Abstract
The aim of this work was the formulation of adhesives for particleboards based on tannins extracted from industrial lignocellulosic wastes, namely chestnut shell, chestnut bur and eucalyptus bark. The interest was centred on the possibility of completely removing formaldehyde from adhesive formulations. For this, hardener alternatives to formaldehyde were used: tris(hydroxymethyl)nitromethane (TRIS), glyoxal (GLY) and hexametilentetramine (HEX). The influence of the type and concentration of the hardener and pH on adhesive gel time and pot-life were studied. A comparative structural characterization of the adhesives was performed by 13C-NMR spectroscopy. Thermomechanical analyses (TMA) tests were carried out as an indication of the final strength of the adhesive systems. Particleboards type 2 of interior use (EN 312) were manufactured with adhesives based on chestnut shell tannins alone or mixed with chestnut bur/or eucalyptus bark tannins. Free-formaldehyde was determined, and boards were prepared with TRIS, GLY and HEX tannin adhesives were classified as E0.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays wood panels are mainly manufactured with thermosetting synthetic resins, most of them based on formaldehyde. However, environmental and health considerations are leading to increasingly severe standards regarding the maximum value allowed for formaldehyde emission from wood boards. This fact coupled with the increasingly higher cost of synthetic resin based on petroleum has intensified the search for alternative resins based on natural materials for the formulation of wood adhesives. In this respect, the use of polyflavonoid tannins as components of wood adhesives is one of the proposed alternatives, not only to prevent the use of formaldehyde but also to reduce its emission from wood panels (Pizzi 2006; Kim 2009).
Chestnut is a tree distributed mainly in the northern hemisphere, in Asia, mainly in China, Korea and Japan, in the south of Europe from Turkey to the Atlantic islands and in the United States. The main varieties are Castanea mollissima in Asia (mainly China), Castanea sativa in Southern Europe and Turkey, and Castanea dentata in North America (Pereira-Lorenzo and Ramos-Cabrer 2004). In Galicia (NW of Spain) the food industry uses about 7,000 t/year of chestnuts (Castanea sativa) for the production of marron-glacé, chestnut purée and other products. The peeling process generates a waste product, the shell, which represents ~10 % of the weight of the whole chestnut, at present used as fuel. Additionally, a second residue is produced, the bur, which remains in the forest after chestnut harvesting.
Eucalyptus (Eucalyptus globulus) is one of the most important forest species in Galicia and represents 27 % of the total volume of processed wood in this community. Eucalyptus wood is used in Galicia to produce, firstly, cellulose pulp and, secondly, panels and boards. In both cases, the bark which represents around 15–19 % of the total wood volume is separated as a waste product and used as fuel in the factory.
In previous work (Vázquez et al. 2009a; 2010; 2011), the extraction of polyphenols from chestnut shell and eucalyptus bark was studied and the results revealed that the former had much better properties for the formulation of wood adhesives. Additionally, the main structural and functional characteristics of chestnut shell (Vázquez et al. 2013), eucalyptus bark (Vázquez et al. 2011) and chestnut bur (Vázquez et al. 2009b) tannin extracts were analysed. These studies demonstrated that chestnut shell tannins are mainly of condensed type whereas eucalyptus bark and chestnut bur tannins are of hydrolysable type.
In this work, the formulation of wood adhesives based on chestnut shell tannins, alone or combined with eucalyptus bark or chestnut bur tannins, was studied. The influence of the type and concentration of the hardener (paraformaldehyde (PAR), tris(hydroxymethyl) nitromethane (TRIS), glyoxal (GLY) or hexametilentetramine (HEX)) and pH on adhesive gel time and pot life were analyzed. Adhesives were characterized by thermomechanical analysis (TMA) and 13C-NMR spectroscopy. Particleboards were manufactured and board quality and formaldehyde emission were evaluated.
2 Materials and methods
2.1 Materials
Chestnut (Castanea sativa) shell (a mixture of the outer brown peel and the inner pellicle) was supplied by a food factory, Marrón Glacé, S.L. (Ourense, Spain), eucalyptus (Eucalyptus globulus) bark was supplied by a pulp factory (ENCE, Pontevedra, Spain), and chestnut bur was collected in a chestnut plantation in Vila de Cruces (Galicia, NW Spain). They were air dried till equilibrium moisture content, ground in a hammer mill, sieved, and the fraction of particle size between 0.1 and 2 mm was selected. Chemical composition of chestnut shell and eucalyptus bark has been determined in a previous work (Vázquez et al. 2008).
2.2 Extraction and concentration
The extraction experiments were carried out in a 10-L Pyrex glass reactor with mechanical stirring and temperature control. Solid/liquid ratio was 1/10 (w/w) for chestnut shell and bur and 1/15 (w/w) for eucalyptus bark. Aqueous solutions of sodium hydroxide and sodium sulphite were used as extraction agents. Alkali concentrations and extraction temperature are shown in Table 1.
The material and water were mixed at room temperature, heated and, once the selected temperature was attained, the alkali was added and contact time begun to run. After 1 h, the suspension was vacuum filtered, the solid residue was washed with water until a nearly colourless filtrate was obtained and the extract together with the first water washings were concentrated by spray-drying.
2.3 Adhesive gel time
In order to study the influence of the hardener type (PAR, GLY, TRIS and HEX), concentration (7.5–20 % on extract weight dry basis, see Table 2) and pH (from natural pH 6–10, see Table 3) on adhesive gel time, 20 % (by weight) aqueous solutions of CS1 chestnut shell tannin extract were prepared and the pH was modified when necessary with a sodium hydroxide aqueous solution. Five grams of the solution were introduced into a test tube which was placed in a boiling water bath, the appropriate amount of hardener was added and well mixed and time began to run. A wire spring was manually moved upwards and downwards until the mixture gelled. The analyses were done in triplicate and the results averaged.
2.4 Adhesive rheological behavior and pot life
The rheological behaviour of a 40 % (by weight) aqueous solution of CS1 chestnut shell tannin extracts (Table 4) and of adhesives prepared with different hardeners (PAR, HEX,GLY and TRIS) (Table 5) was studied at pHs from 6 (natural pH) to 9.
Measurements were performed at 25 °C with a Brookfield DV-II + viscosimeter using the small sample adapter at different times since tannin solution or adhesive preparation [from 0 (solution/adhesive freshly prepared) to 300 min]. Adhesive rheological behaviour was studied analysing the time course of the power law rheological parameters, n (the power law index) and k (the consistence index) at 25 °C. k and n were calculated from the power law by linear regression (R2 > 0.99). Hardener concentrations of 5 and 10 % (on extract weight, dry basis) for PAR and HEX and 10 % for GLY and TRIS were used. These measurements were used to evaluate adhesive pot life.
2.5 Adhesive preparation
For particleboard manufacture, adhesives were prepared as follows: a 40 % (w/w) aqueous solution of the extract or mixture of extracts was prepared and the percentage (on extract weight dry basis) of the selected hardener (TRIS, GLY, HEX or PAR) was added and mixed at room temperature. Adhesive pH was modified when necessary using a sodium hydroxide aqueous solution. The conditions used (type of extract, type and concentration of hardener and pH) are shown in Table 6 for the adhesives based on chestnut shell tannin extracts (CS1 and CS2), and in Table 7 for those prepared with mixtures of chestnut shell tannin extracts (CS1) with eucalyptus bark (EB) or chestnut bur (CB) ones. Adhesives were characterized by TMA and 13C-NMR spectroscopy.
2.6 Solid state 13C-NMR spectroscopy
An aqueous solution of CS1 tannin extract and adhesives prepared with CS1 and the different hardeners (10 % PAR at pH 6; 5 % HEX at pH 8; 10 % TRIS at pH 8; 10 % GLY pH 6) were hardened at 105 °C for 24 h in an oven and finely ground in a ball mill for NMR analysis. The solid state 1D CP-TOSS (Cross Polarization Total Suppression of Spinning Sidebands) 13C-NMR spectrum of adhesives based on the chestnut shell tannin extract were obtained at room temperature with a Varian Inova 750 spectrometer of 17.6 T. The speed of MAS rotation was of 9 kHz. A linear ramp was used for the cross-polarization, with a time contact of 5 ms and a TPPM heteronuclear decoupling was used with a field strength of 74 kHz. The number of scans was 5000 and the relaxation time between scans (d1) was 2.5 s.
2.7 Thermomechanical analysis (TMA)
Adhesives prepared with chestnut shell tannins CS1 and CS2 using different hardeners were characterized by TMA. Analyses were performed with a Mettler Toledo TMA 40 instrument using the following conditions: 30 mg of resin; heating rate 10 °C/min; temperature range 25–250 °C. Bending curves, which allow the determination of modulus of elasticity (MOE), were obtained using the 3-point bending deformation mode. The results were analyzed using the Stare software from Mettler Toledo.
2.8 Particleboard manufacture and testing
Adhesives were prepared as explained before. The amount of adhesive employed for all panels was 10 % based on wood weight (dry basis). Two sets of particleboards were prepared. The first set of panels was one layer 230 mm × 230 mm × 14 mm particleboards prepared in the ENSTIB-LERMAB laboratories with a mixture of beech (Fagus sylvatica) and Norway spruce (Picea abies) wood particles and adhesives based on CS1 or CS2 tannin extracts (Table 6). Boards were prepared using a SMI press at 25 bar maximum pressure and 195 °C press temperature for 7.5 min. The second set of panels was one layer 210 mm × 210 mm × 14 mm particleboards prepared in the laboratories of University of Santiago de Compostela with pine wood particles and adhesives based on mixtures of CS1 with EB or CB tannin extracts (Table 7). The boards were pressed in a laboratory Gumix press at 195 °C and 40 bar for 7.5 min.
Particleboards were evaluated by physical and mechanical tests according to European standards: density (EN 323: 1993), moisture content (EN 322: 1993), thickness swelling (EN 317: 1993), water absorption (EN 317: 1993) and internal bond strength (EN 319: 1993). Internal bond (IB) strength measurements were made for the original board samples and for samples used for the analysis of water resistance to test IB variation after water treatment. The water treated samples were dried in an oven for 24 h followed by 1 h in a desiccator and sanded. Board classification was done according to EN 312: 2010.
2.9 Board formaldehyde emission
Particleboard free-formaldehyde was determined by the flask method (EN 717-3: 1996) within the 24 h following board preparation. The method is based on storing of one to three board pieces (25 mm × 25 mm × 14 mm) with a total mass of around 20 g in a closed polyethylene or glass bottle (Salthammer et al. 2010 ) with 50 ml of distilled water at 40 °C for 180 min. The formaldehyde released by the test samples during this period of time was absorbed by the water. Then, the formaldehyde content was determined by the acetylacetone method (Hantzsch reaction) measuring the absorbance at 412 nm.
3 Results and discussion
3.1 Adhesive gel time
The results for adhesive gel time at natural pH as a function of hardener type and concentration are shown in Table 2. For PAR and HEX adhesives, the gel time was very short at all the concentrations essayed. On the contrary, TRIS adhesives showed significantly higher gel times than all the other adhesive systems. Gel time decreased when increasing hardener concentration from 7.5 to 20 %, except for GLY adhesives. In this case, gel time reached a minimum at a concentration between 10 and 15 %. Then, an intermediate concentration in the range analyzed, 10 %, was selected for analyzing the influence of pH on gel time and comparing its effect on the different hardeners. For GLY and TRIS adhesives, that concentration is close to the lower limit used with these hardeners for other tannin adhesives systems (Trosa and Pizzi 2001; Ballerini et al. 2005). For HEX and PAR lower concentrations, around 5 %, are habitually used (Pichelin et al. 1999).
Table 3 shows the effect of pH on the gel time of tannin adhesives prepared with 10 % of the different hardeners. Gel time diminished with increasing pH for PAR and TRIS adhesives, increased for the HEX ones and reached a minimum for GLY. It is notable the significant drop in the gel time observed for TRIS adhesives when increasing pH. In view of the results obtained, the working pHs selected to allow the employment of these adhesives for wood panels were the natural pH (pH 6) for PAR, pH 7–8 for TRIS and pH 6–8 for HEX and GLY.
3.2 Adhesive rheological behavior and pot-life
Table 4 shows the time course of the power law parameters, k and n, for a 40 % CS1 aqueous solution at various pHs. In addition, the apparent viscosity of the solution at a fixed shear rate was compared. As shown, the tannin solution had a pseudoplastic behavior which became more remarkable with increasing pH and time. In view of the results, it was concluded that pH of the extract solution for the formulation of wood adhesives must be between the natural pH (6) and 8. At higher pHs, the pot-life of the solution is significantly reduced as deduced from the increasingly higher values of apparent viscosity, which limits its application in the formulation of adhesives.
In a second stage, the rheological behavior of adhesives prepared with CS1 extract and the different hardeners was analyzed as a function of time and pH (Table 5). Like the tannin solution, the adhesives showed a pseudoplastic behaviour with n values less than the unity and more pronounced with the progress of the reaction at room temperature. This behaviour is of great interest because when the adhesive is subjected to stress during application its apparent viscosity decreases favouring the flow. With respect to adhesive, apparent viscosity which can limit its applicability generally decreased in the order PAR > HEX ≈ GLY > TRIS. For PAR, pot life decreased significantly with increasing pH, then pH 6 is recommended as previously concluded from gel time analysis. In the case of HEX adhesives, pH 8 was the selected one and the lower concentration, 5 %, allowed to considerably improve adhesive pot life. For GLY the lower pH provided a longer pot life.
The pot life of adhesives prepared using the CS2 tannin extract were analyzed in a previous work (Vázquez et al. 2012). Comparing CS1 and CS2 chestnut shell extracts it was noted that CS2 and the adhesives prepared with it exhibited a more pronounced pseudoplastic character than the CS1 ones. Furthermore, for both extracts TRIS adhesives exhibited the greatest pot lives, while the PAR ones the lowest ones, which limits the applicability of adhesives prepared with PAR.
3.3 CP-MAS 13C-NMR spectroscopy
The 13C-NMR spectra of the CS1 tannin extract and the adhesives prepared with CS1 and different hardeners under previously selected conditions (10 % PAR at pH 6; 5 % HEX at pH 8; 10 % TRIS at pH 8; 10 % GLY at pH 6) are shown in Fig. 1. The assignment of the bands was performed according to Pizzi (1994), Pizzi and Tekely (1995) and Pichelin et al. (1999). The relative intensity of the bands (Online Resource 1) was calculated taking as reference the intensity of the C3′ and C4′ bands (Pizzi and Stephanou 1994).
The presence of low intensity bands due to quinoid and enol structures, characteristic of the catechinic acid (170–200 ppm), both in the spectrum of CS1 cured alone (Fig. 1a) and of the adhesives cured with the four hardeners (Fig. 1b–e), indicates that the acid structure rearrangement has been produced but not to a large degree (Ohara and Hemingway 1991). The 13C-NMR spectrum of the uncured CS1 tannin extract, which was obtained in a previous work (Vázquez et al. 2013), was compared with that of the adhesives prepared with CS1 and the different hardeners. Except when GLY was used as hardener, the slight decrease in the intensity of the band due to the carbons C5 and C7 at 150–154 ppm indicates that the ratio of quinoid structures in the cured adhesives was low, as the intensity of this band indicates the percentage of carbons in these positions which have become nonaromatic quinoid structures (Pizzi and Meikleham 1995).
With respect to the band at around 105 ppm, due to formation of C4–C6 and C4–C8 interflavonoid bonds, a significant increase was detected for the CS1 cured extract (Fig. 1a) with respect to the uncured one (Vázquez et al. 2013), and the increase was more significant when hardeners (PAR, HEX, TRI and GLY) were used in adhesive formulation (Figs. 1b–e).
Comparing the spectra of the CS1 chestnut shell extract cured with PAR (Fig. 1b) and the extract cured without hardener (Fig. 1a), the major differences are the decrease in the intensity of the band due to C5, C7 and C9 carbons of the flavonoid A ring, the increase of the band due to the C1′ carbon which moves slightly to 132.0 ppm, the appearance of bands due to C5′ and C2′ carbons, suggesting a similar behaviour to that of commercial mimosa and pecan nut tannins (Pizzi and Stephanou 1994), and the increased intensity of the bands between 33 and 37 ppm due to the formation of interflavonoid methylene bridges. Furthermore, the relative intensity of the band at 72 ppm due to C3 sites in the interior chain and upper chain-ending positions (Lorenz and Preston 2002) also increased. Finally, when PAR was used as hardener a band at 84 ppm was detected. It is attributed to the presence of free formaldehyde, suggesting that the adhesive can lead to higher formaldehyde emissions than those prepared with the other hardeners (Ferg et al. 1993).
Hexamine presents two possible mechanisms of interflavonoid bonding, which normally co-exist in the final adhesive, that are the bonding by methylene bridges and by benzylamine bridges (Pichelin et al. 1999). The bands at 31 and 41 ppm in the spectrum of the adhesive cured with HEX (Fig. 1c) are due to methylene bridges and are similar to those formed with PAR. The bands at 59, 53 and 49 ppm correspond to tribenzylamine, dibenzylamine and monobenzylamine bridges, respectively (Sojka et al. 1981). The relative intensities of these bands (Online Resource 1) indicate that, in this adhesive, the link between flavonoids units is produced mainly by benzylamine bridges. In general, the more reactive the tannin is, the greater is the proportion of benzylamine bridges to methylene bridges (Pizzi and Tekely 1995). In this case, tribenzylamine bridges are the majority (74.5 % vs. 52.1 and 40.6 %). The intensity of the bands in the region of 83–93 ppm, where the band corresponding to C2 carbon and the bands due to the presence of formaldehyde appear, is lower than for the extract cured without hardener and with the other hardeners, indicating that HEX reacted forming benzylamine bridges instead of decomposing into ammonia and formaldehyde (Pichelin et al. 1999).
In the spectrum of the adhesive cured with TRIS (Fig. 1d), the intensity of the bands due to C6′, C2′ and C5′ carbons increased (as in the case of PAR and GLY) over those of tannins cured without hardener and with HEX. Moreover, the bands at 96–98 ppm for C6 and C8 carbons are less intense due to the participation of these carbons in the formation of interflavonoid bonds by methylene bridges, but were less reduced than when PAR was used. The appearance of bands in the 50–58 ppm region indicates that benzylamine bridges were also formed, although less than when HEX was used.
In the spectrum of the adhesive cured with GLY (Fig. 1e), the catechinic acid rearrangement involving the opening of the A ring was observed and, consequently, the decrease in the relative intensity of the band due to C5 and C7 carbons (band around 155 ppm). Compared with the CS1 cured without hardener, GLY adhesive showed a higher intensity of the bands at 33 and 37 ppm due to methylene bridges formation.
Comparing the relative intensities of the bands characteristic of polymerization reactions, the intensity of interflavonoid bonds (bands at around 105 ppm) and methylene bridges followed the sequence TRIS > PAR > GLY > HEX. The average degree of polymerization (DP) of chestnut shell CS1 tannin extract cured alone and the adhesives prepared with the different hardeners was determined using the equation proposed by Thompson and Pizzi (1995) (Eq. 1). Calculation of y was modified in the case of HEX and TRIS to consider the areas of the bands due to tribenzylamine (Tba), dibenzylamine (Dba) and monobenzylamine (Mba) bridges in addition to the area of the bands due to C4–C8 and C4–C6 bonds according to Eq. 2.
PAR and TRIS are the hardeners which provide adhesives with higher average polymerization degree: 35.9 (CS1 + PAR) >27.1 (CS1 + TRIS) >9.9 (CS1 + HEX) >9.1 (CS1 + GLY) >6.3 (CS1).
3.4 Thermomechanical analysis (TMA)
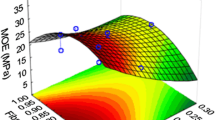
The curing of an adhesive can be evaluated by TMA by analyzing the rigidity of wood-adhesive joints as a function of temperature. The maximum value of the modulus of elasticity (MOE) of a wood-adhesive system and its increase as a function of time or temperature give a good indication of the possible end performance of the adhesive system tested (Lei et al. 2007). Thus, using TMA the technical viability of different adhesive formulations prepared was analyzed according to the tannin extract (CS1 or CS2) and hardener (PAR, GLY, HEX, TRIS) used and the pH selected (6–10). Figure 2 compares the maximum value of the MOE, Fig. 3 the variation undergone by the MOE to reach the maximum (ΔMOE) and Fig. 4 the temperature needed to reach that value.
The highest maximum MOE value was obtained for the adhesive prepared with CS2 and 10 % HEX at pH 8, but very close values were obtained for most of HEX adhesives. The highest ΔMOE value corresponded to the adhesive prepared with CS1 and 5 % HEX at pH 8. In general, MOE and ΔMOE were greater for the adhesives prepared with the CS2 tannin extract. However, for PAR adhesives, the one based on CS1 presented a slightly higher value of MOE, but ΔMOE was considerably lower than that obtained for the CS2 one. For a particular extract and hardener, adhesive pH hardly affected MOE while ΔMOE values varied significantly in most cases and generally decreased with increasing pH.
With respect to the temperature required to reach the maximum MOE value, it varied between 180 °C, for the adhesive prepared with CS1 and 10 % PAR at pH 6, and 110 °C, for the adhesive prepared with CS2 and 10 % TRIS at pH 6. The influence of pH, type of tannin extract and hardener concentration depended on the hardener used.
Garnier et al. (2002) found a very good correlation between the dry internal bond (IB) strength of particleboards bonded with tannin–formaldehyde resins and the results of TMA bending experiments. In this work, the adhesive with the highest ΔMOE value, that prepared with CS1 and 5 % HEX at pH 8 also showed one of the highest dry IB values (Table 6). On the other hand, ΔMOE values for adhesives prepared with CS2 depended on the hardener used in the same way as dry IB, TRIS > PAR > HEX > GLY.
3.5 Evaluation of board quality
Particleboards were manufactured with the adhesives prepared with CS1 and CS2 chestnut shell extracts formulated under the conditions (hardener concentration and pH) previously selected. Table 6 shows the results obtained for density and internal bond strength for dry boards and for boards after immersion in water for 24 h. In addition, the values of thickness swelling (%) and water absorption (%) are presented comparatively for boards after 2 and 24 h of water immersion.
The IB strength of a board is a direct measure of the performance of the adhesive. All boards prepared complied with the dry IB strength requirement of the European norm EN 312 for boards of type P2 for interior use in dry conditions. Comparing the IB values for dry boards, a slight influence of the type of tannin extract and hardener used was found. The best results were obtained for the boards prepared with the adhesives formulated with the CS1 extract and two of the hardeners selected as alternative to PAR, TRIS and HEX, in this order. However, as extraction yield was significantly higher for CS2 extract (Table 1), adhesives formulated with CS2 would be selected for improving process economy. In addition, as HEX is cheaper than TRIS and is used in lower concentration, HEX would be the selected hardener. From the 13C-NMR analysis it was concluded that the adhesive average polymerization degree depended on the hardener used, decreasing in the order TRIS > HEX > GLY for the hardeners selected as alternative to paraformaldehyde, and IB strength values for dry boards followed the same order (Table 6).
Analyzing the water resistance characteristics of the boards, none of them complied with EN 312 standard for boards of type P3. However, those prepared with the adhesives formulated with CS1 showed higher water resistance, with absorption and thickness swelling percentages slightly higher than those formulated with CS2. The differences can be attributed to the higher concentration of sodium sulfite in CS2, which increased tannin solubility but reduced water resistance properties of the adhesives. The best IB results after 24 h of water immersion were obtained for the boards prepared with the adhesives formulated with CS1 using PAR and TRIS as hardeners, which also showed the lowest values of thickness swelling and absorption percentages.
3.6 Board formaldehyde emission
As seen in Table 8, formaldehyde emission of boards prepared with adhesives formulated with HEX, TRIS and GLY were significantly lower than that of the boards prepared with PAR adhesives. Furthermore, HEX was the hardener that led to the boards with the lowest formaldehyde emission.
The formaldehyde emission method used in this work (EN 717-3) does not classify boards based on formaldehyde emission. Then, the results obtained were tested according to Risholm-Sundman and Wallin (1999) who correlated the results analyzed by different official methods. Thus, formaldehyde emission of boards prepared with adhesives based on hardeners alternative to PAR was within the limits established for the E0 commercial label (≤2.4 mg formaldehyde/kg dry board; based on EN 13986: 2004) and considerably below the values obtained for boards manufactured with commercial resins such as urea–formaldehyde or melamine-urea–formaldehyde (Navarrete et al. 2012).
3.7 Adhesives prepared by mixing condensed and hydrolysable tannin extracts
In a second stage, particleboards were prepared with adhesives formulated with mixtures of tannin extracts from chestnut shell (CS1) and eucalyptus bark (EB) or chestnut bur (CB) using 10 % of TRIS as hardener at pH 8. Table 7 shows the results obtained for density and internal bond strength for dry boards and for boards after immersion in water for 24 h, together with the values of thickness swelling (%) and water absorption (%) for boards after 2 and 24 h of water immersion.
Particleboards prepared using adhesives prepared with mixtures of tannin extracts from chestnut shell (CS1) and eucalyptus bark (EB) complied with the dry IB strength requirement of the European norm EN 312 for interior use boards of type P2 for EB tannin contents of up to 40 %. With respect to the results obtained for water resistance, none of the boards overcame the requirements of the EN 312 standard. However, particleboards prepared with 40 % EB slightly exceeded the IB value after 24 h of water immersion obtained for the particleboards prepared with 100 % CS1.
Particleboards prepared using adhesives prepared with mixtures of tannin extracts from chestnut shell (CS1) and chestnut bur tannin extract (CB) complied with the dry IB strength requirement of the European norm EN 312 for interior use boards of type P2, however any board exceeded the standard requirements for exterior use. IB decreased as CB percentage increased from 20 to 40 %.
4 Conclusion
Particleboards were manufactured with adhesives formulated with chestnut shell tannin extracts and different hardeners: tris(hydroxymethyl)nitromethane, glyoxal and hexametilentetramine, which could replace the commercial wood adhesives based on phenol and formaldehyde for interior use applications. Moreover, condensed chestnut shell tannins could be combined with low proportions (<40 %) of hydrolysable type tannins, such as chestnut bur or eucalyptus bark, without reducing board quality. Tannin adhesives prepared have the additional advantage of removing completely formaldehyde from the formulations. Particleboards classified as E0 with respect to formaldehyde emission were obtained, which means an important progress to pass the increasingly severe environmental restrictions of the board industry.
References
Ballerini A, Despres A, Pizzi A (2005) Non-toxic, zero emission tannin-glyoxal adhesives for wood panels. Eur J Wood Wood Prod 63:477–478
EN 13986 (2004) Wood-based panels for use in construction—Characteristics, evaluation of conformity and marking
EN 317 (1993) Particleboards and fibreboards-Determination of swelling in thickness after immersion in water
EN 319 (1993) Particleboards and fibreboards-Determination of tensile strength perpendicular to the plane of the board
EN 322 (1993) Wood-based panels-Determination of moisture content
EN 323 (1993) Wood-based panels-Determination of density
EN 717-3 (1996) Wood-based panels-Determination of formaldehyde release. Formaldehyde release by the flask method
EN 312 (2010) Particleboards-Specifications
Ferg EE, Pizzi A, Levendis DC (1993) 13C NMR Analysis method for urea formaldehyde resin strenght and formaldehyde emission. J Appl Polym Sci 50:907–915
Garnier S, Pizzi A, Huang Z (2002) Dry I.B. forecasting of commercial tannin adhesives-bonded particleboard by TMA bending. Holz Roh Werkst 60:372
Kim S (2009) Environment-friendly adhesives for surface bonding of wood-based flooring using natural tannin to reduce formaldehyde and TVOC emission. Bioresource Technol 100:744–748
Lei H, Pizzi A, Guanben D (2007) Environmentally friendly mixed tannin/lignin wood resins. J Appl Polym Sci 107:203–209
Lorenz K, Preston CM (2002) Characterization of high-tannin fractions from humus by carbon-13 cross-polarization and magic angle spinning nuclear magnetic resonance. J Environ Qual 31:431–436
Navarrete P, Pizzi A, Tapin-Lingua S, Benjelloun-Mlayah B, Pasch H, Rode K, Delmotte L, Rigolet S (2012) Low formaldehyde emitting biobased wood adhesives manufactured from mixtures of tannin and glyoxylated lignin. J Adhes Sci Technol 26:1667–1684
Ohara S, Hemingway RW (1991) Condensed tannins: the formation of a diarylpropanol- catechinic acid dimer from base-catalyzed reactions of (+)-catechin. J Wood Chem Technol 11(2):195–208
Pereira-Lorenzo S, Ramos-Cabrer AM (2004) Chestnut, an ancient crop with future. Prod Pract Quality Assess Food Crops 1:105–161
Pichelin F, Kamoun C, Pizzi A (1999) Hexamine hardener behaviour: effects on wood glueing tannin and other wood adhesives. Holz Roh Werkst 57(5):305–317
Pizzi A (1994) Advanced wood adhesives technology. Marcel Dekker, New York
Pizzi A (2006) Recent developments in eco-efficient bio-based adhesives for wood bonding: opportunities and issues. J Adhes Sci Technol 20(8):829–846
Pizzi A, Meikleham N (1995) Induced accelerated autocondensation of polyflavonoid tannins for phenolic polycondensates. III. CP-MAS 13C-NMR of different tannins and models. J Appl Polym Sci 55:1265–1269
Pizzi A, Stephanou A (1994) A 13C NMR study of polyflavonoid tannin adhesive intermediates. I. Non colloidal performance determining rearrangements. J Appl Polym Sci 51:2109–2124
Pizzi A, Tekely P (1995) Mechanism of polyphenolic tannin resin hardening by hexamethylentetramine: CP-MAS 13C-NMR. J Appl Polym Sci 56:1645–1650
Risholm-Sundman M, Wallin N (1999) Comparison of different laboratory methods for determining the formaldehyde emission from three-layer parquet floors. Holz Roh Werkst 57:319–324
Salthammer T, Mentese S, Marutzky R (2010) Formaldehyde in the indoor environment. Chem Rev 110(4):2536–2572
Sojka SA, Wolfe RA, Guenther GD (1981) Formation of phenolic resins: mechanism and time dependence of the reaction of phenol and hexamethylentetramine as studied by Carbon-13 Nuclear Magnetic Resonance and Fourier Transform Infrared Spectroscopy. Macromolecules 14:1539–1543
Thompson D, Pizzi A (1995) Simple 13C-NMR methods for quantitative determinations of polyflavonoid tannin characteristics. J Appl Polym Sci 55:107–112
Trosa A, Pizzi A (2001) A non-aldehyde emission hardener for tannin-based wood adhesives for exterior panel. Holz Roh Werkst 59:266–271
Vázquez G, Fontenla E, Santos J, Freire MS, González-Alvarez J, Antorrena G (2008) Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind Crop Prod 28:279–285
Vázquez G, González-Álvarez J, Santos J, Freire MS, Antorrena G (2009a) Evaluation of potential applications for chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind Crop Prod 29:279–285
Vázquez G, González-Álvarez J, Freire MS, Fernández-Agulló A, Santos J, Antorrena G (2009b) Chestnut burs as a source of natural antioxidants. Chem Eng Trans 17:855–860
Vázquez G, Freire MS, Santos J, Antorrena G, González-Álvarez J (2010) Optimisation of polyphenols extraction from chestnut shell by response surface methodology. Waste Biomass Valor 1:119–225
Vázquez G, Santos J, Freire MS, Antorrena G, González-Álvarez J (2011) Extraction of antioxidants from eucalyptus (Eucalyptus globulus) bark. Wood Sci Technol 46:443–457
Vázquez G, Santos J, Freire MS, Antorrena G, González-Álvarez J (2012) DSC and DMA study of chestnut shell tannins for their application as wood adhesives without formaldehyde emission. J Therm Anal Calorim 108:605–611
Vázquez G, Pizzi A, Freire MS, Santos J, Antorrena G, González-Álvarez J (2013) MALDI-TOF, HPLC-ESI-TOF and 13C-NMR characterization of chestnut shell tannins for wood adhesives. Wood Sci Technol 47:523–535
Acknowledgments
This work was funded by Ministerio de Ciencia e Innovación, Feder FUNDS and Plan E Fundy (CTQ2009-07539). Authors are also grateful to Ministerio de Ciencia e Innovación for a research grant awarded to J. Santos (BES-2006-13886).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santos, J., Antorrena, G., Freire, M.S. et al. Environmentally friendly wood adhesives based on chestnut (Castanea sativa) shell tannins. Eur. J. Wood Prod. 75, 89–100 (2017). https://doi.org/10.1007/s00107-016-1054-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-016-1054-x