Abstract
Currently there is a growing market for high quality solid wood products in Chile made from Eucalyptus nitens. Thermal modifications have been used to obtain such products out of fast growing species. The chemical and crystallinity changes in the modified wood were investigated using diffuse reflectance FTIR spectroscopy and crystalline analysis by X-ray diffraction to analyze the difference between thermal modifications processes using pressure under wet conditions (closed system) and processes without pressure under drier conditions (open system). In general, the FTIR spectra showed differences in the degradation of the hemicelluloses in the peaks of the C=O linkages, but almost no differences in the peaks that identify the lignin structure of the wood, as it was difficult to separate the different chemical reactions due to the depolymerization of lignin only observing the bands. The degree of crystallinity showed a tendency to increase at high pressure in the closed system modifications and at temperatures above 200 °C in the open system modifications, but no significant differences at low modification pressure and temperatures. Nonetheless, there were differences in FTIR spectra and cellulose crystallinity when directly comparing modifications with the same corrected mass loss under different conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There is interest in Chile to extend the use of solid wood products, especially from fast growing plantation species such as Eucalyptus nitens (Shining Gum). The plantation of this species has gained in importance in recent years due to its excellent growth rates, positioning it into the second fastest growing species in Chile, after Pinus radiata, and better cold and frost resistance than Eucalyptus globulus (Blue Gum), the most used hardwood plantation species (INFOR 2014, 2015). There has been an interest in extending the use to solid wood products to increase the economic returns (Muñoz et al. 2005). Thermal modifications have been used to produce high quality material from plantation eucalypt species (Esteves et al. 2007; de Cademartori et al. 2015; Batista et al. 2016; Wentzel et al. 2019) that can be potentially used for windows, claddings, flooring, decking or garden products (Militz and Altgen 2014).
Thermal modification processes have been developed in the past decades, where the common features are temperatures between 160 and 240 °C in an atmosphere with limited oxygen content (Hill 2006; Esteves and Pereira 2009; Militz and Altgen 2014). The main differences between the processes are the kind of shielding gas used (nitrogen or steam to name a few) and whether the process is open or closed. Usually, open reactor systems work at atmospheric pressure using superheated steam, like the Thermowood® process (Mayes and Oksanen 2002), which has a high temperature drying step at the beginning of the process to avoid excessive drying rates during the actual modification temperature, while the relative humidity (RH) of the wood reaches almost 0% at that step. As for the closed reactor systems, an example is the FirmoLin process (Willems 2009) where the pressure is generated using steam, which enables to control the RH inside the chamber thus preserving the wood moisture content during the process (Willems 2009; Altgen et al. 2016) and preventing excessive vaporization of the degradation products generated during the modification. The differences in the wood moisture content and the duration of the exposition to the peak modification temperature affect the chemical reactions and alter the temperature limit that induces the depolymerization of the cell wall constituents (Stamm and Hansen 1937; Seborg et al. 1953; Borrega and Kärenlampi 2008). The first component to degrade are the hemicelluloses, where there is a hydrolysis of the polymer chains into oligomers and monomers, while also loosing hydrogen bonds (Tjeerdsma et al. 1998; Garrote et al. 1999). Cellulose is the least transformed wood component, as it is not as strongly affected by thermal degradation as the hemicelluloses, even if many chemical reactions are similar between them (Fengel 1967; Alen et al. 2002). However, in the amorphous region of the cellulose microfibril, hydrolytic cleavage takes place, which results in an increase in the relative crystallinity (Bhuiyan et al. 2000; Sivonen et al. 2002). Lignin is the component that is most thermally stable, as its relative percentage after thermal modification usually increases (Alen et al. 2002; Esteves et al. 2008). However, there are still chemical changes happening in the lignin during the modification, such as cleavage of the methoxyl groups and depolymerization of the lignin macromolecule to lower molecular weight compounds (Tjeerdsma et al. 1998; Sivonen et al. 2002) as well as higher concentration of phenolic groups (Runkel 1951; Kollmann and Fengel 1965). These chemical changes influence the loss in mechanical strength of the thermally modified wood (Tjeerdsma et al. 1998; Kubojima et al. 2000), while crystallinity can particularly influence the brittleness (Phuong et al. 2007) and the decrease in flexibility of the material (Kocaefe et al. 2008).
A quick method to measure the chemical changes is the use of Fourier transform infrared spectroscopy (FTIR). The absorption observed in the FTIR spectra can be assigned to a combination of the functional groups C, H, and O that are present in celluloses, hemicelluloses, extractives, lignin and water in woody materials (Rodrigues et al. 1998). In thermally modified wood, FTIR has been used by Tjeerdsma and Militz (2005) analyzing beech (Fagus sylvatica) and Scots pine (Pinus sylvestris), and in the case of species similar to E. nitens, Esteves et al. (2013) used this method when analyzing thermally modified E. globulus wood. They used the KBr embedding method, as described by Faix and Böttcher (1992). Solid wood samples and thin slices have also been used, but to obtain reproducible spectra, the roughness and direction of the cut (radial, tangential or transversal) have to be identical (Pandey and Theagarajan 1997). To minimize the problems of the wood surface during the analysis, diffuse reflectance is a technique that allows the use of samples without previous preparation to obtain the spectra, as it is an in situ measurement that gives fast results in a reliable way.
X-ray diffraction has been used to measure and characterize the crystallinity of the cellulose (Segal et al. 1959; Isogai and Usuda 1990; Thygesen et al. 2005). It has also been used to measure the crystallinity in thermally modified wood (Dwianto et al. 1996; Bhuiyan et al. 2000, 2001) and even in thermally modified eucalypt species (Cheng et al. 2017). It has been shown that the crystallinity increases after modification (Bhuiyan et al. 2000; Cheng et al. 2017), but it is not known if differences in the process conditions have an influence on the crystallinity. There also are no reports on measurements done directly in thermally modified solid wood samples.
The aim of the present study was to use a combination of FTIR and X-ray diffraction to gain deeper insight into the chemical variation and measure the degree of crystallinity to further analyze the changes that occur during the different thermal modification processes.
2 Materials and methods
2.1 Material
Wood from 19-year old Eucalyptus nitens plantations was obtained from the Región del Bío-Bío in Chile. Slats of 20 × 50 × 650 mm3 (radial × tangential × longitudinal) size were prepared from kiln-dried wood, avoiding major defects and large knots. The slats had an average moisture content of 13% prior to the modification process. Ten slats per modification process were used.
2.2 Thermal modification
Thermal modification processes were performed in a laboratory-scale treatment reactor. The samples were placed in a stainless steel vessel with a volume of 65 l that could be heated up to a maximum of 260 °C with electric heating cables and cooled down to room temperature using water cooling coils. Water vapor was produced by heating an external water reservoir connected to the vessel. Exhaust valves were used for the release of excess pressure. The treatment reactor can be used as either an open (without pressure, dry conditions) or a closed (under pressure, wet conditions) system.
The open system modifications were similar to the ThermoWood® process (Mayes and Oksanen 2002). The first step is to rise the temperature 12 °C/h until it reaches 100 °C, followed by increasing the temperature 2 °C/h until 130 °C to emulate the high-temperature drying step of the ThermoWood® process. The third step was to increase the temperature 12 °C/h until it reaches the peak temperatures shown in Table 1. The peak temperature was then held for 3 h. Afterwards the temperature was decreased 20 °C/h until it reached 65 °C, at that point the vessel was opened and the samples were taken out of it.
As for the modifications in the closed system, a similar schedule to the one used in the open system was used, but there was no high-temperature drying step applied, so the temperature was risen 12 °C/h until the peak temperature. It was also held for 3 h and afterwards decreased 20 °C/h up to 65 °C. Pre-vacuum (ca. 13 kPa) was applied at the beginning of each thermal modification process. Peak temperatures, RH and maximum pressure applied in the closed system are shown in Table 1.
The final moisture content (MC) was measured by difference of dry weight using small pieces taken from each slat. The dry mass from the small sections and the mass loss (ML) were calculated using the formulas described by Metsä-Kortelainen et al. (2006), while the corrected mass loss (CML) was measured as described by Altgen et al. (2016), where first the extractives are deducted from the dry weight of the selected wood slats to calculate their corrected weight.
2.3 FTIR analysis
A FTIR chemical imaging system (PerkinElmer) was used to obtain the spectra from each modification and from untreated specimens. The system consists of a spectrophotometer Frontier that has two detectors, type DTGS NIR and MIR, both covering a range between (14,700 and 350 cm−1) with a resolution of 4 cm−1. The imager Spotlight 400, with a detector type MCT MIR (7800–720 cm−1) that has a resolution > 2 cm−1, was used. The system can generate chemical spectra directly on the surface of the wood through chemical images. In this work, diffuse reflectance was used to obtain the spectra with a resolution of 8 cm−1 and 32 scans. The spectra were baseline corrected using an interactive baseline correction and then normalized considering maximum ordinate value in the spectrum.
The samples were cut to blocks of 10 × 10 × 10 mm3 (radial × tangential × longitudinal) and conditioned at 20 °C/65% RH for a month prior to the FTIR analysis. The radial surface was chosen for each analysis.
2.4 X-ray diffraction
The late wood from the radial surface of solid wood Eucalyptus nitens samples was positioned on the sample holder of a multifunctional Smartlab diffractometer (Rigaku Corporation, Japan). The equipment employs Ni-filtered Cu radiation (30 kV and 40 mA), 1 mm divergence slit, 0.3 mm anti-scatter slit, 5° Soller slits and a Rigaku D/teX 250 detector. The alignment was regularly checked against the NIST SRM660c LaB6 powder standard (NISTS 2015). Patterns were collected in the 10–30° range, counting 0.5°/s per step of 0.01°. PDXL 2 v.2.7.3.0 software was used for pattern intensity analysis. The crystallinity index (CI) was calculated according to the method by Segal et al. (1959). Three measurements per sample were taken, as it is a proof of concept for the use of solid wood in the measurement of crystallinity, and due to the difficulty to align the late wood to the beam.
3 Results and discussion
The bands at 1748 cm−1 represent the ketones, which are present in free aldehyde in lignin and hemicelluloses (Rodrigues et al. 1998; Michell and Higgins 2002). The C=O linkage presents strong absorptions in FTIR spectra between 1750 and 1700 cm−1, and the exact wavenumber depends on the functional group (carboxylic acid: at about 1725–1700 cm−1; ester, ketone: 1725–1705 cm−1, aldehyde: 1740–1720 cm−1) and on its structural location (Esteves et al. 2013). As can be seen in Figs. 1, 2 and 3, all thermal modifications show a decrease in absorbance in this band as the temperature increases. The lower reactivity can be related to the decrease in free reactive hydroxyl groups in holocellulose due to the thermal degradation of the hemicelluloses, as it is more reactive than cellulose (Nguila Inari et al. 2007). There is a more noticeable decrease of this band in the modifications at 100% RH (Fig. 2). This is mostly due to the autohydrolysis during the modification, which results in the formation of acetic acid that accelerates the degradation (Garrote et al. 1999, 2001). This decrease is also related to the variations in lignin due to esterification caused by the reaction of the produced acids with the wood cell wall (Tjeerdsma and Militz 2005). Wentzel et al. (2019) showed that the acetic acid concentration was higher in the modifications at 100% RH, causing an increase in the degradation of hemicelluloses, particularly the xylans. This can be seen in the band around 1730 cm−1, which is the band that represents the acetoxy groups in xylan, and in the band around 1468 cm−1, which corresponds to the asymmetric deformation of C-H bond of xylan (Michell and Higgins 2002). There is also a decrease around the peak at 1600 cm−1, which is related to C=C unsaturated linkages and aromatic rings present in lignin (Rodrigues et al. 1998; Michell and Higgins 2002). The changes in this band were related to lignin condensation at the expense of conjugated carbonyl groups and to the carboxylation of polysaccharides (Chow 1971; Gonzalez-Peña et al. 2009).
Lignin in eucalypts can be seen at the band around 1505–1510 cm−1 (Rodrigues et al. 1998), where the aromatic rings of hardwood lignin (Guaiacyl and Syringyl) are represented (Faix 1991), although it can be difficult to differentiate properly its changes, as it can increase or decrease on whether there is an increase in the relative lignin (Kotilainen et al. 2000; Windeisen et al. 2007) or the aromatic ring opening in the lignin is the one dominating (González-Peña and Hale 2009). According to Pandey (1999), guaiacyl and syringyl can also be seen in the peaks around 1250 cm−1, which represent the C-O stretching and bending (Rodrigues et al. 1998; Michell and Higgins 2002). These bands shifted in the modified wood, similar to what happened to E. globulus (Esteves et al. 2013). The peaks show an increase in the band in all the thermal modifications (Figs. 1, 2 and 3), which is related to carbohydrate degradation. Something similar can be seen in the band 1385 cm−1, which represents the O–H bending vibrations in phenols (Rodrigues et al. 1998), where the OH stretching frequencies change and also increase for the thermal modifications. This variation could be influenced by dehydration effects of the modifications (Moharram and Mahmoud 2008; Spiridon et al. 2011). The open system modifications tend to have a lower MC after the process (Table 1). The bands show different peaks when compared directly between samples with same CML from a drier modification at atmospheric pressure (210 °C) and a modification under pressure and 100% RH (160 °C and 0.61 bar) (Fig. 4). There are other vibrations that arise from the aromatic skeletal in lignin (around 1140 cm−1) (Faix 1991; Rodrigues et al. 1998; Michell and Higgins 2002) that decline as the temperature of the treatment increases, which probably contribute towards the increase in the CO band at 1708 cm−1 (González-Peña and Hale 2009). The changes in lignin can be related to the loss of syringyl units, which tend to be liberated easier than the guaiacyl monomers by a thermal degradation (Faix et al. 1990). The decrease in content of this group could be related to the decrease in the methoxyl groups in lignin, which leads to the loss of a monomer. Previous research indicates that a shift occurs in this group, which can be attributed to the breaking of aliphatic sidechains in lignin and/or condensation reactions (Windeisen et al. 2007). Differences between wet and dry modifications, which could be related to the cross-linking reactions from the degradation of hemicelluloses and lignin, are difficult to make out due to the absorbance of the aromatic rings of furan-type intermediates and lignin overlap (Altgen et al. 2018).
The bands at 1335 cm−1 and 1323 cm−1 represent the C–H bending of polysaccharides and the condensed units of syringyl and guaiacyl, respectively, thus combining most of the structural components of wood (Faix 1991). As these bands represent many structures, it is difficult to conclude which is the structure that causes the changes to be able to differentiate between the open and closed system. There were distortions in the intensities of the bands in the region close to 1150–950 cm−1, when DRIFT spectra were measured directly from wood surfaces (Pandey 1999). These represent the C–O stretch in polysaccharides. Similar distortions can be seen in both open and closed system modifications, but no difference between the modifications can be seen, as shown in Fig. 4.
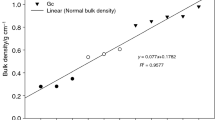
The crystallinity index measured directly on solid wood samples shows a tendency to increase at elevated pressure under wet conditions and at elevated temperatures and drier conditions under atmospheric pressure, but it shows little to no change at the lower pressures and at lower temperatures (Table 2). In the closed system under wet conditions, there was an increase at 150 °C and 0.14 bar and a decrease at 230 °C under drier conditions. It is suspected that this increase is due to the sample surface, which could have had a mixture of early/late wood, and as the measurements are done in situ, it could have caused some issues at the moment of the crystallinity measurement. According to Segal et al. (1959), the difference between the intensities or the height of the angles between 18 and 22° (2θ) of the diffraction pattern in relation to the intensity of the crystalline peak at 22° is the indicator of the degree of crystallinity. A higher difference between the intensity values is an indicator of increasing crystallinity. This could be explained by considering that cellulose chains swell when exposed to high temperature and humidity, thus increasing the distance between the crystal planes, as stated by Cheng et al. (2017).
At higher temperatures, there are more hemicellulose cleavage products due to depolymerisation and the production of more reactive intermediates, such as furfurals, which cause more cross linking reactions that increase the lignin polymer network (Boonstra and Tjeerdsma 2006), causing probably an increase in the relative proportion of crystalline cellulose due to a rearrangement of the cellulose molecules (Bhuiyan et al. 2000). The acetic acid generated by the modification degrades the hemicelluloses and could also degrade the microfibrils of the amorphous region of the cellulose, and even attack the crystalline region (Sivonen et al. 2002). Both chemical changes occur in wet and dry modifications, but the acidity is higher in the wet modification in a closed system (Wentzel et al. 2019). Omitting the crystallinity index at 150 °C and 0.14 bar, the modifications at lower pressures in the wet process showed no significant difference to the untreated samples (Fig. 5), but at higher pressure, there was a noticeable increase in the degree of crystallinity index for the modifications at higher temperatures and pressure (Fig. 6). Bhuiyan et al. (2000) suggested that under moist conditions there is a stimulating effect on the crystallization of amorphous cellulose. As already established by the FTIR spectra and Wentzel et al. (2019), the degradation of hemicelluloses is closely related to the xylans, which crystallize due to the controlled pyrolysis during the modification, thus they could also influence the change in the degree of crystallinity (Akgül et al. 2007). As for the open system modification under drier conditions, there is a steady increase in the crystallinity index as the temperature rises, excluding the modification at 230 °C (Fig. 7), but not a significant difference to the reference with the exception of the modifications at 210 and 220 °C respectively. Besides the increase in acetic acid, the increase in the crystallinity index in the dry modification can be related to the preferred degradation of less ordered molecules (Sivonen et al. 2002). The results obtained in the open system were in contrast to what was shown by Cheng et al. (2017), where there was a decrease in the crystallinity when the temperature rises and an increase at 220 °C. The difference could be due to the different species used and the types of modification.
When directly comparing wet and dry modifications with processes with the same CML (210 °C and atmospheric pressure and 160 °C and 0.61 bar) the wet modification presents a slightly higher crystallinity index than the dry modification, but overall the difference is not significant.
There are differences when directly comparing modifications with the same CML for both FTIR and X-ray diffraction, but in the case of cellulose crystallinity, it is difficult to be certain that there are significant differences between modifications under pressure in wet conditions and without pressure under drier conditions.
4 Conclusion
The biggest chemical differences between modifications under pressure and without pressure were observed in the variations in the peaks of the C=O linkages (1750 and 1700 cm−1). These variations can be related to the formation of acetic acid, that accelerates the degradation of the hemicelluloses, and esterification, which influences the changes in the lignin. The OH bending also showed different peaks between open and closed system (band 1385 cm−1), but it was harder to differentiate the lignin peaks between both processes, as it was difficult to separate the different chemical reactions due to the depolymerization of lignin by only observing the bands. On the other hand, the degree of crystallinity did not show significant differences between both modifications, although the modifications under wet condition had slightly higher values. Further research on the measurement of the crystallinity in solid wood samples should be done, focusing on differences between late and early wood and the direction of the sample (longitudinal, transversal, radial). There were differences in FTIR spectra and cellulose crystallinity when directly comparing modifications with the same CML under different conditions. This should be taken into consideration in further research on the properties of thermally modified wood.
References
Akgül M, Gümüşkaya E, Korkut S (2007) Crystalline structure of heat-treated Scots pine [Pinus sylvestris L.] and Uludağ fir [Abies nordmanniana (Stev.) subsp. bornmuelleriana (Mattf.)] wood. Wood Sci Technol 41:281–289. https://doi.org/10.1007/s00226-006-0110-9
Alen R, Kotilainen R, Zaman A (2002) Thermochemical behavior of Norway spruce (Picea abies) at 180–225 °C. Wood Sci Technol 36:163–171. https://doi.org/10.1007/s00226-001-0133-1
Altgen M, Willems W, Militz H (2016) Wood degradation affected by process conditions during thermal modification of European beech in a high-pressure reactor system. Eur J Wood Prod 74:653–662. https://doi.org/10.1007/s00107-016-1045-y
Altgen M, Uimonen T, Rautkari L (2018) The effect of de-and re-polymerization during heat-treatment on the mechanical behavior of Scots pine sapwood under quasi-static load. Polym Degrad Stabil 147:197–205. https://doi.org/10.1016/j.polymdegradstab.2017.12.007
Batista DC, Bolzón de Muñiz GI, da Silva Oliveira JT, Paes JB, Nisgoski S (2016) Effect of the Brazilian thermal modification process on the chemical composition of Eucalyptus grandis juvenile wood: part 1: cell wall polymers and extractive contents. Maderas-Cienc Tecnol 18:273–284. https://doi.org/10.4067/S0718-221X2016005000025
Bhuiyan MTR, Hirai N, Sobue N (2000) Changes of crystallinity in wood cellulose by heat treatment under dried and moist conditions. J Wood Sci 46:431–436. https://doi.org/10.1007/bf00765800
Bhuiyan MTR, Hirai N, Sobue N (2001) Effect of intermittent heat treatment on crystallinity in wood cellulose. J Wood Sci 47:336–341. https://doi.org/10.1007/bf00766782
Boonstra MJ, Tjeerdsma B (2006) Chemical analysis of heat treated softwoods. Holz Roh Werkst 64:204–211. https://doi.org/10.1007/s00107-005-0078-4
Borrega M, Kärenlampi P (2008) Effect of relative humidity on thermal degradation of Norway spruce (Picea abies) wood. J Wood Sci 54:323–328. https://doi.org/10.1007/s10086-008-0953-9
Cheng XY, Li XJ, Xu K, Huang QT, Sun HN, Wu YQ (2017) Effect of thermal treatment on functional groups and degree of cellulose crystallinity of eucalyptus wood (Eucalyptus grandis × Eucalyptus urophylla). Forest Prod J 67:135–140. https://doi.org/10.13073/FPJ-D-15-00075
Chow S-Z (1971) Infrared spectral characteristics and surface inactivation of wood at high temperatures. Wood Sci Technol 5:27–39. https://doi.org/10.1007/BF00363118
de Cademartori PHG, Missio AL, Mattos BD, Gatto DA (2015) Effect of thermal treatments on technological properties of wood from two Eucalyptus species. An Acad Bras Cienc 87:471–481. https://doi.org/10.1590/0001-3765201520130121
Dwianto W, Tanaka F, Inoue M, Norimoto M (1996) Crystallinity changes of wood by heat or steam treatment. Wood Res 83:47–49
Esteves B, Pereira H (2009) Wood modification by heat treatment: a review. BioRes 4:370–404
Esteves B, Domingos I, Pereira H (2007) Improvement of technological quality of eucalypt wood by heat treatment in air at 170–200 °C. Forest Prod J 57:47–52
Esteves B, Graca J, Pereira H (2008) Extractive composition and summative chemical analysis of thermally treated eucalypt wood. Holzforschung 62:344–351. https://doi.org/10.1515/hf.2008.057
Esteves B, Marques AV, Domingos I, Pereira H (2013) Chemical changes of heat treated pine and eucalypt wood monitored by FTIR. Maderas Cienc Tecnol 15:245–258. https://doi.org/10.4067/s0718-221x2013005000020
Faix O (1991) Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45:21–28. https://doi.org/10.1515/hfsg.1991.45.s1.21
Faix O, Böttcher JH (1992) The influence of particle size and concentration in transmission and diffuse reflectance spectroscopy of wood. Holz Roh Werkst 50:221–226. https://doi.org/10.1007/BF02650312
Faix O, Meier D, Fortmann I (1990) Thermal degradation products of wood. Holz Roh Werkst 48:281–285. https://doi.org/10.1007/BF02626519
Fengel D (1967) Über die Veränderungen des Holzes und seiner Komponenten im Temperaturbereich bis 200 °C—Vierte Mitteilung: Das Verhalten der Cellulose im Fichtenholz bei thermischer Behandlung [On the changes of the wood and its components within the temperature range up to 200 °C—Part IV: the behaviour of cellulose in spruce wood under thermal treatment]. Holz Roh Werkst 25:102–111. https://doi.org/10.1007/bf02608251
Garrote G, Dominguez H, Parajo JC (1999) Hydrothermal processing of lignocellulosic materials. Eur J Wood Prod 57:191–202. https://doi.org/10.1007/s001070050039
Garrote G, Dominguez H, Parajo JC (2001) Study on the deacetylation of hemicelluloses during the hydrothermal processing of Eucalyptus wood. Holz Roh Werkst 59:53–59. https://doi.org/10.1007/s001070050473
González-Peña MM, Hale MDC (2009) Colour in thermally modified wood of beech, Norway spruce and Scots pine. Part 1: colour evolution and colour changes. Holzforschung 63:385–393. https://doi.org/10.1515/HF.2009.078
Gonzalez-Peña MM, Curling SF, Hale MDC (2009) On the effect of heat on the chemical composition and dimensions of thermally-modified wood. Polym Degrad Stabil 94:2184–2193. https://doi.org/10.1016/j.polymdegradstab.2009.09.003
Hill CAS (2006) Wood modification: chemical, thermal and other processes. Wiley, West Sussex
INFOR (2014) Mejoramiento genético de los eucaliptos en Chile [Genetic improvement of Eucalypts in Chile]. Instituto Forestal de Chile, Santiago de Chile
INFOR (2015) Statistical bulletin No 154—Chilean statistical yearbook of forestry 2016. Instituto Forestal de Chile, Santiago de Chile
Isogai A, Usuda M (1990) Crystallinity indexes of cellulosic materials. Sen’i Gakkaishi 46:324–329. https://doi.org/10.2115/fiber.46.8_324
Kocaefe D, Poncsak S, Boluk Y (2008) Effect of thermal treatment on the chemical composition and mechanical properties of birch and aspen. BioRes 3:517–537
Kollmann F, Fengel D (1965) Changes in the chemical composition of wood by thermal treatment. Holz Roh Werkst 23:461. https://doi.org/10.1007/bf02627217 (In German)
Kotilainen RA, Toivanen T-J, Alén RJ (2000) FTIR monitoring of chemical changes in softwood during heating. J Wood Chem Technol 20:307–320. https://doi.org/10.1080/02773810009349638
Kubojima Y, Okano T, Ohta M (2000) Bending strength and toughness of heat-treated wood. J Wood Sci 46:8–15. https://doi.org/10.1007/bf00779547
Mayes D, Oksanen O (2002) ThermoWood handbook. Finnish Thermowood Association, Helsinki
Metsä-Kortelainen S, Antikainen T, Viitaniemi P (2006) The water absorption of sapwood and heartwood of Scots pine and Norway spruce heat-treated at 170 °C, 190 °C, 210 °C and 230 °C. Holz Roh Werkst 64:192–197. https://doi.org/10.1007/s00107-005-0063-y
Michell AJ, Higgins HG (2002) Infrared spectroscopy in Australian forest products research. CSIRO Forestry and Forest Products, Melbourne
Militz H, Altgen M (2014) Processes and properties of thermally modified wood manufactured in Europe. In: Schultz TP, Goodell B, Nicholas DD (eds) Deterioration and protection of sustainable biomaterials. ACS Symposium Series 1158. Oxford University Press, pp 269–285
Moharram MA, Mahmoud OM (2008) FTIR spectroscopic study of the effect of microwave heating on the transformation of cellulose I into cellulose II during mercerization. J Appl Polym Sci 107:30–36. https://doi.org/10.1002/app.26748
Muñoz F, Espinosa M, Herrera MA, Cancino J (2005) Características del crecimiento en diámetro, altura y volumen de una plantación de Eucalyptus nitens sometida a tratamientos silvícolas de poda y raleo [Growth characteristics in diameter, height, and volume of a Eucalyptus nitens plantation with different silvicultural treatment for pruning and thinning]. Bosque 26:93–99. https://doi.org/10.4067/S0717-92002005000100009
Nguila Inari G, Petrissans M, Gerardin P (2007) Chemical reactivity of heat-treated wood. Wood Sci Technol 41:157–168. https://doi.org/10.1007/s00226-006-0092-7
NISTS (2015) Standard reference material 660c: line position and line shape standard for powder diffraction (lanthanum hexaboride powder). National Institute of Standards and Technology, Gaithersburg
Pandey KK (1999) A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J Appl Polym Sci 71:1969–1975. https://doi.org/10.1002/(SICI)1097-4628(19990321)71:12%3c1969:AID-APP6%3e3.0.CO;2-D
Pandey KK, Theagarajan KS (1997) Analysis of wood surfaces and ground wood by diffuse reflectance (DRIFT) and photoacoustic (PAS) Fourier transform infrared spectroscopic techniques. Holz Roh Werkst 55:383–390. https://doi.org/10.1007/s001070050251
Phuong LX, Shida S, Saito Y (2007) Effects of heat treatment on brittleness of Styrax tonkinensis wood. J Wood Sci 53:181–186. https://doi.org/10.1007/s10086-006-0841-0
Rodrigues J, Faix O, Pereira H (1998) Determination of lignin content of Eucalyptus globulus wood using FTIR spectroscopy. Holzforschung 52:46–50. https://doi.org/10.1515/hfsg.1998.52.1.46
Runkel ROH (1951) Zur Kenntnis des thermoplastischen Verhaltens von Holz. Erste Mitteilung. [Information on the thermoplastic behavior of wood. First communication]. Holz Roh Werkst 9:41–53. https://doi.org/10.1007/BF02617537
Seborg RM, Tarkow H, Stamm AJ (1953) Effect of heat upon the dimensional stabilisation of wood. J Forest Prod Res Soc 3:59–67
Segal L, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Sivonen H, Maunu SL, Sundholm F, Jamsa S, Viitaniemi P (2002) Magnetic resonance studies of thermally modified wood. Holzforschung 56:648–654. https://doi.org/10.1515/hf.2002.098
Spiridon I, Teaca CA, Bodîrlău R (2011) Structural changes evidenced by FTIR spectroscopy in cellulose materials after pre-treatment with ionic liquid and enzymatic hydrolysis. BioRes 6:400–413
Stamm AJ, Hansen LA (1937) Minimizing wood shrinkage and swelling effect of heating in various gases. Ind Eng Chem Res 29:831–833. https://doi.org/10.1021/ie50331a021
Thygesen A, Oddershede J, Lilholt H, Thomsen AB, Ståhl K (2005) On the determination of crystallinity and cellulose content in plant fibres. Cellulose 12:563–576. https://doi.org/10.1007/s10570-005-9001-8
Tjeerdsma BF, Militz H (2005) Chemical changes in hydrothermal treated wood: FTIR analysis of combined hydrothermal and dry heat-treated wood. Holz Roh Werkst 63:102–111. https://doi.org/10.1007/s00107-004-0532-8
Tjeerdsma BF, Boonstra M, Pizzi A, Tekely P, Militz H (1998) Characterisation of thermally modified wood: molecular reasons for wood performance improvement. Holz Roh Werkst 56:149–153. https://doi.org/10.1007/s001070050287
Wentzel M, Fleckenstein M, Hofmann T, Militz H (2019) Relation of chemical and mechanical properties of Eucalyptus nitens wood thermally modified in open and closed systems. Wood Mater Sci Eng. 14(3):165–173. https://doi.org/10.1080/17480272.2018.1450783
Willems W (2009) A novel economic large-scale production technology for high quality thermally modified wood. In: Engelund F, Hill CAS, Militz H, Segerholm BK (eds). Proceedings of the 4th European Conference on Wood Modification, Stockholm, Sweden
Windeisen E, Strobel C, Wegener G (2007) Chemical changes during the production of thermo-treated beech wood. Wood Sci Technol 41:523–536. https://doi.org/10.1007/s00226-007-0146-5
Acknowledgements
The authors would like to thank the “Comisión Nacional de Investigación Científica y Tecnológica” Conicyt, through their FONDEQUIP Program, for the financial support for the acquisition of research equipment: EQM150019 “Strengthening of interdisciplinary research in materials and biomaterials, FTIR Infrared Imaging System for non-destructive evaluation of surfaces” and the EQM160152 “Attraction of high-impact International Scientific Collaboration using Advanced X-ray Diffraction techniques to integrate interdisciplinary research in the Araucanía Region”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wentzel, M., Rolleri, A., Pesenti, H. et al. Chemical analysis and cellulose crystallinity of thermally modified Eucalyptus nitens wood from open and closed reactor systems using FTIR and X-ray crystallography. Eur. J. Wood Prod. 77, 517–525 (2019). https://doi.org/10.1007/s00107-019-01411-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-019-01411-0