myeloid cells (Fig. 3). The challenge was performed by intrasplenic injection of mCherry-tagged syngeneic pancreas carcinoma cell lines demonstrating the existence of the premetastatic niche in the liver. Finally, knockdown of MIF, depletion of macrophages, depletion of FN or inhibition of TGFβ signalling, respectively, abolished the described mechanisms of premetastatic niche formation in the liver [6]. This study corroborates in one and the same model the previously described impact of myeloid [30, 35, 45, 46, 62] and macrophage-like [31, 42, 47] BMDCs, FN [5], TGFβ [63], as components of the premetastatic niche in the liver and brings them into a sequential context (Fig. 3). Even this study was confronted with the problem of investigating a clearly defined tumour cell-free premetastatic liver since the employed PDAC mouse models metastasize extremely early [2]. Actually, co-authors of Costa-Silva et al. have shown previously in the very same PKCY mouse model that cells (circulating pancreatic cells, CPCs) disseminate from the pancreas already before ‘malignant’ tumours develop, namely during pancreatitis and early pancreatic intraepithelial neoplasias (PaIN) [2]. Indirect evidence revealed that these CPCs are capable of giving rise to metastases [2]. Consequently, Costa-Silva et al. took the effort to show that the premetastatic niche in the liver could be induced by pre-education with exosomes derived from PDAC cell lines and a subsequent challenge with intrasplenically inoculated mCherry-tagged PAN02 pancreas carcinoma cells [6]. The other data were then interpreted on the basis of this observation [6], although many of them were obtained from mice with spontaneous pancreatic tumour progression [6], i.e. including the possibly early dissemination events even during premalignant stages.
Conclusion and future perspectives
Our knowledge on premetastatic niche formation in the liver is still rather limited. So far, only TIMP-1 and exosomes containing MIF have emerged as single identified TDSFs inducing both infiltration of the liver by CD11b+ BMDCs and link this infiltration to increased permissiveness for circulating DTCs to the liver [6, 46] (Fig. 3). Suppression of the immune response in the liver by MDSCs turned out to be especially crucial for the creation of the premetastatic niche in the liver [8]. Identification of individual responsible TDSFs for MDSC regulation and activity [23, 24] and elucidation of the exact mechanisms leading to increased susceptibility of the liver to metastasis is one important future direction in this research area. Exploration of the impact of individual TDSFs on the architectural and functional aspects of the intrinsic susceptibility of the liver to metastases [9, 19, 21, 22, 25], including the impact on the different liver-specific cell types in the hepatic sinusoids and their adjacent compartments, is only at the beginning. Identification of TDSFs or factors from inflammatory premalignant sites [2] and pathways in the liver will improve diagnosis and possibly pave new ways of therapeutic interference with the infestation of the organ that is most afflicted with metastases of all kinds of tumour diseases. It will be important to establish a link between these mechanisms and clinical situations in which patients are at risk of liver metastasis. Specifically, mouse models suggest that inflammatory and premalignant lesions in the pancreas can establish a premetastatic niche in the liver [2, 6]. This notion should be corroborated by clinical studies correlating measurements of TDSFs in patients with pancreatitis with a prospective follow-up documenting occurrence of liver metastasis. In fact, a correlation between TIMP-1 plasma levels and chronic pancreatitis or tumourigenesis has already been found [64, 65], while there is so far no such correlation for MIF. A clinical perspective to premetastatic niche research would be the early detection of clearly defined TDSFs, i.e. the creators of the premetastatic niche in the liver. As TDSFs are diagnostically more important than the elements within the liver niche, it would not be necessary to access liver tissue in premetastatic phases. In fact, it was recently demonstrated for pancreatic cancer [66] that it should be feasible to detect TDSFs in body fluids during niche-creating inflammation or premalignant phases in a proteomic approach. Regarding the premetastatic niche in the liver, it will be important also to demonstrate the metastasis-promoting activation of specific liver cells by TDSFs in patients. Currently, studies by our group are on the way investigating the correlation of plasma levels of TIMP-1 on HSC activation in patients and the impact of these cells on creation of a premetastatic niche in the liver.
References
Stoecklein NH, Klein CA (2010) Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int J Cancer 126:589–598
Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH et al (2012) EMT and dissemination precede pancreatic tumor formation. Cell 148:349–361
Polzer B, Klein CA (2013) Metastasis awakening: the challenges of targeting minimal residual cancer. Nat Med 19:274–275
Sceneay J, Smyth MJ, Möller H (2013) The pre-metastatic niche: finding common ground. Cancer Metastasis Rev 32:449–464
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA et al (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H et al (2015) Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. doi:10.1038/ncb3169
Yachida S, Jones S, Bozic I, Antal T, Leary A, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA et al (2010) Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467:1114–1117
Mauti LA, Le Bitoux MA, Baumer K, Stehle JC, Golshayan D, Provero P, Stamenkovic I (2011) Myeloid-derived suppressor cells are implicated in regulating permissiveness for tumor metastasis during mouse gestation. J Clin Invest 121:2794–2807
Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Hoyer-Hansen G, Eefsen RL, Reynolds AR, Brodt P (2013) The multifaceted role of the microenvironment in liver metastatsis: biology and clinical implications. Cancer Res 73:2031–2043
Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL (2006) Metastatic patterns in adenocarcinoma. Cancer 106:1624–1633
Jaques DP, Coit DG, Casper ES ES, Brennan MF (1995) Hepatic metastases from soft-tissue sarcoma. Ann Surg 221:392–397
Amankwah EK, Conley AP, Reed DR (2013) Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol 5:147–162
Ryu SW, Saw R, Scolyer RA, Crawford M, Thompson JF, Sandroussi C (2013) Liver resection for metastatic melanoma: equivalent survival for cutaneous and ocular primaries. J Surg Oncol 108:129–135
Bross ID, Viadana E, Pickren JW (1975) The metastatic spread of myeloma and leukemias in men. Virchows Arch A Pathol Anat Histol 365:91–101
Burke D, Allen-Mersh TG (1996) Colorectal liver metastases. Postgrad Med J 72:464–469
Helling TS, Martin M (2014) Cause of death from liver metastases in colorectal cancer. Ann Surg Oncol 21:501–506
Mogrovejo E, Manickam P, Amin M, Cappell MS (2014) Characterization of the syndrome of acute liver failure caused by metastases from breast carcinoma. Dig Dis Sci 59:724–736
Chambers AF, Groom AC, MacDonald IC (2002) Metastasis: dissemination and growth of cancer cells in metastatic sites. Nature Rev Cancer 2:563–572
Vidal-Vanaclocha F (2011) Architectural and functional aspects of the liver with implications for cancer metastasis. In: P. Brodt (ed.) Liver metastasis: biology and clinical management, cancer metastasis—biology and treatment 16, DOI 10.1007/978-94-007-0292-9_2, Springer Science + Business Media B.V 9-42
Paschos KA, Majeed AW, Bird NC (2014) Natural history of hepatic metastases from colorectal cancer—pathobiological pathways with clinical significance. World J Gastroenterol 20:3719–3737
Enns A, Gassmann P, Schlüter K, Korb T, Spiegel HU, Senninger N, Haier J (2004) Integrins can directly mediate metastatic tumor cell adhesion within the liver sinusoids. J Gastrointest Surg 8:1049–1059
Vollmar B, Menger MD (2009) The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev 89:1269–1339
Höchst B, Schildberg FA, Sauerborn P, Gäbel YA, Gevensleben H, Goltz D, Heukamp LC, Türler A, Ballmaier M, Gieseke F et al (2013) Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J Hepatol 59:528–535
Pancione M, Giordano G, Remo A, Febbraro A, Sabatino L, Manfrin E, Ceccarelli M, Colantuoni V (2014) Immune escape mechanisms in colorectal cancer pathogenesis and liver metastasis. J Immunol Res 2014:686879
Vidal-Vanaclocha F (2008) The prometastatic environment of the liver. Cancer Microenviron 1:113–129
Schirrmacher V, Bosslet K (1980) Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. X. Immunoselection of tumor variants differing in tumor antigen expression and metastatic capacity. Int J Cancer 25:781–788
Krüger A, Schirrmacher V, von Hoegen P (1994) Scattered micrometastases visualized at the single-cell level: detection and re-isolation of lacZ-labeled metastasized lymphoma cells. Int J Cancer 58:275–284
Krüger A, Schirrmacher V, Khokha R (1998-1999) The bacterial lacZ gene: an important tool for metastasis research and evaluation of new cancer therapies. Cancer Metastasis Rev 17:285-294
Weinspach D, Seubert B, Schaten S, Honert K, Sebens S, Altevogt P, Krüger A (2014) Role of L1 cell adhesion molecule (L1CAM) in the metastatic cascade: promotion of dissemination, colonization, and metastatic growth. Clin Exp Metastasis 31:87–100
Zhang Y, Davis C, Ryan J, Janney C, Pena MM (2013) Development and characterization of a reliable mouse model of colorectal cancer metastasis to the liver. Clin Exp Metastasis 30:903–918
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M (2009) Carcinoma produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457:102–106
Khatib AM, Auguste P, Fallavollita L, Wang N, Samani A, Kontogiannea M, Meterissian S, Brodt P (2005) Characterization of the host inflammatory response to tumor cells during the initial stages of liver metastasis. Am J Pathol 167:749–759
Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA (2005) Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7:469–483
Hermann PC, Sancho P, Canamero M, Martinelli P, Madriles F, Michl P, Gress T, de Pascual R, Gandia L, Guerra C et al (2014) Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology 147:1119–1133
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T et al (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G + Ly6C+ granulocytes. Proc Natl Acad Sci 107:21248–21255
Ilkovitch D, Lopez DM (2009) The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res 69:5514–5521
Connolly MK, Mallen-St Clair J, Bedrosian AS, Malhotra A, Vera V, Ibrahim J, Henning J, Pachter HL, Bar-Sagi D, Frey AB et al (2010) Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. Leukoc Biol 87:713–725
Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, Zender L, Kapoor V, Felsher DW, Manns MP et al (2013) Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol 59:1007–1013
Eggert T, Medina-Echeverz J, Kapanadze T, Kruhlak MJ, Korangy F, Greten TF (2014) Tumor induced hepatic myeloid derived suppressor cells can cause moderate liver damage. PLoS One 9, e112717
Li H, Fan X, Stoicov C, Liu JH, Zubair S, Tsai E, Ste Marie R, Wang TC, Lyle S, Kurt-Jones E et al (2009) Human and mouse colon cancer utilizes CD95 signaling for local growth and metastatic spread to liver. Gastroenterology 137:934–944
Ballas ZK, Buchta CM, Rosean TR, Heusel JW, Shey MR (2013) Role of NK cell subsets in organ-specific murine melanoma metastasis. PLoS One 8, e65599
Sato S, Hanibuchi M, Kuramoto T, Yamamori N, Goto H, Ogawa H, Mitsuhashi A, Van TT, Kakiuchi S et al (2013) Macrophage stimulating protein promotes liver metastases of small cell lung cancer cells by affecting the organ microenvironment. Clin Exp Metastasis 30:333–344
Park YJ, Song B, Kim YS, Kim EK, Lee JM, Lee GE, Kim JO, Kim YJ, Chang WS, Kang CY (2013) Tumor microenvironmental conversion of natural killer cells into myeloid-derived suppressor cells. Cancer Res 73:5669–5681
Sato Y, Shimzu K, Shinga J, Hidaka M, Kawano F, Kakimi K, Yamasaki S, Asakura M, Fujii SI (2015) Characterization of the myeloid-derived suppressor cell subset regulated by NK cells in malignant lymphoma. Oncoimmunology 4, e995541
Ling X, Spaeth E, Chen Y, Shi Y, Zhang W, Schober W, Hail N, Konopleva M, Andreeff M (2013) The CXCR4 antagonist AMD3465 regulates oncogenic signalling and invasiveness in vitro and prevents breast cancer growth and metastasis in vivo. PLoS One 8, e58426
Seubert B, Grünwald B, Kobuch J, Cui H, Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N et al (2015) TIMP1 creates a pre-metastatic niche in the liver through SDF/CXCR4-dependent neutrophil recruitment in mice. Hepatology 61:238–248
Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD et al (2013) Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 19:3404–3415
Würtz SØ, Møller S, Mouridsen H, Hertel PB, Friis E, Brünner N (2008) Plasma and serum levels of tissue inhibitor of metalloproteinases-1 are associated with prognosis in node-negative breast cancer: a prospective study. Mol Cell Proteomics 7:424–430
Holten-Andersen MN, Nielsen HJ, Sørensen S, Jensen V, Brünner N, Christensen IJ (2006) Tissue inhibitor of metalloproteinases-1 in the postoperative monitoring of colorectal cancer. Eur J Cancer 42:1889–1896
Pesta M, Kulda V, Kucera R, Pesek M, Vrzalova J, Liska V, Pecen L, Treska V, Safranek J, Prazakova M et al (2011) Prognostic significance of TIMP-1 in non-small cell lung cancer. Anticancer Res 31:4031–4038
Pennanen H, Kuittinen O, Soini Y, Turpeenniemi-Hujanen T (2004) Clinicopathological correlations of TIMP-1 and TIMP-2 in Hodgkin’s lymphoma. Eur J Haematol 72:1–9
Bunatova K, Pesta M, Kulda V, Topolcan O, Vrzalova J, Sutnar A, Treska V, Pecen L, Liska V (2012) Plasma TIMP1 level is a prognostic factor in patients with liver metastases. Anticancer Res 32:4601–4606
Chirco R, Liu XW, Jung KK, Kim HR (2006) Novel functions of TIMPs in cell signalling. Cancer Metastasis Rev 25:99–113
Lee SJ, Yoo HJ, Bae YS, Kim HJ, Lee ST (2003) TIMP-1 inhibits apoptosis in breast carcinoma cells via a pathway involving pertussis toxin-sensitive G protein and c-Src. Biochem Biophys Res Commun 312:1196–1201
Porter JF, Shen S, Denhardt D (2004) Tissue inhibitor of metalloproteinase-1 stimulates proliferation of human cancer cells by inhibiting a metalloproteinase. Br J Cancer 90:463–470
Egea V, Zahler S, Rieth N, Neth P, Popp T, Kehe K, Jochum M, Ries C (2012) Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/β-catenin signaling. Proc Natl Acad Sci U S A 109:E309–E316
Cui H, Seubert B, Stahl E, Dietz H, Reuning U, Moreno-Leon L, Ilie M, Hofman P, Nagase H, Mari B et al (2014) Tissue inhibitor of metalloproteinase-1 induces a pro-tumorigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene. doi:10.1038/onc.2014.300
Schelter F, Grandl M, Seubert B, Schaten S, Hauser S, Gerg M, Boccaccio C, Comoglio P, Krüger A (2011) Tumor cell-derived Timp-1 is necessary for maintaining metastasis-promoting Met-signaling via inhibition of Adam-10. Clin Exp Metastasis 28:793–802
Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S, Flechsig C, Krell HW, Antolovic D, Brew K et al (2007) Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signalling. Cancer Res 67:8615–8623
Schrötzlmair F, Kopitz C, Halbgewachs B, Lu F, Algül H, Brünner N, Gänsbacher B, Krüger A (2010) Tissue inhibitor of metalloproteinases-1-induced scattered liver metastasis is mediated by host-derived urokinase-type plasminogen activator. J Cell Mol Med 14:2760–2770
Kobuch J, Cui H, Grünwald B, Saftig P, Knolle PA, Krüger A (2015) TIMP-1 signaling via CD63 triggers granulopoiesis and neutrophilia in mice. Haematologica 100:1005–1013
Yamamoto M, Kikuchi H, Ohta M, Kawabata T, Hiramatsu Y, Kondo K, Baba M, Kamiya K, Tanaka T, Kitagawa M et al (2008) TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res 68:9754–9762
Medina-Echeverz J, Fioravanti J, Díaz-Valdés N, Frank K, Aranda F, Gomar C, Ardaiz N, Dotor J, Umansky V, Prieto J et al (2014) Harnessing high density lipoproteins to block transforming growth factor beta and to inhibit the growth of liver tumor metastases. PLoS One 9, e96799
Pan S, Chen R, Crispin DA, May D, Stevens T, McIntosh MW, Bronner MP, Ziogas A, Anton-Culver H, Brentnall TA (2011) Protein alterations associated with pancreatic cancer and chronic pancreatitis found in human plasma using global quantitative proteomics profiling. J Proteome Res 10:2359–2376
Pan S, Chen R, Brand RE, Hawley S, Tamura Y, Gafken PR, Milless BP, Goodlett DR, Rush J, Brentnall TA (2012) A multiplex targeted proteomic assay for biomarker detection in plasma: a pancreatic cancer biomarker case study. J Proteome Res 11:1937–1948
Pan S, Brentnall TA, Chen R (2015) Proteomics analysis of bodily fluids in pancreatic cancer. Proteomics. doi:10.1002/pmic.201400476
Acknowledgment
This work was supported by grants from Deutsche Forschungsgemeinschaft (KR2047 1–2 and KR2047 1–3). Ulrike Ludwig is acknowledged for the support in the artwork.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krüger, A. Premetastatic niche formation in the liver: emerging mechanisms and mouse models. J Mol Med 93, 1193–1201 (2015). https://doi.org/10.1007/s00109-015-1342-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1342-7