Abstract
The antitermitic activity of extracts from the branch heartwood of Chamaecyparis obtusa (hinoki) against Japanese termites (Reticulitermes speratus) was compared with that of the trunk. Samples of branch and trunk heartwood were extracted with n-hexane, ethyl acetate, and methanol successively. n-Hexane extracts of branch and trunk heartwood were strongly antitermitic, and branch heartwood contained greater quantities of active n-hexane extracts than trunk heartwood. Germacra-1-(10), 5-dien-4β-ol, t-cadinol, α-cadinol, hinokiresinol, and hinokinin were separated from the branch extracts and the termiticidal and antifeedant activity of these compounds was tested by no-choice and dual-choice test methods. The sesquiterpenoids, germacra-1-(10), 5-dien-4β-ol, t-cadinol and α-cadinol were strongly termiticidal. The norlignan hinokiresinol and lignan hinokinin had weak termiticidal, and strong antifeedant and repellent activity. High concentrations of germacra-1-(10), 5-dien-4β-ol and hinokiresinol were present in branch heartwood. These compounds protect hinoki branches from termites and other harmful organisms. Hinoki branch heartwood, which is usually unused, is a potential source of active antitermitic compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chamaecyparis obtusa Endl. (hinoki) has been a common and traditional plantation conifer for many years, and covers about 25 % of plantation forest in Japan (Japan Forestry agency 2011). Hinoki is a durable building timber and the timber is well known for antitermitic properties (Matsushima et al. 1990; Kinjo et al. 1988; Ohtani et al. 1996). Several bioactive compounds, such as mono- and sesquiterpenoids, have been extracted from the wood (Kondo and Imamura 1986; Kinjo and Yaga 1986; Hong et al. 2004; Yanga et al. 2007). Previous studies identified several of these chemicals and investigated the bioactivities of hinoki heartwood, leaves, bark, and nuts (Yanga et al. 2007; Shiei et al. 1981; Ishii and Kadoya 1993). However, there are few reports on the bioactivity of hinoki branch heartwood. Here, the allelopathy and activity of chemicals extracted from hinoki branch heartwood against harmful organisms were investigated and several strongly antifungal sesquiterpenoids were found. The characteristic components of branch heartwood, germacra-1-(10), 5-dien-4β-ol and hinokiresinol, also killed brine shrimp (Artemia salina) (Morikawa et al. 2012).
Antitermitic activity of branch is found in essential oils extracted from other plants, such as Melaleuca spp. (Sakasegawa et al. 2003). Kijidani et al. (2012) reported that antitermitic activity of hinoki depended on the color of the heartwood; red varieties exhibited higher termite resistance than yellow varieties. In an earlier study, it was found that trunk and branch heartwood color differs, and it was hypothesized that the vivid red hinoki branch heartwood is more resistant to termites than the paler trunk heartwood.
At present, hinoki branch wood is considered valueless and is not used. As the branch occupies approximately 20–30 % of the mass of the tree, the value of this resource will increase if bioactive compounds can be extracted from the branches. The aim of this study was to investigate the antitermitic activity against a subterranean termite (Reticulitermes speratus), which damages wooden structures in Japan, comparing the activity of branch and trunk heartwood.
2 Materials and methods
2.1 Plant materials and sample separation
Sample materials from a previous study were used (Morikawa et al. 2012). Hinoki trees (93 years) were felled from the Yamagata Field Science Center (Faculty of Agriculture, Yamagata University, Japan) in Tsuruoka City. Heartwood (3.5 cm diameter of a branch of 5 cm id) was sampled from trunk logs (ca. 28 cm id × 35 cm) and branches (ca. 5 cm id × 20 cm). Samples (50.0 g) of branch and trunk heartwood were crushed using a Wiley mill and extracted with n-hexane, ethyl acetate, and methanol successively at ambient temperature for 7 days.
Branch heartwood extracts (n-hexane 5.20 g; ethyl acetate 3.72 g; methanol 0.66 g) and trunk heartwood extracts (n-hexane 1.04 g; ethyl acetate 0.36 g; methanol 0.48 g) were collected. The n-hexane extracts of branch (4.01 g) was passed through a silica gel (60 N, spherical 63–210 µm, neutral; Kanto Chemical Co., Inc., Japan) chromatography column, and the n-hexane fraction (893 mg) was collected and labeled ‘H-1’. The column was further eluted with successive mixtures of n-hexane:ethyl acetate (ranging from 100:1 n-hexane:ethyl acetate to ethyl acetate only) and 40 fractions (labeled fr. h1–h40) were collected. Fr. h15 and h16 (321 mg, n-hexane:ethyl acetate, 6:1) were isolated as germacra-1-(10), 5-dien-4β-ol. Fr. h18 (238 mg, n-hexane:ethyl acetate, 4:1) was further purified by passing through an activated alumina (spherical 75 µm; Wako pure Chemical Industries, Ltd., Japan) chromatography column with n-hexane to isolate t-cadinol (195 mg). Fr. h20 and h21 (445 mg, n-hexane:ethyl acetate, 3:1) contained α-cadinol. Similarly, the ethyl acetate extract (2.46 g) was fractionated through a silica gel 60 N chromatography column with successive solvent mixes of n-hexane:acetone (100:1 to acetone only), and 27 fractions were collected (fr. a1–a27). Fr. a6–a13 (1.09 g) was passed through a silica gel 60 N chromatography column with successive mixes of n-hexane:acetone (1:6 n-hexane:acetone to acetone only) and 25 fractions were collected (fr. b1–b25). Fr. b8 was a colorless crystal of hinokinin (38 mg). Similarly, fr. b10–b17 (0.61 g) was passed through a silica gel 60 N chromatography column using successive mixtures of n-hexane: acetone (1:3 to acetone only) and 28 fractions (fr. c1–c28) were collected. Fr. c23–c25 was a vivid red powder of hinokiresinol (130 mg).
The purity of compounds in these fractions was analyzed by means of gas–liquid chromatography (GC) with a flame ionization detector (FID). Germacra-1-(10), 5-dien-4β-ol (81.7 % GC), t-cadinol (85.5 % GC), hinokiresinol (92.2 % GC) and hinokinin (95.4 % GC) were analyzed with impurities of less than 1.0 % GC. α-Cadinol (77.8 % GC) sample contained t-cadinol (8.9 % GC) and other impurities (not more than 1.0 % GC). The structures of the compounds in hinoki branch heartwood extracts are shown in Fig. 1.
2.2 Analysis of samples
n-Hexane and ethyl acetate extracts of heartwoods were analyzed by means of GC-FID with a HITACHI G-3000 gas chromatograph (HITACHI Ltd., Japan). Each compound was analyzed by gas–liquid chromatography-mass spectroscopy (GC–MS). The above compounds, except for germacra-1-(10), 5-dien-4β-ol and α-cadinol, were identified by comparison to GC–MS data of standard compounds stocked in the laboratory. GC–FID analysis was performed with a HITACHI G-3000 gas chromatograph (HITACHI Ltd., Japan) under the following conditions: DB-1 capillary column (30 m × 0.32 mm id; 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA); starting temperature was 100 °C (1 min) and increased with a gradient of 4 °C/min up to 280 °C (15 min); injection and detection temperature were 250 °C. Helium was used as the carrier gas with a column head pressure of 50 kPa. GC–MS data were measured with a Shimadzu QP-5000 GC–MS (SHIMADZU Corp., Japan): DB-1 capillary column (30 m × 0.32 mm id; 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA); column temperature was started at 100 °C (1 min) and increased with a gradient of 4 °C/min up to 280 °C (15 min); injection temperature of 250 °C; and interface temperature of 250 °C. The acquisition mass range was 50–450 amu. Helium was used as the carrier gas with a column head pressure of 50 kPa. The identification of peaks, except for the isolated compounds, on the chromatogram was based on comparison of mass spectra with those in the NIST 62 Mass spectral library.
Nuclear magnetic resonance (NMR) data were compared with the published data (Medarde et al. 1995; El-Shazly and Hussein 2004) using a Shimadzu QP-5000 (SHIMADZU Corp., Japan) GC–MS and JEOL JNM-EX400 (1H 400 MHz/13C 100 MHz) spectrometer (JEOL Ltd., Japan).
2.3 Termites
Colonies of R. speratus Kolbe were obtained from the wild in Tsuruoka, Yamagata in 2010 and maintained in humidified chambers at 26 ± 1 °C until start of the test.
2.4 Antitermitic test
Termiticidal and antifeedant activity were tested by no-choice and dual choice methods. Test samples were dissolved in acetone and applied to paper discs (8 mm diameter, 1.5 mm thickness, ca. 30 mg weight) at 0.25, 0.5 and 1.0 %, or 0.5, 1.0 and 2.0 % (sample weight/paper disc weight ratios); discs were dried in a vacuum desiccator for 24 h. Control disks were dosed with 120 µl pure acetone and dried. The no-feed experiments were completed at the same time as the feeding test samples, but no paper discs were placed in the Petri dishes.
The no-choice test followed previous methods (Kusumoto et al. 2009; Seru et al. 2004). Briefly, impregnated paper discs were placed on 2.0 g sterile sea sand (particle size 0.1–0.3 mm; Kanto Chemical Co., Inc., Japan) in Petri dishes (50 mm diameter, 10 mm high). The dual-choice test was performed according to previous studies (Ashitani et al. 2013; Ohmura et al. 2000). A control disc was put together with an impregnated paper disc on the sea sand in the petri dish allowing termites to feed on either control or impregnated disc. The sand was moistened to supply water. Ten R. speratus workers were placed in each Petri dish, and the dishes were kept in a dark room for 21 days at 26 ± 1 °C and 60 % relative humidity. Three replicates of each treatment were prepared. Dead termites were removed daily and percent mortality rate was calculated. After 21 days, the sample discs were vacuum dried, weighed, and feeding activity was estimated by calculating the mass loss (percent weight loss) by:
-
Termite mortality (%) = 100 × (Number of dead termites/Number of initial termites)
-
Mass loss (%) = 100 × (pre-treatment paper disc weight−post-treatment paper disc weight)/pre-treatment paper disc weight.
2.5 Statistical analysis
Mean and SE (n = 3) of mortality and weight loss for each treatment were calculated and compared by analysis of variance (ANOVA) and a protected Tukey–Kramer test using Statcel 2 software (OMS Inc., Japan). Weight losses in each treatment were compared by t test, and p < 0.05 was considered significant.
3 Results and discussion
3.1 Antitermitic activity of successive extracts
Both branch and trunk heartwood n-hexane extracts exhibited termiticidal activity and inhibited termite feeding (Fig. 2). The effect of the branch extracts was the strongest after 7 days (Fig. 2a). All extracts significantly inhibited feeding (Fig. 2b).
a Lethal and b antifeedant activities of extracts from hinoki branch and trunk heartwood. No-choice tests. Bars = mean ± SE. ‘NF’ means no-feed experiments. Common letters denote no significant difference. Tukey–Kramer, p < 0.05, small letter after 7 days, capital letter after 22 days. Asterisk means significantly different from control mass loss. t test, p < 0.05
In a previous study, it was found that branch heartwood yielded six times more n-hexane extract and 12 times more ethyl acetate extract than trunk heartwood (Morikawa et al. 2012), and branch heartwood contained more active extracts than the trunk heartwood.
3.2 Active components in extracts from branch heartwood
In a previous work, it was reported that branch and trunk heartwood n-hexane extracts contained t-muurolol (Morikawa et al. 2012). In this study, NMR analysis was used and this component of the trunk heartwood n-hexane extract was compared with other published data and identified as α-cadinol (El-Shazly and Hussein 2004). The two compounds t-muurolol and α-cadinol are diastereomers and have similar mass spectra in GC–MS, but can be identified by NMR analysis.
β-Thujaplicin (hinkitiol) which is known to have antitermitic (Nakashima and Shimizu 1972) and antifungal properties (Sakasegawa et al. 2003; Morita et al. 2004) was used as the positive control.
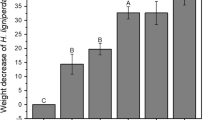
The H-1 extract obtained in this study was a mixture of hydrocarbon compounds (β-elemene, copaene, α-muurolene, γ-cadinene, and δ-cadinene) having little or no termiticidal activity (Fig. 3a). The sesquiterpene compounds, germacra-1-(10), 5-dien-4β-ol, t-cadinol, and α-cadinol are strongly termiticidal (Fig. 3a). After 7 days, high concentrations (0.5, 1.0 %) of these compounds had killed all termites, whereas high concentrations of hinokinin and β-thujaplicin were less lethal after 21 days. All extracts depressed feeding activity compared with the controls (Fig. 3b); sesquiterpene compounds inhibited feeding and killed the termites. Hinokiresinol and hinokinin inhibited feeding, but these compounds did not kill termites (Fig. 3a, b). In the dual-choice test, the sesquiterpene compounds (germacra-1-(10),5-dien-4β-ol, t-cadinol, and α-cadinol) killed termites faster than hinokiresinol (Fig. 4a), hinokinin, and β-thujaplicin, although feeding was inhibited at all concentration levels (Figs. 3, 4b). These data indicate hinokiresinol and hinokinin repel termites but are not lethal. It was observed that termites covered hinokiresinol impregnated paper discs with sterile sea sand in the no-choice and dual-choice tests and concluded that hinokiresinol was strongly antifeedant.
a Lethal and b antifeedant activities of compounds isolated from hinoki branch heartwood. No-choice tests. Bars = mean ± SE. NF means no-feed experiments. Common letters denote no significant difference. Tukey–Kramer, p < 0.05, small letter after 7 days, capital letter after 22 days. Asterisk means significantly different from control mass loss. t test, p < 0.05
a Lethal and b repellent feedant activities of compounds isolated from hinoki branch heartwood. Dual-choice tests. Bars = mean ± SE. NF means no-feed experiments. Common letters denote no significant difference. Tukey–Kramer, p < 0.05, small letter after 7 days, capital letter after 22 days. Asterisk mean differs from control. T-test, p < 0.05. Double asterisk mean differs from the control put in same petri dish. t test, p < 0.05
Several compounds are abundant in hinoki branch heartwood. The norlignan hinokiresinol and the lignan hinokinin have weak termiticidal activities, but protect heartwood by repelling termites and antifeedant activities. Active sesquiterpenes, germacra-1-(10), 5-dien-4β-ol, t-cadinol and α-cadinol are termiticidal. The purities of sesquiterpene samples used in this study were not so high, because the samples were obtained by fractionation from the branch extract directly. The compounds t-cadinol and α-cadinol are known as antitermitic compounds in the heartwood of C. obtuse (Ohtani et al. 1996; McDaniel 1989). Germacra-1-(10), 5-dien-4β-ol and the norlignan hinokiresinol are characteristic components of branch heartwood. From the above results, it can be seen that branch heartwood protects itself by termiticidal activities of sesquiterpenes and antifeedant activities of hinokiresinol and hinokinin. These compounds also inhibit other organisms, and t-cadinol, α-cadinol and hinokiresinol inhibit the growth of wood rotting fungi (Morikawa et al. 2012). Although germacra-1-(10), 5-dien-4β-ol is weakly antifungal, this compound has been shown to kill brine shrimp, a species that can be used to monitor allelopathic compounds (Ohira and Yatagai 1994; Tanaka et al. 1979; Ali et al. 2011). Hinokiresinol can also kill brine shrimp.
4 Conclusion
The combination of different compounds protects hinoki branches from attack by a wide range of harmful organisms. Hinoki branch heartwood contains greater quantities of active n-hexane extracts than trunk heartwood. The n-hexane extracts yield mainly active sesquiterpenes, germacra-1-(10), 5-dien-4β-ol, t-cadinol and α-cadinol. The norlignan hinokiresinol and lignan hinokinin had weak termiticidal, but strong antifeedant and repellent activity. Branch heartwood contains more germacra-1-(10), 5-dien-4β-ol and hinokiresinol than trunk heartwood and may increase resistance to termite attack. Some of these compounds may also be a source of antifungal and anti-insect chemicals that may be used to protect wooden structures.
References
Ali N, Ahmed G, Shah SWA, Ismail Shah, Ghias M, Imran Khan (2011) Acute toxicity, brine shrimp cytotoxicity and relaxant activity of fruits of Callistemon citrinus curtis. BMC Compl Alternative Med 11:99
Ashitani T, Kusumoto N, Borg-Karlson Anna-Karin, Koki Fujita, Takahashi K (2013) Antitermite activity of β-caryophyllene epoxide and episulfide. Z Naturforsch C 68(7–8):302–306
El-Shazly AM, Hussein KT (2004) Chemical analysis and biological activity of the essential oil of Teucrium leucocladum Boiss (Lamiaceae). Biochem Syst Ecol 32(7):665–674
Hong EJ, Na KJ, Choi IG, Choi KC, Jeung EB (2004) Antibacterial and antifungal effects of essential oils from coniferous trees. Biol Pharm Bull 27(6):863–866
Ishii T, Kadoya K ((1993)) Phytotoxic constituents in the bark and sawdust extracts of Chamaecyparis obtusa and Cryptomeria japonica and their effects on the growth of seedlings of trifoliate orange (Poncirus trifoliata Raf.) and rice (Oryza sativa L.). Engei Gakkai Zasshi 62(2):285–294
Japan Forestry agency (2011) Annual report on trends in forests and forestry fiscal year 2011. Tokyo, Japan, pp 55–56
Kijidani Y, Sakai N, Kimura K, Fujisawa Y, Hiraoka Y, Matsumura J, Koga S (2012) Termite resistance and color of heartwood of hinoki (Chamaecyparis obtusa) trees in five half-sib families in a progeny test stand in Kyushu. Japan. J Wood Sci 58(6):471–478
Kinjo K, Yaga S (1986) Study on the cultivation culture media of basidiomycetes IV. Antifungal activity of hinoki. Mokuzai Gakkaishi 32(8):632–634
Kinjo K, Doufuku Y, Yaga S (1988) Termiticidal substances from the wood of Chamaecyparis obtusa Endl. Mokuzai Gakkaishi 34(5):451–455
Kondo R, Imamura H (1986) Antifungal compounds in heartwood extractives of hinoki (Chamaecyparis obtusa Endl). Mokuzai Gakkaishi 32(3):213–217
Kusumoto N, Ashitani T, Hayasaka Y, Murayama T, Ogiyama K, Takahashi K (2009) Antitermitic activity of abietane-type diterpenes from Taxodium distichum Cones. J Chem Ecol 35(12):635–642
Matsushima N, Kang HY, Sameshima K, Takamura N (1990) The complexity of termiticidal activity in hinoki (Chamaecyparis obtusa Endl) wood. Mokuzai Gakkaishi 36(7):559–564
McDaniel CA (1989) Major termiticidal components of heartwood of Port-Orford-cedar, Chamaecyparis lawsoniana (A. Murr.) Parl. Mat Organismen 24:1–15
Medarde M, Gordaliza M, Lucas MJ (1995) Structure elucidation of germacrane alcohols from Juniperus communis subsp. Hemisphaerica. J Nat Prod 58(7):1059–1064
Morikawa T, Ashitani T, Sekine N, Kusumoto N, Takahashi K (2012) Bioactivity of extracts from Chamaecyparis obtusa branch heartwood. J Wood Sci 58(6):544–549
Morita Y, Matsumura E, Okabe T, Fukui T, Shibata M, Sugiura M, Ohe T, Tsujibo H, Ishida N, Inamori Y (2004) Biological activity of α-thujaplicin, the isomer of hinokitiol. Biol Pharm Bull 27(6):899–902
Nakashima Y, Shimizu K (1972) Studies on an antitermitic activity of Hinokiasunaro (Thujopsis dolabrata Sieb. et Zucc. var. Hondai Makino). III. The components with a termiticidal activity. Miyazaki Daigaku Nogakubu Kenkyu Hokoku 19:251–259
Ohira T, Yatagai M (1994) Allelopathic compounds produced by forest plants II. The relationships between the inhibition effects on plant growth and killing activity of brine shrimp on phenolic compounds. Mokuzai Gakkaishi 40(5):541–548
Ohmura W, Doi S, Aoyama M, Ohara S (2000) Antifeedant activity of flavonoids and related compounds against the subterranean termite Coptotermes formosanus Shiraki. J Wood Sci 46(2):149–153
Ohtani Y, Hazama M, Sameshima K (1996) Crucial Chemical Factors for Termiticidal Activity of Hinoki Wood (Chamaecyparis obtusa) II. Variations in termiticidal activity among five individual samples of hinoki wood. Mokuzai Gakkaishi 42(12):1228–1233
Sakasegawa M, Hori K, Yatagai M (2003) Composition and antitermite activity of essential oils from Melaleuca species. J Wood Sci 49(2):181–187
Seru G, Pannakal ST, Serge F, Hartmut L (2004) Antitermitic quinones from Diospyros sylvatica. Phytochemistry 65(9):1265–1271
Shiei B, Iizuka Y, Matsubara Y (1981) Monoterpenoid and sesquiterpenoid constituents of the essential oil of hinoki (Chamaecyparis obtusa (Sieb. et Zucc.) Endl.). Agric Biol Chem 45(6):1497–1499
Tanaka K, Manabe M, Matsuura S (1979) Biological test using brine shrimp (part2). Rept Natl Food Res Inst 34:84–88
Yanga JK, Choia MS, Seob WT, Rinker DL, Hand SW, Cheonge GW (2007) Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 78(2):149–152
Acknowledgments
The study was supported by LIXIL JS Foundation (Grant-in-Aid for Research 12–85).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morikawa, T., Ashitani, T., Kofujita, H. et al. Antitermitic activity of extracts from Chamaecyparis obtusa branch heartwood. Eur. J. Wood Prod. 72, 651–657 (2014). https://doi.org/10.1007/s00107-014-0830-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-014-0830-8