Abstract
Eight known abietane-type diterpenes were isolated from the weak acidic fraction of the n-hexane extract from cones of Taxodium distichum, one of the extant, living fossil conifers. They were identified as 6,7-dehydroroyleanone (1), taxodal (2), taxodione (3), salvinolone (4), 14-deoxycoleon U (5), 5,6-dehydrosugiol (6), sandaracopimaric acid (7), and xanthoperol (8). The structures of these compounds were determined by comparison of NMR spectral data with published data. The antitermitic (termicidal and antifeedant) activities of the compounds 1–8 against the subterranean termite, Reticulitermes speratus Kolbe, were evaluated. Compounds 1 and 3 showed potent termicidal activity, and 5 and 8 showed potent antifeedant activity. Compound 1 was found to be one of the representative bioactive compounds in the n-hexane extract of T. distichum cones. Compounds 1–8, with the exception of 7, were oxides of ferruginol (9). Therefore, the presence of various oxidation forms of the abietane-type structure reflects their various bioactivities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Taxodium distichum Rich
(Taxodiaceae), commonly known as “bald” or “swamp” cypress, is well known as an extant deciduous, living fossil conifer indigenous to the southern part of North America. T. distichum heartwood is used for building materials, and has been reported to resist the attacks of the subterranean termite, Coptotermes formosanus Shiraki (Scheffrahn et al. 1988). The cones and seeds of T. distichum tend to be discovered from ancient stratum, and there are several reports concerning the fossil conifer and sediments of ancient flora (Otto et al. 2003, 2005). The cones produced by the conifers are essential parts for self-propagation. Consequently, the potential for having antifeedant, antifungal, as well as other phytochemical activities against external influences is suggested (Yano and Furuno 1994).
Abietane-type diterpenes are widely distributed in the plant kingdom as natural compounds. They reveal characteristic bioactivities including cytotoxic, anti-tumor, anti-microbial, and anti-bacterial effects (Gao and Han 1997; Ulubelen et al. 1999; Gigante et al. 2003; Son et al. 2005; Marques et al. 2006). From previous studies, it is known that the abietane-type diterpenes are the major compounds in the cones of Taxodium species (Otto et al. 2003). The isolation of several kinds of abietane-type diterpenes from the cones of T. distichum has been reported (Yamamoto et al. 2003). Some of the compounds isolated, such as taxodione, taxodone, and taxodistine A and B, have been reported to have anti-tumor and cytotoxic activity (Kupchan et al. 1969; Hirasawa et al. 2007). Taxodione has been reported to exhibit antibacterial activities against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) as well (Yang et al. 2001). In addition, nezukol, ferruginol, and manool, which have been isolated from the T. distichum heartwood (Scheffrahn et al. 1988), were shown to have antitermitic activities (Kang et al. 1993; Kano et al. 2004).
Natural compounds isolated from Taxodiaceae extant fossil conifers, such as from the genus Metasequoia and Taxodium, mostly have been taken from the leaf (Si et al. 2005) or the heartwood (Sato et al. 1966; Enoki et al. 1977). Only a few reports have considered bioactive compounds in living fossil conifer cones. Their bioactive compounds might have been important for the persistence, and thus, evolutionary success of T. distichum.
The aim of this research was to investigate the bioactive compounds of T. distichum cones and to evaluate their antitermitic activities by using a bioassay with the subterranean termite Reticulitermes speratus Kolbe. Subterranean termites are important decomposers in the terrestrial environment, and there has been interest in the activities of natural compounds against these termites (Bultman et al. 1979; Cornelius et al. 1997; Bläske and Hertel 2001). The termites R. speratus, C. formosanus, and Cryptotermes domesticus are harmful insects that damage wooden structures of buildings in Japan (Kang et al. 1993; Fukumoto et al. 2007).
Methods and Materials
Termite Collections
Colonies of Reticulitermes speratus Kolbe were collected in Tsuruoka city, Yamagata prefecture, and maintained in well-humidified chambers at 27°C ± 1°C for 1 month before the bioassay.
Termicidal and Antifeedant Tests
Termicidal and antifeedant activities were tested simultaneously. Samples were applied to paper discs (Advantec, 8 mm diam, 1.5 mm thickness, ca. 30 mg disk weight) according to a previously described method (Tellez et al. 2002; Ganapaty et al. 2004). Paper discs were treated with 60 μl of each sample solution (5.0 mg/ml in MeOH). After being dried in a vacuum desiccator for 24 h, the weights of the paper discs were measured. The concentration of the sample in the dried paper disc was prepared to 1.0% (sample weight/paper disc weight × 100). This dried paper disc was put on top of sea sand (3 g) that was spread uniformly at the bottom of a glass petri dish (45 mm diam, 20 mm high). Ten R. speratus workers were placed in the petri dish. The sea sand was kept moistened with water by using a sprayer. These petri dishes were maintained in a well-humidified chamber at 27°C ± 1°C for 10 d together with blank paper discs (no samples) and controls (no paper discs). Each of the tests included three replicates. The mortality of termites was recorded every 24 h, and termicidal activities were evaluated from the mortality average. The mass loss of each paper disc was measured at the end of the experimental period. Antifeedant activities were evaluated from the average of the mass that one termite fed per 24 h (mass loss of paper disc in 10 d/total number of termites in 10 d), and the relative rates were calculated from the blank readings.
Plant Material
The fallen cones of T. distichum (Taxodiaceae) were gathered in the Yamagata Field Science Center (Faculty of Agriculture, Yamagata University, Japan). Identification was confirmed by the above institution.
General Experimental Procedures
GLC analysis was performed with a HITACHI G-3000 Gas Chromatograph under the following conditions: DB-1 capillary column (30 m × 0.32 mm i.d.; 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA); column temperature from 180°C (0 min) to 280°C (15 min) at 4°C/min; injection temperature 250°C: detection temperature 250°C. GC/MS data were measured with a SHIMADZU QP-5000 GC-MS: DB-1 capillary column (30 m × 0.32 mm i.d.; 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA); column temperature from 100°C (1 min) to 320°C (10 min) at 5°C/min; injection temperature of 230°C: detection temperature of 250°C; acquisition mass range of 450-50 amu using helium as the carrier gas (3.6 ml/min). 1D and 2D NMR spectra were measured with a JEOL JNM-EX400 (1H 400 MHz/13C 100 MHz) spectrometer. The IR spectra were taken by a HORIBA FT-710 IR spectrometer with KBr pellets. UV spectra were taken by a SHIMADZU UV-1600PC spectrometer. Optical rotation values were measured by a HORIBA SEPA-300 polarimeter.
Extraction and Isolation
The n-hexane extract of air-dried T. distichum cones (800.0 g) was extracted by the same method as previously reported (Kusumoto et al. 2008). The n-hexane soluble fraction (88.6 g) was extracted by partition extraction with saturated NaHCO3, 10% NaCO3, and 1% NaOH aqueous solution in a separatory funnel to yield 1.4 g, 1.2 g, and 11.2 g fractions, respectively, along with 74.8 g residue fraction. Part of the weak acidic fraction (11.0 g) was applied to silica gel 60 N (spherical 63–210 μm, neutral, Kanto Chemical Co., Japan) column chromatography (CC) with solvent systems of n-hexane / EtOAc (100:1 to EtOAc only), and eight fractions (fr. A–H) were collected. Fractions C, D, and E (566.3 mg, n-hexane / EtOAc, 100:1) were recrystallized by using benzene to give a red needle of 6,7-dehydroroyleanone (1, 306.3 mg). Further, fr. G (541.9 mg, n-hexane / EtOAc, 100:3) was recrystallized by using n-hexane to give a colorless needle of taxodal (2, 44.6 mg). Fraction F (304.7 mg, n-hexane / EtOAc, 100:1 to 50:1) was purified by silica gel 60 N CC with a solvent system of n-hexane / chloroform (3:1 to chloroform only), and 40 fractions (fr. 1–40) were collected. Fractions 9, 10, 11, and 12 (110.0 mg, n-hexane / chloroform, 3:1) were purified by silica gel 60 N CC using a solvent system of n-hexane / chloroform (3:1) to give a dark yellow amorphous solid of taxodione (3, 29.0 mg). Fraction H (10.2 g) contained a polymerized mixture, which was separated to yield the EtOAc soluble (9.8 g) and insoluble fractions (333.7 mg). The EtOAc soluble fraction (6 g) was applied to silica gel 60 N CC using chloroform, and 48 fractions (fr. H1-H48) were collected. Fractions H9, H10, and H11 (233.1 mg) were recrystallized by using benzene, which gave a pale yellow powder of salvinolone (4, 123.2 mg). Successively, fractions H16, H17, H18, and H19 (461.1 mg) were recrystallized by using benzene, which yielded a pale yellow crystal of 14-deoxycoleone U (5, 182.8 mg). Fractions H23 to H27 (921.8 mg) were applied to silica gel 60 N CC using a solvent system of benzene / acetone (9:1) to a give pale yellow needle of 5,6-dehydrosugiol (6, 21.3 mg), a dark brown needle of sandaracopimaricacid (7, 11.6 mg), and a yellow needle of xanthoperol (8, 37.6 mg), respectively. Ferruginol (9) and sugiol (11) were isolated from Cryptomeria japonica in previous studies (Nagahama et al. 2000; Ashitani et al. 2001). 6,7-Dehydroferruginol (10) was synthesized from 11 by a chemical conversion according to a previous report (Matsui et al. 2004).
6,7-Dehydroroyleanone (1)
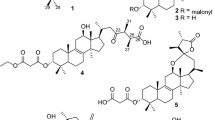
306.3 mg. Red needle. IR γmax (KBr) cm−1: 3360.4, 2961.2, 2925.5, 2910.1, 2869.6, 1663.3, 1642.1, 1625.7, 1551.5, 1457.9, 1377.9, 1329.7, 1298.8, 1272.8, 1253.5, 1164.8, 1106.0, 913.1, 769.5, 756.0, 714.5, 651.8; UV (C = 0.05 mg/ml, n-hexane) nm: 451.0, 327.0, 255.5; mp: 171°C; [α] 24.3D = −364.8° (C = 0.3 mg/ml); EI-MS: m/z 314 (M+, C20H26O3, 79%), 299 (27), 281 (6), 271 (21), 258 (16), 253 (16), 246 (15), 245 (68), 244 (74), 243 (23), 232 (100), 231 (45), 229 (22), 217 (23), 213 (25), 201 (15), 187 (25), 185 (19), 129 (18), 128 (21), 115 (32), 91 (24), 83 (41), 69 (30), 55 (75); 1H-NMR (CDCl3): δ 0.98 (3H, s, 18-CH3), 1.01 (3H, s, 19-CH3), 1.03 (3H, s, 20-CH3), 1.21 (3H, d, J = 7.1 Hz, 16-CH3), 1.22 (3H, d, J = 7.2 Hz, 17-CH3), 1.25 (2H, m, 3-CH2), 1.43 (2H, td, J = 13.3 Hz, 3.9 Hz, 1-CH2), 1.51 (2H, m, 3-CH2), 1.62 (2H, m, 2-CH2), 1.71 (2H, m, 2-CH2), 2.14 (1H, t, J = 3.1 Hz, 5-CH), 2.89 (2H, d, J = 3.4 Hz, 1-CH2), 3.17 (1H, hept, J = 7.1 Hz, 15-CH), 6.46 (1H, dd, J = 3.0 Hz, 9.7 Hz, 6-CH), 6.81 (1H, dd, J = 3.1 Hz, 9.8 Hz, 7-CH), 7.34 (1H, s, 12-OH); 13C-NMR (CDCl3): δ 15.2 (20-CH3), 18.7 (2-CH2), 19.8 (16-CH3), 20.0 (17-CH3), 22.8 (19-CH3), 24.1 (15-CH), 32.6 (18-CH3), 33.3 (4-C), 35.2 (1-CH2), 39.3 (10-C), 40.5 (-CH2), 52.1 (5-CH), 121.1 (7-CH), 122.6 (13-C), 138.5 (8-C), 139.6 (6-CH), 140.5 (9-C), 151.2 (12-C), 183.5 (11-C), 186.1 (14-C). (Figs. 1 and 2).
Route of ferruginol (9) oxidation via 6,7-dehydroferruginol (10) and sugiol (11), and bioactivities of each compound. a Relative mortality (%) of Reticulitermes speratus caused by the compounds; mortality caused by compound 9 in 10 d = 100 (A higher rate means a higher termicidal activity). b Relative mass (μg) of paper disc that one termite fed in 24 h, mass of disc treated with 9 = 1 (A lower rate means a higher antifeedant activity)
Taxodal (2, Fig. 1)
44.6 mg. Colorless needle. IR γmax (KBr) cm−1: 3012.3, 2969.8, 2948.6, 2861.8, 2775.1, 2751.9, 2503.2, 1689.3, 1666.2, 1614.1, 1581.3, 1469.5, 1461.8, 1425.1, 1400.1, 1346.1, 1276.7, 1253.5, 1211.1, 1172.5, 979.7, 898.7, 763.7; UV (C = 0.005 mg/ml, EtOAc) nm: 285.5; mp: 234–240°C; [α] 20D = −131.4 o (C = 0.5 mg/ml); EI-MS: m/z 302 (M+, C19H26O3, 26%), 287 (3), 274 (8), 269 (2), 259 (9), 256 (3), 241 (5), 232 (9), 231 (51), 220 (55), 219 (36), 205 (14), 204 (18), 203 (100), 191 (29), 190 (22), 189 (15), 187 (16), 177 (17), 175 (16), 161 (22), 159 (10), 147 (12), 128 (14), 115 (15), 91 (16), 77 (11), 69 (11), 55 (27); 1H-NMR (aceton-d6): δ 1.17 (3H, s, 17-CH3), 1.22 (3H, s, 18-CH3), 1.26 (3H, d, J = 7.0 Hz, 16-CH3), 1.27 (3H, d, J = 7.0 Hz, 15-CH3), 1.55 (3H, s, 19-CH3), 1.62 (2H, m, 1-CH2), 1.67 (2H, m, 2-CH2), 2.43 (2H, ddd, J = 13.3 Hz, 13.3 Hz, 4.0 Hz, 3-CH2), 3.31 (1H, hept, J = 7.0 Hz, 14-CH), 7.16 (1H, s, 10-CH), 7.73 (1H, s, 13-CH), 9.20 (1H, s, 11-OH), 9.68 (1H, s, 6-CH); 13C-NMR (CDCl3): δ 17.6 (2-CH2), 20.8 (15-CH3), 20.8 (16-CH3), 24.0 (19-CH3), 25.5 (14-CH), 25.6 (17-CH3), 28.4 (18-CH3), 36.9 (3-CH2), 38.9 (1-CH2), 43.1 (4-C), 51.4 (9-C), 114.3 (10-CH), 124.8 (7-C), 131.4 (12-C), 136.5 (13-CH), 146.9 (8-C), 158.7 (11-C), 190.2 (6-CH), 213.2 (5-C).
Taxodione (3, Figs. 1 and 2)
29.0 mg. Dark yellow amorphous solid. IR γmax (KBr) cm−1: 3322.8, 2935.1, 2361.4, 1669.1, 1612.2, 1594.84, 1354.8, 639.3; mp: 104-109°C; EI-MS m/z: 314 (M+, C20H26O3, 100%), 299 (17), 286 (58), 272 (19), 271 (83), 253 (13), 245 (69), 245 (69), 244 (25), 243 (25), 232 (38), 231 (44), 229 (25), 217 (33), 215 (26), 206 (22), 203 (25), 189 (22), 187 (22), 175 (14), 173 (15), 165 (12), 157 (11), 141 (17), 129 (20), 128 (24), 115 (30), 109 (41), 91 (26), 77 (24), 69 (31), 55 (51); 1H-NMR (CDCl3): δ 1.12 (3H, s, 18-CH3), 1.16 (3H, d, J = 7.0 Hz, 17-CH3), 1.18 (3H, d, J = 7.0 Hz, 16-CH3), 1.22 (2H, m, 3-CH2), 1.27 (3H, s, 19-CH3), 1.27 (3H, s, 20-CH3), 1.40 (2H, m, 3-CH2), 1.61 (2H, m, 2-CH2), 1.73 (2H, m, 1-CH2), 1.75 (2H, m, 2-CH2), 2.60 (1H, s, 5-CH), 2.93 (2H, m, 1-CH2), 3.07 (1H, hept, J = 7.0 Hz, 15-CH), 6.21 (1H, s, 7-CH), 6.88 (1H, s, 14-CH), 7.58 (1H, s, 11-OH); 13C-NMR (CDCl3): δ 18.5 (2-CH2), 21.2 (16-CH), 21.6 (17-CH3), 21.8 (20-CH3), 22.1 (19-CH3), 27.1 (15-CH), 32.8 (4-C), 33.3 (18-CH3), 37.0 (1-CH2), 42.9 (10-C), 63.0 (5-CH), 125.6 (9-C), 134.0 (7-CH), 136.1 (14-CH), 139.9 (8-C), 145.0 (11-C), 145.3 (13-C), 181.7 (12-C), 201.0 (6-C).
Salvinolone (4, Figs. 1 and 2)
123.2 mg. Pale yellow powder. IR γmax (KBr) cm−1: 3373.9, 3250.4, 2960.2, 2869.6, 2361.4, 2341.2, 1625.7, 1583.3, 1506.1, 1456.0, 1371.1, 1314.3, 1260.3, 1178.3, 1048.1; mp: 204–206°C; EI-MS m/z: 314 (M+, C20H26O3, 99%), 299 (11), 286 (5), 272 (11), 271 (44), 258 (10), 255 (5), 245 (89), 244 (100), 229 (34), 215 (29), 203 (20), 175 (14), 128 (10), 115 (12), 83 (18), 55 (24); 1H-NMR (CDCl3): δ 1.27 (3H, d, J = 7.0 Hz, 16-CH3), 1.30 (3H, d, J = 7.0 Hz, 17-CH3), 1.43 (3H, s, 18-CH3), 1.43 (3H, s, 19-CH3), 1.44 (2H, m, 3-CH2), 1.50 (3H, s, 20-CH3), 1.75 (2H, m, 2-CH2), 1.78 (2H, m, 1-CH2), 1.90 (2H, m, 2-CH2), 1.93 (2H, m, 3-CH2), 2.28 (2H, m, 1-CH2), 3.19 (1H, hept, J = 7.0 Hz, 15-CH), 5.78 (1H, s, 11-OH), 6.86 (1H, s, 6-CH), 7.15 (1H, s, 12-OH), 8.01 (1H, s, 14-CH); 13C-NMR (CDCl3): δ 17.6 (2-CH2), 22.3 (16-CH3), 22.5 (17-CH3), 26.9 (15-CH), 27.6 (18-CH3), 28.2 (19-CH3), 33.6 (1-CH2), 35.9 (4-C), 37.9 (3-CH2), 40.3 (10-C), 111.4 (14-CH), 120.9 (8-C), 125.6 (6-CH), 133.8 (13-C), 141.0 (11-C), 143.8 (9-C), 154.9 (12-C), 157.7 (5-C), 179.7 (7-C).
14-Deoxycoleon U (5, Figs. 1 and 2)
182.8 mg. Pale yellow crystal. IR γmax (KBr) cm−1: 3523.3, 3380.6, 3232.1, 2962.1, 2935.1, 2875.3, 1768.4, 1631.5, 1583.3, 1554.3, 1488.8, 1467.6, 1411.6, 1342.2, 1270.9, 1187.9, 1135.9, 1060.7, 997.0, 908.3, 885.2, 784.9, 572.8, 464.8; mp: 210–212°C; EI-MS m/z: 330 (M+, C20H26O4, 35%), 315 (6), 287 (12), 274 (9), 262 (16), 261 (94), 260 (100), 248 (19), 247 (12), 245 (28), 233 (15), 232 (12), 231 (15), 219 (14), 217 (14), 191 (8), 128 (8), 115 (9), 82 (15), 77 (8), 69 (8), 55 (17); 1H-NMR (pyridine-d5): δ 1.30 (3H, d, J = 7.0 Hz, 16-CH3), 1.30 (3H, d, J = 7.0 Hz, 17-CH3), 1.64 (3H, s, 18-CH3), 1.68 (3H, s, 19-CH3), 1.95 (3H, s, 20-CH3), 3.64 (1H, hept, J = 7.0 Hz, 15-CH), 7.10 (1H, s, -OH), 8.24 (1H, s, 14-CH), 8.39 (1H, s, -OH); 13C-NMR (pyridine-d5): δ 18.3 (2-CH2), 22.8 (16-CH3), 23.1 (17-CH3), 27.5 (19-CH3), 27.6 (15-CH), 28.3 (18-CH3), 28.4 (20-CH3), 30.6 (1-CH2), 36.7 (4-C), 36.9 (3-CH2), 41.4 (10-C), 116.4 (14-CH), 121.6 (8-C), 135.6 (13-C), 140.3 (11-C), 142.4 (9-C), 144.2 (5-C), 144.3 (6-C), 150.2 (12-C), 180.7 (7-C).
5,6-Dehydrosugiol (6, Figs. 1 and 2)
21.3 mg. Pale yellow needle. IR γmax (KBr) cm−1: 2964.1, 2935.1, 2867.6, 1637.3, 1612.2, 1560.1, 1504.2, 1459.9, 1388.5, 1324.9, 1263.2, 1184.1, 891.0, 879.4, 869.7, 651.8; mp: 256°C; EI-MS m/z: 298 (M+, C20H26O2, 61%), 283 (25), 255 (32), 242 (14), 241 (16), 230 (35), 229 (84), 228 (42), 214 (18), 213 (100), 199 (34), 187 (30), 185 (10), 171 (11), 170 (12), 165 (15), 157 (13), 152 (11), 128 (11), 115 (12), 83 (10), 55 (23); 1H-NMR (acetone-d6): δ 1.18 (3H, d, J = 7.0 Hz, 16-CH3), 1.20 (3H, d, J = 7.0 Hz, 17-CH3), 1.21 (3H, s, 18-CH3), 1.32 (3H, s, 19-CH3), 1.47 (3H, s, 20-CH3), 2.40 (2H, m, 1-CH2), 3.23 (1H, t, J = 1.7 Hz, 15-CH), 6.30 (1H, s, 6-CH), 6.95 (1H, s, 11-CH), 7.84 (1H, s, 14-CH); 13C-NMR (acetone-d6): δ 19.2 (2-CH2), 22.6 (16-CH3), 22.7 (17-CH3), 27.5 (15-CH), 29.7 (20-CH3), 32.8 (18-CH3), 32.8 (19-CH3), 38.0 (4-C), 38.5 (1-CH2), 41.0 (3-CH2), 41.9 (10-C), 111.4 (11-CH), 123.2 (8-C), 124.6 (6-CH), 124.9 (14-CH), 135.0 (13-C), 155.3 (9-C), 160.5 (12-C), 174.5 (5-C), 185.7 (7-C).
Sandaracopimaric acid (7, Fig. 1)
11.6 mg. Dark brown needle. IR γmax (KBr) cm−1: 2929.3, 2869.6, 1695.1, 1637.3, 1560.1, 1540.9, 1508.1, 1457.9, 1382.7, 1363.4, 1276.7, 997.0, 908.3; mp: 157–163°C; EI-MS m/z: 302 (M+, C20H30O2, 17%), 287 (30), 257 (8), 241 (8), 167 (15), 159 (9), 148 (12), 139 (22), 135 (21), 134 (18), 133 (28), 123 (25), 121 (100), 119 (28), 107 (34), 105 (33), 93 (42), 91 (51), 81 (30), 79 (43), 77 (24), 67 (27), 55 (45); 1H-NMR (CDCl3): δ 0.84 (3H, s, 20-CH3), 1.04 (3H, s, 17-CH3), 1.14 (2H, m, 1-CH2), 1.21 (3H, s, 19-CH3), 1.26 (2H, m, 6-CH2), 1.36 (2H, m, 12-CH2), 1.54 (2H, m, 11-CH2), 1.60 (2H, m, 2-CH2), 1.62 (2H, m, 3-CH2), 1.66 (2H, m, 1-CH2), 1.77 (1H, m, 9-CH), 1.93 (1H, dd, J = 2.5 Hz, 12.4 Hz, 5-CH), 2.21 (2H, d, J = 7.0 Hz, 7-CH2), 4.89 (2H, dd, J = 1.5 Hz, 10.4 Hz, 16-CH2), 4.91 (2H, dd, J = 1.5 Hz, 17.6 Hz, 16-CH2), 5.22 (1H, s, 14-CH), 5.77 (1H, dd, J = 10.5 Hz, 17.4 Hz, 15-CH); 13C-NMR (CDCl3): δ 15.5 (20-CH3), 17.0 (19-CH3), 18.4 (2-CH2), 18.8 (11-CH2), 24.9 (6-CH2), 26.3 (17-CH3), 34.7 (12-CH2), 35.7 (7-CH2), 37.3 (3-CH2), 37.7 (10-C), 38.0 (13-C), 38.6 (1-CH2), 47.6 (4-C), 49.1 (5-CH), 50.8 (9-CH), 110.4 (16-CH2), 129.4 (14-CH), 136.9 (8-C), 149.2 (15-CH), 185.2 (18-COOH).
Xanthoperol (8, Figs. 1 and 2)
37.6 mg. Yellow needle. IR γmax (KBr) cm−1: 3371.0, 2961.2, 2938.0, 2903.3, 2871.5, 2361.4, 1715.4, 1655.6, 1592.0, 1566.9, 1466.6, 1328.7, 1290.1, 1263.2; mp: 246–249°C; EI-MS m/z: 314 (M+, C20H26O3, 28%), 286 (7), 272 (9), 271 (43), 229 (11), 217 (25), 205 (22), 204 (100), 203 (33), 187 (12), 173 (7), 161 (33), 128 (8), 115 (14), 91 (10), 77 (8), 69 (11), 55 (22); 1H-NMR (CDCl3): δ 0.46 (3H, s, 19-CH3), 0.97 (3H, s, 18-CH3), 1.22 (3H, s, 20-CH3), 1.28 (2H, s, 1-CH2), 1.28 (3H, d, J = 7.0 Hz, 17-CH3), 1.30 (3H, d, J = 7.0 Hz, 16-CH3), 1.31 (2H, m, 3-CH2), 1.45 (2H, m, 3-CH2), 1.57 (2H, m, 2-CH2), 2.47 (2H, d, J = 14.8 Hz, 1-CH2), 2.64 (1H, s, 5-CH), 3.22 (1H, hept, J = 7.0 Hz, 15-CH), 6.86 (1H, s, 11-CH), 8.04 (1H, s, 14-CH); 13C-NMR (CDCl3): δ 18.9 (2-CH2), 22.3 (16-CH3), 22.3 (17-CH3), 24.1 (19-CH3), 27.0 (15-CH), 31.4 (18-CH3), 35.4 (4-C), 36.3 (1-CH2), 38.5 (20-CH3), 39.4 (10-C), 42.0 (3-CH2), 68.9 (5-CH), 111.2 (11-CH), 127.2 (8-C), 129.8 (14-CH), 134.7 (13-C), 150.6 (9-C), 160.5 (12-C), 179.9 (7-C), 200.1 (6-C).
Results and Discussion
Bioactivities of the Compounds from T. distichum Cones
The n-hexane soluble fraction (88.6 g) of T. distichum cones was separated by partition extraction and yielded strong (1.59%), medium (1.39%), and weak (12.61%) acidic fractions and a neutral fraction (84.41%). Termicidal activities of these four fractions were tested, and the results are shown in Table 1. The medium (33.3 ± 20.3%) and the weak (33.3 ± 12.0%) acidic fractions exhibited potent termicidal activity during a 10-d bioassay period. Based on the amount of extraction and the results of the bioassays, the weak acidic fraction seemed to contain the bioactive compounds. Therefore, it was further examined by silica gel column chromatography.
On a preparative scale, compounds 1–8 (Fig. 1) were isolated from the weak acidic fraction. The structures of these compounds, 6,7-dehydroroyleanone (1) (Hensch et al. 1975; Tezuka et al. 1998), taxodal (2) (Kusumoto et al. 2008), taxodione (3) (Kupchan et al. 1969; Tezuka et al. 1998), salvinolone (4) (Lin et al. 1989; Yamamoto et al. 2003), 14-deoxycoleon U (5) (Hueso-Rodriguez et al. 1983; Yamamoto et al. 2003), 5,6-dehydrosugiol (6) (Kupchan et al. 1969; Lin et al. 1989), sandaracopimaric acid (7) (Wenkert and Buckwalter 1972), and xanthoperol (8) (Kondo et al. 1963; Li et al. 2003), were determined by EI mass spectra, IR data, melting points, and NMR spectral data, and these were compared with published data. This is the first report of 2, 7, and 8 from T. distichum cones. Compounds 1–8, except 2 and 7, were diterpenes with an abietane-type structure.
The termicidal and antifeedant activities of compounds 1–8 against R. speratus were tested, (except for 7, which was isolated in too low amounts), together with ferruginol (9), 6,7-dehydroferruginol (10), and sugiol (11). The results are shown in Table 2. Compounds 9–11 are well-known abietane-type diterpenes that exist in Taxodiaceae (Otto et al. 2002). Compound 9 has been shown previously to have antitermitic and antifungal activities (Kofujita et al. 2001; Kano et al. 2004).
When testing termicidal activity, 1 caused strong mortality (46.7 ± 3.33% in 5 d, 70.0 ± 10.0% in 10 d). Compound 3 also caused notable mortality after 10 d (20.0 ± 11.6%), with the same value as 9. The mortality rates caused by 8 after 10 d (10.0 ± 5.77%) and the rates observed in the “no feed” assay (13.3 ± 3.33%) were similar.
When testing antifeedant activity, 8 showed effective activitiy against R. speratus. The termites fed on paper treated with 8. Compounds 3 and 5 also showed potent relative activities in the antifeedant test.
From these results, we conclude that T. distichum cones contain strong bioactive compounds (1, 3, 5, and 8). In particular, 1 is a specific bioactive compound in the n-hexane extract of T. distichum cones, and has a quinone structure at the C ring (Fig. 2). The abietane-type diterpenes, which have quinone structures at the C rings, such as cryptoquinone and 7-hydroxy-11,14-dioxo-8,12-abietadiene, previously have been reported to show antifungal and cytotoxic activities (Kofujita et al. 2002, 2006). Herein, we report that 1, the abietane-type quinone, exhibited strong activities against subterranean termite.
Bioactivities of the Oxidized Abietane-type Compounds
It was assumed that the bioactive compounds 1–8 were oxides of 9 (Yamamoto et al. 2003). In order to compare the bioactive value of 9 with its oxides, the oxidation routes of 9 and the relative values on the basis of 9, as a standard compound, are indicated in Fig. 2.
First, we discuss the termicidal activity (Fig. 2a). The activity decreased with dehydrogenation of C-6 and C-7 at 10, but the activity increased with the synthesis of a quinone structure in the oxidation progression to 1 or 3. Activity also decreased at 11, but increased again through oxidation to 4, 6, and 8. Moreover, oxidation of 4 to 5 resulted in loss of all activity.
Second, a similar change was observed with antifeedant activity (Fig. 2b). The activity of 3 was stronger than that of 1. Furthermore, the activity completely disappeared at 11, but high activities were obtained through oxidation to 5 or 8.
These findings suggest that the various forms of the abietane-type structure due to the degree of oxidation reflect their various bioactivities. As several compounds that are active against R. speratus were discovered, they may be related to the defense of T. distichum. Hence, we consider that this may be one of the important factors that explains why T. distichum have prospered on the earth.
References
ASHITANI, T., UJIKE, M., NAGAHAMA, S., UENO, T., and SAKAI, K. 2001. Characterization of Sugi (Cryptomeria japonica) bark extracts. Mokuzai Gakkaishi 47:276–281.
BLÄSKE, V. U. and HERTEL, H. 2001. Repellent and toxic effects of plant extracts on subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol. 94:1200–1208.
BULTMAN, J. D., BEAL, R. H., and AMPONG, F. F. K. 1979. Natural resistance of some tropical African woods to Coptotermes formosanus Shiraki. Forest Prod. J. 29:46–51.
CORNELIUS, M. L., GRACE, J. K., and YATES, J. R. III 1997. Toxicity of monoterpenoids and other natural products to the formosan subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol. 90:320–325.
ENOKI, A., TAKAHAMA, S., and KITAO, K. 1977. The Extractives of Metasequoia, Metasequoia glyptostroboides Hu et Cheng. The isolation of Metasequirin-A, Athrotaxin and Agatharesinol from the heartwood. Mokuzai Gakkaishi 23:579–586.
FUKUMOTO, H., MORIMOTO, M., FUKUDA, Y., KITAYAMA, T., and KOMAI, K. 2007. Antifeedants in tropical asian species, resak, against a subterranean termite, Reticulitermes speratus (Kolbe). Bull. Fac. Agrc. Kinki Univ. 40:31–38.
GANAPATY, S., THOMAS, P. S., FOTSO, S., and LAATSCH, H. 2004. Antitermitic quinones from Diospyros sylvatica. Phytochemistry 65:1265–1271.
GAO, J. and HAN, G. 1997. Cytotoxic abietane diterpenoids from Caryopteris incana. Phytochemistry 44:759–761.
GIGANTE, B., SANTOS, C., SILVA, A. M., CURTO, M. J. M., NASCIMENTO, M. S. J., PINTO, E., PEDRO, M., CERQUEIRA, F., PINTO, M. M., DUARTE, M. P., LAIRES, A., RUEFF, J., GONÇALVES, J., PEGADO, M. I., and VALDEIRA, M. L. 2003. Catechols from abietic acid: synthesis and evaluation as bioactive compounds. Bioorg. Med. Chem. 11:1631–1638.
HENSCH, M., RÜEDI, P., and EUGSTER, C. H. 1975. Horminon, Taxochinon und weitere Royleanone aus 2 abessinischen Plectranthus-Spezies (Labiatae). Helv. Chim. Acta 58:1921–1934.
HIRASAWA, Y., IZAWA, E., MATSUNO, Y., KAWAHARA, N., GODA, Y., and MORITA, H. 2007. Taxodistines A and B, abietane-type diterpenes from Taxodium distichum. Bioorg. Med. Chem. Lett. 17:5868–5871.
HUESO-RODRIGUEZ, J. A., JIMENO, M. L., RODRIGUEZ, B., SAVONA, G., and BRUNO, M. 1983. Abietane diterpenoids from the root of Salvia phlomoides. Phytochemistry 22:2005–2009.
KANG, H. Y., Sameshima, K., and Takamura, N. 1993. Termite resistance test of hardwoods of Kochi prefecture growth. Mokuzai Gakkaishi 39:345–351.
KANO, H., SHIBUTANI, S., HAYASHI, K., IIJIMA, Y., and DOI, S. 2004. Effect of high-temperature drying processes on termite resistance of Sugi (Cryptomeria japonica) heartwood. Mokuzai Gakkaishi 50:91–98.
KOFUJITA, H., FUJINO, Y., SASAKI, T., HASEBE, M., OTA, M., and SUZUKI, K. 2001. Antifungal activity of the bark of Cryptomeria japonica and its relevant components. Mokuzai Gakkaishi 47:479–486.
KOFUJITA, H., OTA, M., TAKAHASHI, K., KAWAI, Y., and HAYASHI, Y. 2002. A diterpene quinone from the bark of Cryptomeria japonica. Phytochemistry 61:895–898.
KOFUJITA, H., FUJINO, Y., OTA, M., and TAKAHASHI, K. 2006. Antifungal diterpenes from the bark of Cryptomeria japonica D. Don. Holzforschung 60:20–23.
KONDO, Y., IKENOUE, T., and TAKEMOTO, T. 1963. Structure of Xanthoperol. Chem. Pharm. Bull. 11:678–680.
KUPCHAN, S. M., KARIM, A., and MARCKS, C. 1969. Taxodione and Taxodone, two novel diterpenoid quinone methide tumor inhibitors from Taxodium distichum. J. Org. Chem. 34:3912–3919.
KUSUMOTO, N., MURAYAMA, T., KAWAI, Y., ASHITANI, T., OGIYAMA, K., and TAKAHASHI, K. 2008. Taxodal, a novel irregular abietane-type diterpene from the cones of Taxodium distichum. Tetrahedron Lett. 49:4845–4847.
LI, A., SHE, X., ZHANG, J., WU, T., and PAN, X. 2003. Synthesis of C-7 oxidized abietane diterpenes from racemic ferruginyl methyl ether. Tetrahedron 59:5737–5741.
LIN, L.Z., BLASKÓ, G., and CORDELL, G. A. 1989. Diterpenes of Salvia prionitis. Phytochemistry 28:177–181.
MATSUI, T., MATSUSHITA, Y., SUGAMOTO, K., and YANO, H. 2004. Isolation of terpenoids from Sugi (Cryptomeria japonica) wood and chemical conversion of ferruginol. Memoirs of the Fac. Eng. Miyazaki Univ. 33:63–73.
MARQUES, C. G., RIJO, P., SIMÕES, M. F., DUARTE, M. A., and RODRIGUEZ, B. 2006. Abietanes from Plectranthus grandidentatus and P. Hereroensis against methicillin- and vancomycin-resistant bacteria. Phytomed. 13:267–271.
NAGAHAMA, S., IWAOKA, T., and ASHITANI, T. 2000. Terpenoids of the wood oil of Sugi (Cryptomeria japonica) VI. Mokuzai Gakkaishi 46:225–230.
OTTO, A., WHITE, J. D., and SIMONEIT, B. R. T. 2002. Natural product terpenoids in Eocene and Miocene conifer fossils. Science 297:1543–1545.
OTTO, A., SIMONEIT, B. R. T., and REMBER, W. C. 2003. Resin compounds from the seed cones of three fossil conifer species from the Miocene Clarkia flora, Emerald Creek, Idaho, USA, and from related extant species. Rev. Palaeobot. Palynol. 126:225–241.
OTTO, A., SIMONEIT, B. R. T., and REMBER, W. C. 2005. Conifer and angiosperm biomarkers in clay sediments and fossil plants from the Miocene Clarkia Formation, Idaho, USA. Org. Geochem. 36:907–922.
SATO, A., SENDA, M., KAKUTANI, T., WATANABE, Y., and KITAO, K. 1966. Extractives from heartwood of Metasequoia glyptostroboides Hu et Cheng (part 1). Bull. Wood Res. Inst. Kyoto univ. 39:13–21.
SCHEFFRAHN, R. H., HSU, R. C., SU, N. Y., HUFFMAN, J. B., MIDLAND, S. L., and SIMS, J. J. 1988. Allelochemical resistance of Bald Cypress, Taxodium distichum, heartwood to the subterranean termite, Coptotermes formosanus. J. Chem. Ecol. 14:765–776.
SI, Y., YAO, X. H., ZHANG, C. K., and TU, Z. B. 2005. C-32 Triterpenes from Taxodium ascendens. Biochem. Syst. Ecol. 33:211–214.
SON, K. H., OH, H. M., CHOI, S. K., HAN, D. C., and KWON, B. M. 2005. Anti-tumor abietane diterpenes from the cones of Sequoia sempervirens. Bioorg. Med. Chem. Lett. 15:2019–2021.
TELLEZ, M. R., KHAN, I. A., KOBAISY, M., SCHRADER, K. K., DAYAN, F. E., and OSBRINK, W. 2002. Composition of the essential oil of Lepidium meyenii (Walp.). Phytochemistry 61:149–155.
TEZUKA, Y., KASIMU, R., LI, J. X., BASNET, P., TANAKA, K., NAMBA, T., and KADOTA, S. 1998. Constituents of roots of Salvia deserta Schang. (Xinjiang-Danshen). Chem. Pharm. Bull. 46:107–112.
ULUBELEN, A., ÖKSÜZ, S., KOLAK, U., TAN, N., BOZOK-JOHANSSON, C., ÇELIK, C., KOHLBAU, H. J., and VOELTER, W. 1999. Diterpenoids from the roots of Salvia bracteata. Phytochemistry 52:1455–1459.
WENKERT, E. and BUCKWALTER, B. L. 1972. Carbon-13 nuclear magnetic resonance spectroscopy of naturally occurring substances. X. Pimaradienes. J. Am. Chem. Soc. 94:4367–4369.
YANO, S. and FURUNO, T. 1994. Resin acids from extracts of pine cones of Kuromatsu (Pinus thunbergii). Mokuzai Gakkaishi 40:72–77.
YAMAMOTO, H., HIRAO, T., WAKAYAMA, K., and CHIDA, T. 2003. Abietane diterpenes from cones of Taxodium distichum Rich. Bull. Fac. Edu. Ibaraki Univ. 52:31–39.
YANG, Z., KITANO, Y., CHIBA, K., SHIBATA, N., KUROKAWA, H., DOI, Y., ARAKAWA, Y., and TADA, M. 2001. Synthesis of variously oxidized abietane diterpenes and their antibacterial activities against MRSA and VRE. Bioorg. Med. Chem. 9:347–356.
Acknowledgements
The authors thank Nobuhiro Sekine and Ganis Lukmandaru (The United Graduate School of Agricultural Science, Iwate University) for research counsel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kusumoto, N., Ashitani, T., Hayasaka, Y. et al. Antitermitic Activities of Abietane-type Diterpenes from Taxodium distichum Cones. J Chem Ecol 35, 635–642 (2009). https://doi.org/10.1007/s10886-009-9646-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9646-0