Abstract
Inflammatory lesions, ischemic tissues, or solid tumors are characterized by the occurrence of severe tissue hypoxia within the diseased tissue. Subsequent stabilization of hypoxia-inducible transcription factors—particularly of hypoxia-inducible factor 1α (HIF1A)—results in significant alterations of gene expression of resident cells or inflammatory cells that have been recruited into such lesions. Interestingly, studies of hypoxia-induced changes of gene expression identified a transcriptional program that promotes extracellular adenosine signaling. Adenosine is a signaling molecule that functions through the activation of four distinct adenosine receptors—the ADORA1, ADORA2A, ADORA2B, and ADORA3 receptors. Extracellular adenosine is predominantly derived from the phosphohydrolysis of precursor nucleotides, such as adenosine triphosphate or adenosine monophosphate. HIF1A-elicited alterations in gene expression enhance the enzymatic capacity within inflamed tissues to produce extracellular adenosine. Moreover, hypoxia-elicited induction of adenosine receptors—particularly of ADORA2B—results in increased signal transduction. Functional studies in genetic models for HIF1A or adenosine receptors implicate this pathway in an endogenous feedback loop that dampens excessive inflammation and promotes injury resolution, while at the same time enhancing ischemia tolerance. Therefore, pharmacological strategies to enhance HIF-elicited adenosine production or to promote adenosine signaling through adenosine receptors are being investigated for the treatment of acute inflammatory or ischemic diseases characterized by tissue hypoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The field of hypoxia changed its direction dramatically when Gregg Semenza performed studies of the erythropoietin promoter that led to the subsequent identification of hypoxia-inducible factor 1α (HIF1A)—the key transcription factor of hypoxia adaptation [1–5]. It turned out that this transcription factor controls numerous hypoxia-inducible genes and has been implicated in a number of physiological and pathological changes that are closely associated with hypoxic conditions such as those that occur during ischemic or inflammatory diseases [6]. Subsequently, it has been appreciated that tissue hypoxia is a distinct feature of a wide variety of diseases, including ischemia, inflammatory diseases, and cancer [7, 8], and that the induction of hypoxia-inducible transcription factors under these disease conditions is not simply a bystander effect, but significantly impacts the inflammatory or ischemic microenvironment [9–12]. One of the important functions of HIF1A-dependent alterations of gene expression during hypoxia is its transcriptional effect on extracellular adenosine signaling. In addition, there are also transcription factors other than HIF involved in the adaptive response to hypoxia (for example, the Sp1 transcription factor) [13, 14]. In the present review, we discuss the mechanisms of how HIF-dependent changes in gene expression are associated with enhanced adenosine responses. Moreover, we give examples of how hypoxia-elicited increases in extracellular adenosine provide an endogenous feedback signal that dampens excessive inflammation and promotes tissue repair and healing. This pathway has been implicated in tissue protection during acute lung injury (ALI), inflammatory bowel disease, or ischemic disorders (discussed and referenced in the succeeding sections).
Tissue hypoxia occurs in a wide range of clinical disorders
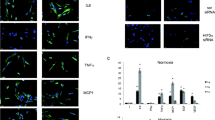
Many clinically relevant diseases are characterized by significant tissue hypoxia [3, 4, 8]. For example, ischemic tissue injury—such as that occurring in the context of myocardial infarction [15, 16], acute kidney injury (AKI) [17], or stroke [18]—is characterized by a profound increase in tissue hypoxia due to lack of arterial supply with oxygen from the blood [7, 19]. Experimental evidence for the existence of tissue hypoxia within ischemic tissues comes from histologic staining studies [17]. Indeed, tissue hypoxia can be made visible on a histologic level by utilizing nitroimidazole compounds that are retained in hypoxic cells. Figure 1 describes how these compounds are retained in hypoxic cells when intracellular oxygen levels are low [20]. As such, studies utilizing hypoxia staining have shown dramatic increases of tissue hypoxia in ischemic organs [17]. Interestingly, the occurrence of tissue hypoxia (i.e., positive hypoxia staining) in an ischemic organ can persist beyond the actual ischemic period, even if the vascular supply is restored. In some instances, this can be due to a postischemic no-reflow phenomenon [17, 21–23]. Other studies indicate that inflammatory diseases are characterized by significant tissue hypoxia. For example, studies from the laboratory of Sean Colgan have revealed that the inflamed intestinal mucosa becomes severely hypoxic during experimentally induced colitis—an animal model for inflammatory bowel disease [10, 24–26]. The cyclic occurrence of intermittent hypoxia has been implicated in the pathogenesis of sleep apnea and leads to subsequent inflammatory activation [27]. Other diseases characterized by tissue hypoxia include infections with pathogens [28–31], cancer [32–34], and obesity [35]. The interdependent relationship between hypoxia and inflammation within a tumor leads to primarily hypoxic areas that become infiltrated by inflammatory cells, while cancer inflammation further exacerbates tissue hypoxia within tumors by causing alterations in the supply and demand for metabolites, particularly for oxygen [8]. Figure 2 demonstrates how inflammation and hypoxia, as well as oxygen supply and demand, are interconnected in several different clinical disorders.
2-Nitroimidazole compounds (NO2-R) stain hypoxic tissues in vivo. a Schematic of NO2-R metabolism in the cell: After passive uptake (top left), NO2-R undergoes a single-electron reduction to a nitro radical anion intermediate (lower left). Oxygen regenerates the native compound by electron uptake (marked in red) and subsequent reaction to H2O2 (not shown). In the absence of oxygen, the activated compound intermediate is processed to a hydroxylamine intermediate (bottom middle) which then stably binds SH-containing molecules, such as proteins (bottom right). These adducts accumulate in the cell and can be visualized using labeled antibodies (right). b C57bl/6 mice were subjected to sham procedure (upper panel) or kidney ischemia (occlusion of the renal artery for 30 min; lower panel). After 5 min of reperfusion, mice were injected with pimonidazole. Antibody staining was performed after an additional 15 min
Hypoxia-induced transcription factors
Research stimulated by the discovery of HIF1A subsequently identified the molecular pathway that promotes hypoxia-elicited alterations of gene expression [3–5, 8, 36]. HIF is a heterodimeric transcription factor. It consists of a constitutively expressed beta-subunit (HIF1B) and an alpha-subunit (expressed as one of two isoforms—HIF1A or HIF2A), which is highly regulated on a posttranslational level. When oxygen levels are high, HIF1A and HIF2A are subjected to immediate proteasomal degradation involving two hydroxylation steps (Fig. 3). Factor inhibiting HIF (FIH) hydroxylates an asparagine residue in the C-terminal activation domain of HIF [37]. This first step prevents coactivator binding, and thereby functionally inhibits HIF activity. A second hydroxylation step is mediated by a group of enzymes that function as prolyl hydroxylases PHD1, PHD2, or PHD3 [38–40]. PHDs hydroxylate conserved proline residues within the N-terminal activation domain of the HIF1A or HIF2A protein. This second hydroxylation step facilitates binding of the von Hippel–Lindau gene product, thereby promoting ubiquitination and subsequent proteasomal degradation of HIF1A or HIF2A [41–44]. Key observations on the pathway of normoxic HIF degradation via proline hydroxylation and proteasomal degradation were made in the research laboratories of William Kaelin and Peter Ratcliff.
Hypoxia-dependent stabilization of the transcription factor hypoxia-inducible factor (HIF). In normoxic conditions (left side of schematic), hydroxylases inactivate HIFA subunits. FIH hydroxylates an asparaginyl residue in the carboxy-terminal activation domain (CAD), preventing coactivator (p300) recruitment. PHDs hydroxylate a proline residues in the N-terminal activation domain (in the oxygen-dependent degradation domain (ODD)), facilitating pVHL-dependent ubiquitination and proteasomal degradation. In hypoxia, PHDs and FIH are inhibited and the coactivator p300 is recruited to the HIFα subunit, which forms a heterodimer with HIFβ. This complex is transcriptionally active
Since PHDs and FIH require oxygen as cofactor for their individual hydroxylation reactions, hypoxia is associated with a functional inhibition of FIH and PHDs, respectively. Therefore, hypoxic conditions are associated with the posttranslational stabilization of HIF1A and HIF2A. It should be noted that the levels of other cosubstrates and products can also modulate FIH and PHD activities (e.g., 2-oxoglutarate, succinate) [45]. While HIF1A is expressed ubiquitously, HIF2A expression is limited to certain tissues (e.g., vascular endothelia). Understanding the differential effects of HIF1A versus HIF2A is currently an area of intense investigation. Indeed, there is emerging evidence that a specific hypoxia-elicited response is predominantly mediated by either HIF1A or HIF2A [46]. For example, a study in patients with familial erythrocytosis discovered a gain-of-function mutation in the HIF2A gene as cause for the disease [47].
Following stabilization of HIF1A/HIF2A and coactivator binding, the alpha-subunit forms a heterodimer with HIF1B, translocates into the nucleus, and binds to promoter regions within HIF target genes. This promoter region is conserved and referred to as hypoxia response element (HRE). The consensus core sequence for an HRE is RCGTG (where R is A or G) [48]. In most instances, binding of the HIF heterodimer to an HRE causes transcriptional induction of the gene [7, 8, 49]. However, there are also examples for HIF-mediated gene repression either by HIF-dependent induction of a gene repressor—such as microRNAs [50] or other inhibitor pathways—or by the same mechanism that also induces expression: direct binding of HIF to an HRE within the promoter region of the gene [17, 51–54]. It is presently not understood why direct binding of HIF to an HRE can, in some instances, function as gene repressor. HIF-dependent alteration in gene expression have been implicated in many hypoxia-adaptive responses, such as the induction of erythropoietin during anemia or the induction of vascular endothelial growth factor in hypoxic tissues. In addition to hypoxia-dependent stabilization of HIF, there are also examples of hypoxia-independent HIF stabilization during inflammatory or infectious diseases [28, 29]. In the present review, we discuss how HIF functions increase extracellular adenosine signaling events and concomitant tissue protection from hypoxia, ischemia, and inflammation.
Adenosine
Adenosine belongs to the family of purine nucleosides and is composed of the nucleobase adenine attached to a single ribose sugar molecule [24, 38–40, 55, 56]. It is well-known for its biological function as molecular building block of the universal energy currency adenosine triphosphate (ATP) [57]. In contrast, extracellular adenosine is known for its function as signaling molecule. It can activate four known adenosine receptors, the ADORA1, ADORA2A, ADORA2B, or ADORA3. These are G protein-coupled receptors with intracellular second messenger systems, one of which is cAMP. While signaling through ADORA1 and ADORA3 decreases intracellular cAMP, the activation of ADORA2A and ADORA2B increases intracellular cAMP levels [32, 33, 49, 58]. Many biological functions have been attributed to adenosine signaling (Fig. 4). For example, the heart rate-slowing effects of intravenous adenosine that is used for patient treatment of supraventricular tachycardia are mediated through the ADORA1A receptor [59]. The ADORA2A receptor is expressed on inflammatory cells: pharmacologic studies from the laboratory of Bruce Cronstein provided critical evidence that the activation of ADORA2A on neutrophils attenuates inflammatory responses [60]. Moreover, a landmark paper from the laboratory of Michail Sitkovsky was the first to provide genetic in vivo evidence for the anti-inflammatory functions of ADORA2A signaling by functioning as endogenous feedback loop in dampening acute inflammatory responses [61]. Other studies from the laboratories of Joel Linden and Mark Okusa confirmed the anti-inflammatory role of ADORA2A signaling and provided insight into its tissue-specific origin. Indeed, these studies indicated a functional role of ADORA2A signaling on inflammatory cells, including T cells [62–64] or dendritic cells [65]. Studies from the laboratory of Sean Colgan and our research group implicated the ADORA2B receptor in hypoxia-adaptive responses [66–68], for example, during myocardial ischemia [15, 16, 69], AKI [17, 38, 70], or intestinal inflammation [55, 71–74]—such as that occurring during inflammatory bowel disease. ADORA3A has been suggested to play a functional role in histamine release from rodent mast cells [75].
Hypoxia induces signaling through the ADORA2A and ADORA2B adenosine receptors. Four adenosine receptors (AR) have been described, mediating the effects of extracellular adenosine: ADORA1, ADORA2A, ADORA2B, and ADORA3. All of these receptors modulate intracellular cAMP levels. ADORA1 and ADORA3 signaling lowers cAMP concentrations, while signaling through ADORA2A and ADORA2B—which are both transcriptionally induced in hypoxia—increases cAMP level. The schematic gives examples for the biological effects of AR signaling
Influence of hypoxia on extracellular adenosine signaling events
The magnitude of extracellular adenosine signaling events is determined by the concentration of adenosine in the extracellular space—available to activate the receptor—and the concentration of an individual adenosine receptor. This is a highly dynamic process and many different controls contribute to this process. During conditions of hypoxia or inflammation, extracellular adenosine signaling is a reflection of (1) adenosine production from precursor molecules, (2) expression of adenosine receptors, and (3) adenosine breakdown (Figs. 4 and 5).
Hypoxia attenuates adenosine transport and metabolism, thereby enhancing extracellular adenosine signaling. a Extracellular ATP and ADP are released from different cell types upon stimulation (e.g., thrombocytes, neutrophils) or when they undergo necrosis or pyroptosis (danger-associated molecular pattern molecules (DAMPs)). The ecto-apyrase CD39—which is expressed on epithelia, endothelia, and immune cells—converts ATP and ADP to AMP. Ecto-5′-nucleotidase (CD73) rapidly converts extracellular AMP to ADO. Both enzymes are transcriptionally upregulated in hypoxic conditions, thereby promoting extracellular adenosine production during hypoxia. b Breakdown of extracellular adenosine is initiated by its uptake into the cell by equilibrative nucleoside transporters (ENT1 and ENT2). Intracellular ADO is either phosphorylated by adenosine kinase (ADK) or processed to inosine by adenosine deaminase (ADA). ENTs and ADK are transcriptionally repressed during hypoxia, thereby prolonging adenosine signaling effects during conditions of hypoxia
Extracellular adenosine production
During injurious conditions, many cell types release adenosine precursor molecules into the extracellular compartment [40, 76]. This occurs predominantly in the form of the adenosine precursor nucleotides ATP and adenosine diphosphate (ADP). Since intracellular ATP concentrations are generally high, many cells release ATP upon stimulation. For example, inflammatory cells or vascular endothelia release ATP during inflammation or hypoxia [68, 76–78]. Other sources for extracellular nucleotides include platelets that release ADP via granular release [7]. ATP can function as a signaling molecule itself, via activation of ATP receptors [79]. In many instances, ATP signaling drives proinflammatory responses [80], and hypoxia-dependent enhancement of ATP conversion to adenosine functions as an endogenous feedback loop to dampen excessive inflammatory injury [6, 24]. Extracellular ATP and ADP are rapidly converted to adenosine through a two-step enzymatic process, including CD39-dependent conversion of ATP/ADP to adenosine monophosphate (AMP) and CD73-dependent conversion of AMP to adenosine [72, 81–83]. Studies on the transcriptional effects of hypoxia revealed that CD39 is transcriptionally induced by hypoxia through an SP1-dependent transcriptional pathway [13, 14]. Similarly, CD73 is transcriptionally induced by HIF1A binding to the CD73 promoter and subsequent induction of CD73 transcript and protein levels [67, 68, 84, 85] (Fig. 5a). As such, hypoxia and inflammation are associated with increased production of adenosine from its precursor molecules, thereby shifting the balance from proinflammatory ATP signaling towards anti-inflammatory adenosine signaling.
Adenosine receptor expression during hypoxia
Comparative studies of adenosine receptor expression revealed that ADORA2B is selectively induced in different tissues during inflammation or hypoxia, including vascular endothelia [68], intestinal epithelia [66, 72–74], the kidneys [17, 70], the heart [15, 16, 69], and the lungs [86]. Similarly, studies from human tissues—e.g., biopsies from ischemic myocardial tissues—revealed a selective induction of ADORA2B transcript levels [15]. HIF1A binding to an HRE within the ADORA2B promoter results in increased ADORA2B transcription, protein expression, and function during conditions of hypoxia [66, 68]. Other studies identified a transcriptional pathway for ADORA2A, indicating that this receptor is transcriptionally induced in pulmonary endothelial cells during hypoxic conditions via a transcriptional pathway under the control of HIF2A [87]. Taken together, these studies demonstrate that hypoxia drives extracellular adenosine signaling events on the receptor level by HIF-dependent induction of ADORA2A and ADORA2B [38–40].
Influence of hypoxia on the termination of adenosine signaling
Extracellular adenosine signaling is terminated by transport of adenosine from the extracellular towards the intracellular compartment through equilibrative nucleoside transporters (ENT1 and ENT2) [17, 51, 53, 54], followed by intracellular metabolism to inosine (via enzymatic activity of intracellular adenosine deaminase) [88] or to AMP (via enzymatic activity of adenosine kinase (ADK)) [52]. Conditions of hypoxia or inflammation result in a functional attenuation of adenosine breakdown by repressing adenosine transporter activity and metabolism. Indeed, studies on the transcriptional control of ENTs demonstrate an HIF1A-dependent pathway of ENT1 [17, 54] and ENT2 repression [51, 53, 89], resulting in attenuated adenosine uptake and prolonged signaling effects during conditions of hypoxia. In addition, intracellular adenosine metabolism via ADK is attenuated due to HIF1A-dependent repression of ADK transcript, protein, and function [52] (Fig. 5b). Taken together, hypoxia-elicited repression of adenosine transporters (particularly ENT1 and ENT2) and ADK results in enhanced extracellular adenosine levels and signaling events.
Hypoxia enhances adenosine signaling through netrin-1
Several reports indicate that, during conditions of hypoxia, additional molecular signals can influence adenosine signaling. For example, hypoxia-elicited induction of netrin-1 (NTN1) has been implicated in enhancing extracellular adenosine signaling during inflammatory hypoxia [71, 90, 91]. NTN1 was originally described as a neuronal guidance molecule. Recent studies, however, also discovered an anti-inflammatory role of NTN1 in different diseases (e.g., inflammatory bowel disease, ALI) [55, 71, 91–94]. Just like hypoxia increases levels of extracellular adenosine, so it increases NTN1 levels, albeit by direct transcriptional induction of gene expression [91]. Surprisingly, several studies indicate that NTN1 exerts its immunological effects by enhancing signaling events through the ADORA2B receptor [91]. As such, migration of leukocytes is inhibited, making NTN1 a strong chemorepulsive molecule, which can dampen the influx of inflammatory cells during conditions of limited oxygen availability. The mechanism by which NTN1 signaling enhances extracellular adenosine signaling remains unclear (e.g., direct activation of the receptor, indirect enhancement of adenosine levels, or allosteric enhancement of Adora2b signaling).
Functional role of hypoxia-elicited adenosine elevations during acute inflammatory diseases
Inflammatory bowel disease
Many studies have implicated hypoxia-elicited elevations of extracellular adenosine levels in an endogenous feedback loop to dampen intestinal inflammation as it occurs during inflammatory bowel disease [24]. The intestinal mucosa is already under physiologic conditions among the more “hypoxic” organs of the body. This phenomenon is referred to as “physiological hypoxia” and relates to the fact that the intestinal lumen is anaerobic, thereby causing an extremely steep oxygen gradient across the intestinal epithelial monolayer covering the mucosal surface. Due to alterations in oxygen supply and demand caused by intestinal inflammation, the degree of hypoxia becomes far more severe during intestinal inflammation [25]. Interestingly, genetic studies on the role of HIF uncovered that stabilization of HIF provides a protective and anti-inflammatory pathway during intestinal inflammation [10, 11, 25, 95–97]. Moreover, studies with pharmacologic compounds that achieve HIF stabilization (PHD inhibitors) demonstrate robust protection from intestinal inflammation. As discussed previously, HIF stabilization results in enhanced extracellular adenosine production and signaling. As such, several studies show that mice with failure to produce extracellular adenosine (CD39 −/−or CD73 −/−mice) develop a more severe course of disease when exposed to experimentally induced inflammatory bowel disease [98, 99]. Moreover, extracellular adenosine signaling through ADORA2B or ADORA2B has been shown to protect from intestinal inflammation [71, 74, 100–102].
Intestinal ischemia
Similar to intestinal inflammation, intestinal ischemia is characterized by profound intestinal tissue hypoxia in the context of intermittent loss of perfusion to the gut, resulting in dramatic increases in morbidity and mortality [103]. In this context, different studies have provided evidence for an anti-inflammatory role for HIF1A-elicited enhancement of extracellular adenosine production via CD73 [81] and signaling through ADORA2B [73]. In addition, a recent study targeted HIF1A during intestinal ischemia using pharmacological or genetic approaches. Initial studies with the pharmacological HIF activator (PHD inhibitor) dimethyloxallyl glycine (DMOG) indicated attenuation of intestinal injury with DMOG treatment in intestinal ischemia and reperfusion injury (IR) [72]. In this murine model, DMOG treatment was associated with induction of CD73 and ADORA2B transcript and protein levels, while DMOG protection was abolished in CD73 −/− or Adora2b −/− mice. Finally, studies of mice with tissue-specific deletion of Hif1a in intestinal epithelia or pharmacological inhibition of Hif1a with 17-dimethylaminoethylamino-17-demethoxygeldanamycin revealed enhanced tissue injury during IR, thereby providing strong evidence for the Hif–adenosine pathway in gut protection from ischemia [72].
Acute kidney injury
Similar to intestinal IR, several studies have shown a protective role of hypoxia-elicited increases in extracellular adenosine production and signaling during IR in other organs, including the heart [7, 14–16, 69, 104] and the liver [13, 82, 89]. Particularly during ischemic tissue injury of the kidneys—a life-threatening condition that frequently complicates the care of hospitalized patients—hypoxia-elicited increases in adenosine production have been shown to protect from AKI. Studies in mice deficient in the pathway for extracellular adenosine production or deficient for signaling through Adora2b experience more severe kidney injury [70, 105–107]. Additional studies on the termination of extracellular adenosine signaling revealed a functional role for hypoxia-elicited elevations of extracellular adenosine in preventing a no-reflow phenomenon [17]. Indeed, a complex biologic network regulates kidney perfusion under physiologic conditions. This system is profoundly perturbed during ischemic AKI [17]. Due to its role in adapting tissues to hypoxia, one of our recent studies pursued the hypothesis that extracellular adenosine has a regulatory function in the postischemic control of renal perfusion [17]. Consistent with the notion that ENTs terminate adenosine signaling, this study found that pharmacologic ENT inhibition in mice elevated renal adenosine levels and dampened AKI. Deletion of the ENTs resulted in selective protection in Ent1 −/− mice. Comprehensive examination of adenosine receptor knockout mice exposed to AKI demonstrated that renal protection by ENT inhibitors involves Adora2b. Subsequent studies in mice with tissue-specific deletion of the Adora2b and Adora2b reporter mice revealed a crosstalk between renal Ent1 and Adora2b expressed on vascular endothelia to effectively prevent a postischemic no-reflow phenomenon. Together, these studies identify ENT1 and adenosine receptors as key to the process of reestablishing renal perfusion following ischemic AKI [17].
Acute lung injury
ALI is characterized by alveolar injury and uncontrolled inflammation [108, 109]. Several studies have provided evidence for a protective role for the hypoxia–adenosine pathway also during ALI [86, 110–112]. Very compelling evidence comes from the laboratory of Michail Sitkovsky and his team [32, 33, 113]. The group examined the consequences of different levels of oxygenation and concomitant alterations of the adenosine pathway on outcome parameters during murine ALI [113]. ALI frequently requires symptomatic supportive therapy by intubation and mechanical ventilation with the supplemental use of high oxygen concentrations. Although oxygen therapy represents a life-saving measure, the discovery of hypoxia-elicited adenosine production and concomitant lung protection would predict that administration of oxygen to ALI patients with uncontrolled pulmonary inflammation could have dangerous side effects [113]. As such, oxygenation could weaken tissue hypoxia-driven adenosine production and signaling, and thereby dampen the hypoxia-mediated anti-inflammatory pathway and further exacerbate lung injury. To examine this hypothesis, the authors used a mouse model of ALI induced by bacterial infection. Thiel et al. exposed one group of mice to 100 % oxygen, mimicking therapeutic oxygenation, and left another group at normal ambient levels (21 % oxygen). Five times more mice died after receiving 100 % oxygen than died breathing normal oxygen levels [113]. Mice given 60 % oxygen—considered clinically safe—developed severe pneumonia, but did not die. Indeed, the authors went on to demonstrate that hypoxia-dependent lung protection during ALI involves the enhancement of extracellular adenosine signaling through Adora2a.
Other studies from the laboratory of Sean Colgan have implicated extracellular adenosine production and signaling through ADORA2B in lung protection during hypoxia—as occurs in the setting of hypoxic preconditioning. They found that ADORA2B-dependent adenosine signaling helps to protect the lungs via inhibition of the proinflammatory transcription factor nuclear factor NF-κB [114]. Indeed, adenosine-dependent inhibition of NF-κB involved the proteasomal pathway and was mediated by adenosine-mediated cullin-1 deneddylation [114]. As such, these findings implicate hypoxia-elicited elevations of extracellular adenosine and ADORA2B-dependent adenosine signaling in lung protection from excessive inflammation [114, 115].
Conclusions
Research over the past decade indicates that several disease conditions, such as IR, inflammatory bowel disease, or ALI are associated with the stabilization of hypoxia-inducible transcription factors. One of the key outcomes of HIF activation during these disease conditions includes the activation of extracellular adenosine signaling—particularly through ADORA2B. Many functional studies demonstrate that this pathway resembles an endogenous feedback loop to dampen hypoxia-induced inflammation and to provide organ protection during conditions of limited oxygen availability. It will be critical for the next years to translate these basic research findings from the bench towards patient treatment. For example, this could be achieved by pharmacological means of HIF activation (such as PHD inhibitors) or adenosine receptor agonists. We anticipate that targeting hypoxia-induced increases in extracellular adenosine signaling will provide novel therapies for a wide range of acute inflammatory diseases.
References
Wang G, Jiang B, Rue E, Semenza G (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. PNAS 92:5510–5514
Semenza GL, Roth PH, Fang HM, Wang GL (1994) Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269:23757–23763
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408
Semenza GL (2011) Oxygen sensing, homeostasis, and disease. N Engl J Med 365:537–547
Semenza GL (2007) Life with oxygen. Science 318:62–64
Eltzschig HK, Sitkovsky MV, Robson SC (2012) Purinergic signaling during Inflammation. N Engl J Med 367:2322–2333
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion—from mechanism to translation. Nat Med 17:1391–1401
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364:656–665
Taylor CT (2008) Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol 586:4055–4059
Taylor CT, Colgan SP (2007) Hypoxia and gastrointestinal disease. J Mol Med 85:1295–1300
Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP et al (2010) Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. doi:10.1053/j.gastro.2010.06.068
Cummins EP, Taylor CT (2005) Hypoxia-responsive transcription factors. Pflugers Arch 450:363–371
Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK (2010) SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol 184:4017–4024
Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP (2009) Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113:224–232
Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM et al (2012) Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 18:774–782
Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK (2008) Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118:166–175
Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G et al (2012) Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest 122:693–710
Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17:796–808
Eltzschig HK, Collard CD (2004) Vascular ischaemia and reperfusion injury. Br Med Bull 70:71–86
Arteel GE, Thurman RG, Yates JM, Raleigh JA (1995) Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer 72:889–895
Reffelmann T, Hale SL, Dow JS, Kloner RA (2003) No-reflow phenomenon persists long-term after ischemia/reperfusion in the rat and predicts infarct expansion. Circulation 108:2911–2917
Kloner RA, Ganote CE, Jennings RB (1974) The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 54:1496–1508
Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T (2009) Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 15:1031–1037
Colgan SP, Eltzschig HK (2012) Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol 74:153–175
Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH (2004) Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114:1098–1106
Colgan SP, Taylor CT (2010) Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7:281–287
Ryan S, Taylor CT, McNicholas WT (2005) Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112:2660–2667
Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, Yazdi AS, Matteoli G, Bohn E, Autenrieth IB, Karhausen J et al (2008) Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology 134:756–767
Haeberle HA, Durrstein C, Rosenberger P, Hosakote YM, Kuhlicke J, Kempf VA, Garofalo RP, Eltzschig HK (2008) Oxygen-independent stabilization of hypoxia inducible factor (HIF)-1 during RSV infection. PLoS One 3:e3352
Nizet V, Johnson RS (2009) Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9:609–617
Kempf VA, Lebiedziejewski M, Alitalo K, Walzlein JH, Ehehalt U, Fiebig J, Huber S, Schutt B, Sander CA, Muller S et al (2005) Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation 111:1054–1062
Sitkovsky M, Lukashev D (2005) Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol 5:712–721
Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M (2004) Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol 22:657–682
Semenza GL (2009) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29:625–634
Ye J (2009) Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 33:54–66
Fraisl P, Aragones J, Carmeliet P (2009) Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov 8:139–152
Mahon PC, Hirota K, Semenza GL (2001) FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15:2675–2686
Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK (2011) Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 22:14–20
Aherne CM, Kewley EM, Eltzschig HK (2011) The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta 1808:1329–1339
Eltzschig HK, Macmanus CF, Colgan SP (2008) Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med 18:103–107
Kaelin WG Jr (2008) The von Hippel–Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8:865–873
Kaelin WG (2007) Von Hippel–Lindau disease. Annu Rev Pathol 2:145–173
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464–468
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A et al (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54
Aragones J, Fraisl P, Baes M, Carmeliet P (2009) Oxygen sensors at the crossroad of metabolism. Cell Metab 9:11–22
Ratcliffe PJ (2007) HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest 117:862–865
Eltzschig HK, El Kasmi KC, Eckle T (2008) The HIF2A gene in familial erythrocytosis. N Engl J Med 358:1965–1966, author reply 1966–1967
Wenger RH, Stiehl DP, Camenisch G (2005) Integration of oxygen signaling at the consensus HRE. Sci STKE 2005:re12. doi:10.1126/stke.3062005re12
Eltzschig HK (2009) Adenosine: an old drug newly discovered. Anesthesiology 111:904–915
Loscalzo J (2010) The cellular response to hypoxia: tuning the system with microRNAs. J Clin Invest 120:3815–3817
Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK (2009) Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136:607–618
Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK (2008) HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 111:5571–5580
Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK (2007) Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol 27:1004–1013
Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J et al (2005) HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med 202:1493–1505
Grenz A, Clambey E, Eltzschig HK (2012) Hypoxia signaling during intestinal ischemia and inflammation. Curr Opin Crit Care. doi:10.1097/MCC.0b013e3283514bd0
Koeppen M, Eckle T, Eltzschig HK (2011) Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol 61:145–186
Khakh BS, Burnstock G (2009) The double life of ATP. Sci Am 301(84–90):92
Fredholm BB (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14:1315–1323
Delacretaz E (2006) Clinical practice. Supraventricular tachycardia. N Engl J Med 354:1039–1051
Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M (1990) The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest 85:1150–1157
Ohta A, Sitkovsky M (2001) Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414:916–920
Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD (2003) Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest 112:883–891
Hasko G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7:759–770
Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J (2006) Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 203:2639–2648
Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ et al (2012) Dendritic cells tolerized with adenosine A2AR agonist attenuate acute kidney injury. J Clin Invest 122:3931–3942
Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP (2006) HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J 20:2242–2250
Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP (2004) Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood 104:3986–3992
Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP (2003) Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 198:783–796
Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K et al (2007) Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115:1581–1590
Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK (2008) The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5:e137
Aherne CM, Collins CB, Masterson JC, Tizzano M, Boyle TA, Westrich JA, Parnes JA, Furuta GT, Rivera-Nieves J, Eltzschig HK (2012) Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut 61:695–705
Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK (2011) Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol 186:4367–4374
Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK (2009) Cutting edge: A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol 182:3965–3968
Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP (2009) Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol 182:4957–4964
Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR (2003) Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol 171:338–345
Eltzschig HK, Weissmuller T, Mager A, Eckle T (2006) Nucleotide metabolism and cell–cell interactions. Methods Mol Biol 341:73–87
Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK (2008) ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One 3:e2801
Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP (2006) ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res 99:1100–1108
Burnstock G, Fredholm BB, Verkhratsky A (2011) Adenosine and ATP receptors in the brain. Curr Top Med Chem 11:973–1011
Riegel AK, Faigle M, Zug S, Rosenberger P, Robaye B, Boeynaems JM, Idzko M, Eltzschig HK (2011) Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood 117:2548–2555
Hart ML, Henn M, Kohler D, Kloor D, Mittelbronn M, Gorzolla IC, Stahl GL, Eltzschig HK (2008) Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia–reperfusion injury. FASEB J 22:2784–2797
Hart ML, Much C, Gorzolla IC, Schittenhelm J, Kloor D, Stahl GL, Eltzschig HK (2008) Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology 135:1739.e3–1750.e3
Hart ML, Kohler D, Eckle T, Kloor D, Stahl GL, Eltzschig HK (2008) Direct treatment of mouse or human blood with soluble 5′-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol 28:1477–1483
Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP (2004) Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med 200:1395–1405
Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP (2002) Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110:993–1002
Eckle T, Grenz A, Laucher S, Eltzschig HK (2008) A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118:3301–3315
Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP et al (2009) Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci U S A 106:10684–10689
Eltzschig HK, Faigle M, Knapp S, Karhausen J, Ibla J, Rosenberger P, Odegard KC, Laussen PC, Thompson LF, Colgan SP (2006) Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood 108:1602–1610
Hart ML, Much C, Kohler D, Schittenhelm J, Gorzolla IC, Stahl GL, Eltzschig HK (2008) Use of a hanging-weight system for liver ischemic preconditioning in mice. Am J Physiol Gastrointest Liver Physiol 294:G1431–G1440
Grenz A, Dalton JH, Bauerle JD, Badulak A, Ridyard D, Gandjeva A, Aherne CM, Brodsky KS, Kim JH, Tuder RM et al (2011) Partial netrin-1 deficiency aggravates acute kidney injury. PLoS One 6:e14812
Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK (2009) Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol 10:195–202
Mirakaj V, Gatidou D, Potzsch C, Konig K, Rosenberger P (2011) Netrin-1 signaling dampens inflammatory peritonitis. J Immunol 186:549–555
Mirakaj V, Thix CA, Laucher S, Mielke C, Morote-Garcia JC, Schmit MA, Henes J, Unertl KE, Kohler D, Rosenberger P (2010) Netrin-1 dampens pulmonary inflammation during acute lung injury. Am J Respir Crit Care Med 181:815–824
Mutz C, Mirakaj V, Vagts DA, Westermann P, Waibler K, Konig K, Iber T, Noldge-Schomburg G, Rosenberger P (2010) The neuronal guidance protein netrin-1 reduces alveolar inflammation in a porcine model of acute lung injury. Crit Care 14:R189
Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT (2008) The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134:156–165
Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP et al (2012) Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. doi:10.1073/pnas.1202366109
Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP (2008) Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134:145–155
Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP (2008) Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol 180:4246–4255
Friedman DJ, Kunzli BM, A-Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC (2009) From the cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A 106:16788–16793
Eltzschig HK, Rivera-Nieves J, Colgan SP (2009) Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin Ther Targets 13:1267–1277
Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB (2006) Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol 177:2765–2769
Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M et al (2005) Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129:26–33
Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP et al (2012) Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A 109:E2784–E2793
Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK (2007) CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 116:1784–1794
Grenz A, Kim JH, Bauerle JD, Tak E, Eltzschig HK, Clambey ET (2012) Adora2b adenosine receptor signaling protects during acute kidney injury via inhibition of neutrophil-dependent TNF-alpha release. J Immunol. doi:10.4049/jimmunol.1201651
Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK (2007) Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18:833–845
Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK (2007) Contribution of E-NTPDase1 (CD39) to renal protection from ischemia–reperfusion injury. FASEB J 21:2863–2873
Koeppen M, McNamee EN, Brodsky KS, Aherne CM, Faigle M, Downey GP, Colgan SP, Evans CM, Schwartz DA, Eltzschig HK (2012) Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal Immunol. doi:10.1038/mi.2012.114
Eckle T, Koeppen M, Eltzschig HK (2009) Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 24:298–306
Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK (2010) Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol 184:5271–5279
Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK (2009) Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J 23:473–482
Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK (2007) Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol 178:8127–8137
Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV (2005) Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol 3:e174
Khoury J, Ibla JC, Neish AS, Colgan SP (2007) Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Invest 117:703–711
Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK (2008) A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111:2024–2035
Acknowledgments
The present research work was supported by Grant D/10/52531 from the German Academic Exchange Service (DAAD) to J.M.P., a German Research Foundation (DFG) Grant (EH401/1-1) to H.E., an American Heart Association Grant to A.G., and National Heart Institute Grants R01-HL0921, R01-DK083385, and R01-HL098294 and a grant by the Crohn’s and Colitis Foundation of America (CCFA) to H.K.E.
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poth, J.M., Brodsky, K., Ehrentraut, H. et al. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J Mol Med 91, 183–193 (2013). https://doi.org/10.1007/s00109-012-0988-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0988-7