Abstract
Melamine-urea-formaldehyde resin (MUF) and phenol–formaldehyde resin (PF) were prepared in the laboratory. Their curing behavior was analyzed by differential scanning calorimetry (DSC). MUF resin was then cured at 110, 120, and 130 °C, while PF resin was cured at 135, 150, and 165 °C. The dry and wet bonding strength of plywood made by hot-pressing at different temperatures and heat-treatment at 200 and 250 °C were measured. DSC results show that the PF resin had a more obvious exothermic peak than the MUF resin during heat scanning. Fourier transform infrared spectroscopy analysis shows that MUF and PF resins cured at higher temperature undergo more condensation reactions. Solvent dissolution test shows that PF resin cured at 135 °C has a weight retention similar to that of resins cured at 150 and 165 °C. However, using a temperature of 120 °C was better than 110 and 130 °C for MUF resin. Thermogravimetric analysis results show that PF resin had better heat resistance than MUF resin. The curing temperature did not influence the thermal degradation behavior of cured resins. However, increasing the curing temperature resulted in higher thermal stability. Heat treatment decreased the bonding strength of plywood. However, the bonding strength still met the requirement of the CNS 1349 standard when heat-treated at 200 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Wood is a renewable biomaterial that is widely used in wooden constructions, interior decorations, furniture manufacturing, and diverse wooden products. However, the hygroscopicity and anisotropy makes wood dimensionally unstable. In addition, the biomaterials are easily attacked by fungi, which decrease wood durability. Many wood modification techniques have been invented to overcome these drawbacks. These techniques can be divided into chemical, physical, and biological methods. After modification, the dimensional stability, fungal resistance, and durability can be enhanced.
Chemical modification is an efficient method to improve the properties of wood. The most common method includes esterification and etherification, which can decrease the hydrophilicity and improve the dimensional stability of wood (Rowell 1986; Jebrane and Sèbe 2007; Jebrane et al. 2011). Nevertheless, this method not only increases costs, but also causes environmental pollution problems (Mamiński et al. 2013). On the other hand, biological modification is an environmentally friendly, green technique, which improves the properties of wood mainly through the catalyst reaction by enzyme (Kudanga et al. 2010, 2011; Widsten et al. 2010). However, its efficiency has yet to be confirmed.
Heat treatment is a physical modification of wood. Owing to the rising awareness of environmental protection, interest in the heat treatment of wood has been renewed recently (Esteves and Pereira 2009). Hill (2006) indicated that most of the temperatures used for heat treatment of wood are between 180 and 260 °C. Temperatures lower than 140 °C resulted in no significant change in properties, and temperatures higher than 260 °C resulted in unsuitable degradation of wood. Esteves et al. (2008) noted that there are mainly four commercial heat treatment processes such as the Finnish process (Thermowood), Dutch process (Plato Wood), French (Rectification), and German (OHT), which use steam, combination of steam and heated air, inert gas, and heated oil, respectively. Candelier et al. (2016) collected papers and reports published between 1970 and 2015 which concerned the thermal treatment of wood and carried out a literature review of the main industrial methods used to evaluate the conferred wood properties. The conclusion pointed out that each method has its limits and drawbacks, such as the required investment for the equipment, reliability and accuracy of the results and ease of use at industrial scale. Sandberg and Kutnar (2016) reviewed the state-of-the-art reports that presented the basic concepts in manufacturing thermally modified timber. They indicated that the thermo-hydro and thermo-hydro-mechanical techniques have a growing interest in Europe and the United States.
Nakano and Miyazaki (2003) indicated that heat treatment could reduce the hydroxyl groups and decrease the hygroscopicity of wood with increasing heating temperature up to 250 °C. Bekhta and Niemz (2003) reported that the heat treatment time and temperature had more influence than relative humidity. They also pointed out the heat treatment mainly resulted in darkening of the wood tissues, improving the dimensional stability of wood, and reducing the mechanical properties of wood. Pétrissans et al. (2003) heat-treated pine, spruce, beech, and poplar wood at 240 °C under an inert atmosphere. The results showed that the degree of crystallinity and the hydrophobicity of wood increased after the heat treatment. Ayadi et al. (2003) showed heat-treated wood had better color stability than untreated wood. Rapp et al. (2006) indicated that the thermal modification processes improved the durability and dimensional stability of wood, but the strength properties decreased. Shi et al. (2007) examined the mechanical behavior of heat-treated spruce, pine, fir, aspen, and birch. They found that the variation in properties depended on the species and treatment schedules used. Borrega and Kärenlampi (2008, 2010) found that the hygroscopicity, strength, failure strain and toughness of heat-treated wood were reduced with the mass loss increased. Dubey et al. (2011) treated Pinus radiata wood in linseed oil at 180 °C for 3 h. The results showed that the stability of wood improved significantly but the wood tended to be darker. Sernek et al. (2008) investigated the bonding performance of the heat-treated wood and indicated that the bonding strength of heat-treated wood was lower than that of untreated wood, and was influenced by the adhesive used for gluing. Zigon et al. (2015) also indicated heat-treated wood had lower shear bonding strength than untreated wood.
As mentioned above, most researches of heat-treatment were focused on the influence of the properties of solid wood. However, wood composites made by bonding the mid-small diameter of wood with adhesives are the developing trend in the wood industry. Boonstra et al. (2006) noted steam pre-treatment could effectively improve the dimensional stability of particleboard and fiberboard, but the internal bonding strength would be reduced. Nevertheless, studies that relate to the impact of heat treatment on the bonding strength of wood composites are absent. In this study, the effect of the curing temperature on the bonding strength of heat-treated plywood that was glued with MUF and PF adhesives was investigated.
2 Materials and methods
2.1 Materials
Lauan (Shorea spp; veneer) was used for plywood manufacturing. Chemicals such as phenol, melamine, urea, formalin (37%), hexamethylene-tetramine, sodium hydroxide (NaOH), ammonium chloride (NH4Cl), hydroxylamine hydrochloride, and acetone were used for synthesizing resins and determining their properties. All chemicals are reagent grade and were used without further purification.
2.2 Preparation of MUF and PF resins
MUF was prepared by reacting urea-melamine with formalin. The molar ratio of formaldehyde to urea was set as 1.8/1, and 35% by weight of urea was substituted with melamine. The pH of formalin was adjusted to 7.5 by hexamethylenetetramine, followed by adding the calculated amount of urea and melamine. The reaction was conducted in a four-neck round-bottomed reactor equipped with a stirrer, thermometer, and reflux condenser, and heated at 85 °C with constant stirring until the target reaction time. Then, the reaction mixture was cooled and the pH was adjusted to 9.0. The PF was prepared with a 2.0/1.0/0.4 molar ratio of formaldehyde/phenol/NaOH. To prepare PF resin, phenol, formalin, and one-third of the NaOH(aq) were placed in the round-bottomed reactor and heated at 85 °C for 10 min followed by gradual addition of the remaining two-thirds of the NaOH(aq) dropwise. After all the NaOH(aq) had been added, the reaction proceeded at 85 °C for 2 h.
2.3 Characterization of MUF and PF resins
About 2 g of MUF and PF resin were poured into an aluminum foil dish and dried in an oven at 105 °C for 3 h and 135 °C for 1 h, respectively. The solid content was determined by measuring the weight before and after drying. The viscosity of resins was measured using the Brookfield-DV-E viscometer at 25 °C. The pH was detected with an electronic pH meter at 25 °C. The quantity of free formaldehyde in the MUF and PF resins was determined using the ammonium chloride method and the hydroxylamine hydrochloride method, respectively (Walker 1975). The thermosetting behavior was determined by differential scanning calorimetry (DSC; Perkin-Elmer DSC-7) with a large-volume O-ring-sealed stainless steel sample pan. The sample was heated from 30 to 250 °C at a heating rate of 10 °C/min in nitrogen environment.
2.4 Properties of cured MUF and PF resin
MUF resin was cured at 110, 120, and 130 °C. PF resin was hot-set at 135, 150, and 165 °C. The cured resins were ground to powders. The degree of gelation was determined by measuring the weight retention after the resin powders immersed in acetone for 24 h. Fourier transform infrared spectroscopy (FTIR; Perkin–Elmer Spectrum 100) analysis of the cured resins was carried out by an attenuated total reflection method (ATR) between 650 and 4000 cm−1. Thermal degradation was measured using TGA (Thermogravimetric analysis; Perkin–Elmer; Pyris 1) from 50 to 800 °C with a heating rate of 10 °C/min in N2 atmosphere.
2.5 Manufacturing of plywood
Three-layer plywood with dimensions of 30 × 30 × 0.4 cm3 was made with Lauan veneer. The thickness of the face and core veneer was 1 and 2 mm, respectively. To avoid over-penetration because of low viscosity, PF resin was mixed with Acacia confuse bark powder (passing through a 200-mesh) to adjust the viscosity to 1000–2000 cps prior to glue application. The amount of glue used for a single glue line was 250 g/m2. The MUF plywood was made by hot-pressing at 110, 120, and 130 °C for 5 min under a pressure of 1.2 MPa. The PF plywood was made at the same conditions except the hot-press temperature used was 135, 150, and 165 °C. All the plywood was stored at 20 °C and 65% RH environment for 1 week to adjust the moisture content.
2.6 Heat treatment and bonding strength test of plywood
The heat treatment of plywood was conducted in a carbonization furnace at temperatures of 200 and 250 °C for 2 h in N2 atmosphere. The tensile shear bonding strength under dry and wet conditions was measured according to the CNS 1349 standard with a universal strength-testing machine (Shimadzu UEH-10) at a loading speed of 1 mm/min. For MUF plywood, the wet bonding strength was measured after the specimens were immersed in 60 °C water for 3 h. For PF plywood, the wet bonding strength was measured after the specimens were immersed in boiling water for 4 h, followed by drying in an oven at 60 °C for 20 h, and finally immersion in boiling water again for 4 h. The wood failure of the test samples was estimated by naked eye to the nearest 5% of the shear area. Eight specimens were tested for each test set.
3 Results and discussion
3.1 Properties of synthetic resins
MUF and PF resins that are widely used in the wood industry were prepared in this study. Table 1 shows their basic properties. MUF resin has a viscosity, pH, solid content, and free-formaldehyde content of 1500 cps, 6.6, 60.8%, and 0.36%, respectively. While PF resin has lower viscosity and solid content, and higher pH than MUF resin, no free formaldehyde was detected.
3.2 DSC thermosetting of resins
Figure 1 shows the heat flow curves of MUF and PF resins. Both show an exothermic peak. However, the peak for the PF resin is more obvious than that of the MUF resin, indicating that the PF resin undergoes more crosslinking reaction than the MUF resin during heat scanning. Table 2 shows the DSC thermosetting parameters of MUF and PF resin. The beginning and ending temperatures for the reaction of the MUF resin are 115 and 161 °C, respectively. These values are 121 and 170 °C for the PF resin, which are higher than those for the MUF resin. This result indicates that the MUF resin has higher heat reactivity than the PF resin. However, MUF resin released less heat during the DSC analysis. This is because of the higher viscosity, corresponding to a higher molecular weight, which caused MUF to form a three-dimensional structure more easily and release less heat (Siimer et al. 2006).
3.3 FTIR analysis of cured resins
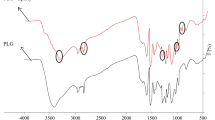
Figure 2 shows the FTIR spectra of MUF resins that cured at different temperatures. The broad absorption band at 3700 − 3000 cm−1 is attributed to the associated stretching vibration of N–H and O–H. Wherein the stretching vibration of O–H appears at a higher wavenumber than that of the N–H. The intensity of O–H absorption (between 3700 − 3300 cm−1) decreases with increasing curing temperature, indicating more condensation reactions may occur because of the hydroxymethyl groups at higher curing temperature. The absorption peak appearing at 1640 cm−1 is attributed to the C=O stretching vibration of urea. The peak at 1360 cm−1 is attributed to the presence of asymmetric N–C–N in the melamine. The peaks at 1540 cm−1 and 1495 cm−1 represent the secondary N–H bending vibration and methylene bridge (–CH2–), respectively. The relative intensity of the 1540 cm−1 peak to the 1495 cm−1 peak decreased as the curing temperature increases, indicating more methylene bridges have been formed at the higher temperature (Kandelbauer et al. 2007).
Figure 3 shows the FTIR spectra of the cured PF resins. The broad absorbance peak at 3200–3500 cm−1 is attributed to the O–H stretching vibration of phenol. The peaks at 2920 and 2850 cm−1 are the stretching vibrations of the asymmetric and symmetric CH2, respectively. The peaks at 1600 and 1470 cm−1 are the characteristic absorptions of the benzene ring. The 1440 cm−1 peak is attributed to the CH2 bending vibration. The peak at 1350 cm−1 corresponds to the in-plane bending vibration of the phenol O–H. The peak at 1217 cm−1 is attributed to the C–O vibration. The 880 cm−1 peak is attributed to the four substituted benzene rings (Costa et al. 2007). The peak that appears at around 1600 cm−1 can be distinguished into 1590 and 1610 cm−1, which corresponds to the stretching of the unsubstituted benzene ring and the 1,2,4-substituted ring, respectively (Křístková et al. 2004). The PF resin cured at 165 °C has the largest relative intensity of the peak ratio of 1610 cm−1/1590 cm−1, indicating more crosslinking reaction occurred at higher curing temperature.
3.4 Degree of gelation of cured resins
The weight retention ratio (WR) of cured resins after solvent immersion can be used as an index of the degree of gel formation (Chauvet et al. 2005). The unreacted components as well as the incompletely crosslinked structures inside the cured resin will be dissolved and cause a weight loss. Table 3 shows the WR of the cured resins after immersion in acetone. PF resins have a WR of over 95%, which is higher than that of MUF resins. Increasing the curing temperature has no effect on increasing the WR, indicating that 135 °C is enough for curing the PF resin. Conversely, the WR of MUF resins is dependent on the curing temperature. There is an obvious increase in WR with temperature from 110 to 120 °C, but is not clear for the temperature increase from 120 to 130 °C, indicating that MUF resin cured at 120 °C is the most efficient.
3.5 TGA of cured resins
Figure 4 shows the TG and DTG curves of MUF resin cured at different temperatures. The TG curves show that all have a similar thermal degradation behavior. A drastic weight loss appears at a temperature between 200 and 350 °C, followed by a more moderate weight loss between 450 and 680 °C. However, the resin cured at 120 °C has better heat stability than others at temperatures lower than 250 °C. DTG curves show that the broad peak at temperatures lower than 400 °C includes many small peaks, indicating that a complex pyrolysis reaction occurred in this temperature region.
Figure 5 shows the TG and DTG curves of cured PF resins. They have a higher heat resistance than MUF resins, and have a char yield of over 65% at a temperature of 800 °C. Their pyrolysis can be divided into four stages, i.e., lower than 300, 350–460, 460–600, and 700–800 °C. The first stage is due to the evaporation of absorbed water and formaldehyde from the hydroxymethyl groups. The second stage is caused by the breaking of the terminal phenol or methylene-phenol bond, which leads to the formation of phenol and cresol. At the same time, a condensation reaction between the hydroxyl groups and the methylene may release water. At the third stage, some complex reactions have occurred and form a polyarylated structure. These reactions include the etherification between the hydroxyl groups, the dehydrogenation between methylene and C–H to form a carbon link, the reaction between the methylene link with H2O and H2, and the breaking of the ether link. The main reaction in the last stage is dehydrogenation of the aromatic nucleus (Trick et al. 1997; Trick and Saliba 1995). However, the TG curves show that the resin cured at 165 °C has less weight loss, while the one cured at 135 °C has the highest weight loss. Besides, DTG curves indicate that the pyrolysis speed decreased with increase in curing temperature. Based on the above, it can be seen that PF resins cured at a higher temperature have a more complete crosslinking structure and better thermal stability.
3.6 Bonding strength of heat-treated plywood
Table 4 shows the dry bonding strength of untreated and heat-treated MUF and PF plywood made at different hot-press temperatures. The CNS 1349 standard requires that the bonding strength of Lauan plywood should exceed 0.7 MPa. As can be seen, plywood without heat treatment has a dry bonding strength between 0.98 and 1.52 MPa, which can meet the requirement of the CNS standard. Although heat treatment is a useful method to improve the dimensional stability of wood, the strength will decrease because of the thermal degradation of wood components, especially hemicellulose (Ramiah 1970; Alén et al. 1996; Weiland and Guyonnet 2003). When heat treatment has been performed for the glued wood composite, thermal degradation may occur both in wood and the resins. In addition, an internal stress may occur in the glue line, which also decreases the bonding strength. As can be seen, heat treatment decreases the bonding strength of plywood. However, except for PF −135 °C, plywood heat-treated at 200 °C for 2 h has a dry bonding strength that still meets the requirement. However, when heated at 250 °C, the dry bonding is lower than the requirement. However, plywood made with PF resin has wood failures greater than 75%, indicating that the low value of the bonding strength is due to the lower wood strength rather than the weakened glue line strength.
Table 5 shows the wet bonding strength of untreated and heat-treated plywood. MUF plywood is tested after 60 °C water immersion, whereas PF plywood is tested after repeated soaking in boiling water. This trend is similar to that of the dry bonding strength. All the plywood without heat treatment has the wet bonding strength that meets the requirement of the CNS standard. Most of the plywood treated at 200 °C meets the requirement, but none of those treated at 250 °C meet the requirement.
4 Conclusion
MUF and PF resins were prepared in this study. DSC results show that PF resin has a more obvious exothermic peak compared to the MUF resin. FTIR analysis shows that MUF resin cured at 130 °C and PF resin cured at 165 °C undergo more condensation reactions than the others. Cured PF resins have a higher degree of gelation than MUF resins. PF resins cured at 135 °C have a weight retention that is similar to those cured at 150 and 165 °C after dissolution in solvent. However, curing at 120 °C will be more suitable for the MUF resin. TGA results show that the thermal degradation behavior is not influenced by the curing temperature. A drastic weight loss appears at 200–350 and 450–680 °C for MUF resins. PF resins have better heat resistance than MUF resins, which has a char yield of over 65% at a temperature of 800 °C. An obvious weight loss appears at 350–460 and 460–600 °C. Increasing the setting temperature can result in better heat resistance. MUF and PF resin plywood have a dry and wet bonding strength that meet the requirement of the CNS 1349 standard. Heat treatment decreases the bonding strength of plywood. However, it still meets the CNS standard when heat-treated at 200 °C, but none meet the requirement when heat-treated at 250 °C.
References
Alén R, Kuoppala E, Oesch P (1996) Formation of the main degradation compound groups from wood and its components during pyrolysis. J Anal Appl Pyrol 36:137–148
Ayadi N, Lejeune F, Charrier F, Charrier B, Merlin A (2003) Color stability of heat-treated wood during artificial weathering. Holz Roh Werkst 61:221–226
Bekhta P, Niemz P (2003) Effect of high temperature on the change in color, dimensional stability and mechanical properties of spruce wood. Holzforschung 57:539–546
Boonstra MJ, Pizzi A, Zomers F, Ohlmeyer M, Paul W (2006) The effects of a two stage heat treatment process on the properties of particleboard. Holz Roh Werkst 64:157–164
Borrega M, Kärenlampi PP (2008) Mechanical behavior of heat-treated spruce (Picea abies) wood at constant moisture content and ambient humidity. Holz Roh Werkst 66:63–69
Borrega M, Kärenlampi PP (2010) Hygroscopicity of heat-treated Norway spruce (Picea abies) wood. Eur J Wood Prod (2010) 68:233–235
Candelier K, Thevenon MF, Petrissans A, Dumarcay S, Gerardin P, Petrissans M (2016) Control of wood thermal treatment and its effects on decay resistance: a review. Ann Forest Sci 73:571–583
Chauvet J, Asua JM, Leiza JR (2005) Independent control of sol molar mass and gel content in acrylate polymer/latexes. Polymer 46:9555–9561
Costa L, di Montelera LR, Camino G, Weil ED, Pearce EM (2007) Structure-charring relationship in phenol-formaldehyde type resins. Polym Degrad Stabil 56:23–35
Dubey MK, Pang S, Walker J (2011) Effect of oil heating age on colour and dimensional stability of heat treated Pinus radiate. Eur J Wood Prod 69:255–262
Esteves BM, Pereira HM (2009) Wood modification by heat treatment: a review. BioResources 4:370–404
Esteves BM, Domingos IJ, Pereira HM (2008) Pine wood modification by heat treatment in air. BioResources 3:142–154
Hill CAS (2006) Wood modification: chemical, thermal and other processes. Wiley, Chichester
Jebrane M, Sèbe G (2007) A novel simple route to wood acetylation by transesterification with vinyl acetate. Holzforschung 61:143–147
Jebrane M, Pichavant F, Sèbe G (2011) A comparative study on the acetylation of wood by reaction with vinyl acetate and acetic anhydride. Carbohyd Polym 83:339–345
Kandelbauer A, Despres A, Pizzi A, Taudes I (2007) Testing by fourier transform infrared species variation during melamine-urea-formaldehyde resin preparation. J Appl Polym Sci 106:2192–2197
Křístková M, Filipb P, Weissa Z, Peterc R (2004) Influence of metals on the phenol–formaldehyde resin degradation in friction composites. Polym Degrad Stabil 84:49–60
Kudanga T, Prasetyo EN, Widsten P, Kandelbauer A, Jury S, Heathcote C, Sipilä J, Weber H, Nyanhongob GS, Guebitzb GM (2010) Laccase catalyzed covalent coupling of fluorophenols increases lignocellulose surface hydrophobicity. Bioresour Technol 101:2793–2899
Kudanga T, Nyanhongob GS, Guebitzb GM, Burtona S (2011) Potential applications of laccase-mediated coupling and grafting reactions: a review. Enzyme Microb Technol 48:195–208
Mamiński MŁ, Król M, McDonald AG, McIlroy DN, Niraula IB, Czechowska J, Parzuchowski P (2013) Thermally initiated solvent-free radical modification of beech (Fagus sylvatica) wood. Wood Sci Technol 47:1019–1031
Nakano T, Miyazaki J (2003) Surface fractal dimensionality and hygroscopicity for heated wood. Holzforschung 57:289–294
Pétrissans M, Gérardin P, El bakali I, Serraj M (2003) Wettability of heat-treated wood. Holzforschung 57:301–307
Ramiah MV (1970) Thermogravimetric and differential thermal analysis of cellulose, hemicellulose, and lignin. J Appl Polym Sci 14:1323–1337
Rapp AO, Brischke C, Welzbacher CR (2006) Interrelationship between the severity of heat treatments and sieve fractions after impact ball milling: a mechanical test for quality control of thermally modified wood. Holzforschung 60:64–70
Rowell RM (1986) Vapor phase acetylation of southern pine, douglas-fir, and aspen wood flakes. J Wood Chem Technol 6:293–309
Sandberg D, Kutnar, A (2016) Thermally modified timber: recent developments in Europe and north America. Wood Fiber Sci 48:28–39
Sernek M, Boonstra M, Pizzi A, Despres A (2008) Bonding performance of heat treated wood with structural adhesives. Holz Roh Werkst 66:173–180
Shi JL, Kocaefe D, Zhang J (2007) Mechanical behaviour of Québecwood species heat-treated using ThermoWood process. Holz Roh Werkst 65:255–259
Siimer K, Kaljuvee T, Christjanson P, Lasn I (2006) Curing of urea-formaldehyde resins on a wood substrate. J Therm Anal Calorim 84:71–77
Trick KA, Saliba TE (1995) Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite. Carbon 33:1509–1515
Trick KA, Saliba TE, Sandhu SS (1997) A kinetic model of the pyrolysis of phenolic resin in a carbon/phenolic composite. Carbon 35:393–401
Walker JF (1975) Formaldehyde. Huntinton, New York
Weiland JJ, Guyonnet R (2003) Study of chemical modifications and fungi degradation of thermally modified wood using DRIFT spectroscopy. Holz Roh Werkst 61:216–220
Widsten P, Heathcote C, Kandelbauer A, Guebitz G, Nyanhongo GS, Prasetyo EN, Kudanga T (2010) Enzymatic surface functionalisation of lignocellulosic materials with tannins for enhancing antibacterial properties. Process Biochem 45:1072–1081
Zigon J, Pizzi A, Zhang H, Sega B, Cop M, Sernek, M (2015) The influence of heat and chemical treatments of beech wood on the shear strength of welded and UF bonded specimens. Eur J Wood Prod 73:685–687
Acknowledgements
We thank the Ministry of Science and Technology for financial support (NSC102-2815-C-005-042-B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, WS., Lee, WJ. Influence of curing temperature on the bonding strength of heat-treated plywood made with melamine-urea-formaldehyde and phenol–formaldehyde resins. Eur. J. Wood Prod. 76, 297–303 (2018). https://doi.org/10.1007/s00107-016-1154-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-016-1154-7