Abstract
The cure properties of cure-accelerated phenol-urea-formaldehyde (PUF) resins with different catalysts [calcium oxide (CaO), sodium carbonate (Na2CO3), zinc oxide (ZnO), and magnesium oxide (MgO)] were investigated by gelation test and differential scanning calorimetry (DSC) analysis. The results indicated that catalysts such as Na2CO3, ZnO, and MgO were capable of increasing the curing rate and decreasing the curing temperature of PUF resins, however, the CaO inhibited the cure reaction. The formation of methylene bridges was considered to be the main reaction during curing. For the ZnO- and MgO-accelerated PUF resins, the addition reaction of formaldehyde with free phenolic site may act as subsidiary reaction. The activation energies (E a ) of cure-accelerated PUF resins other than CaO-acceleration were much lower than the control resin. The effects of catalysts and hot press temperature on adhesive performances of PUF resins were also discussed by plywood test. The PUF resins with Na2CO3, ZnO, and MgO had higher wet shear strength than the control resin. Hot press temperature had a strong influence on the wet shear strength as well as the catalysts. Among the catalysts, MgO had more significant improving effect on both the curing process and the wet shear strength of PUF resin.
Zusammenfassung
Mittels Geliertest und Differentialrasterkalorimetrie (DSC) wurden die Aushärtungseigenschaften von Phenol-Harnstoff-Formaldehydharzen (PUF), denen verschiedene Katalysatoren (Calciumoxid (CaO), Natriumcarbonat (Na2CO3), Zinkoxid (ZnO) und Magnesiumoxid (MgO)) als Härtungsbeschleuniger zugesetzt wurden, untersucht. Die Ergebnisse zeigten, dass die Katalysatoren Na2CO3, ZnO und MgO in der Lage sind, die Aushärtungsgeschwindigkeit zu erhöhen und die Aushärtungstemperatur von PUF Harzen zu senken, wohingegen CaO die Aushärtungsreaktion hemmte. Die Bildung von Methylenbrücken wurde als Hauptreaktion bei der Aushärtung angesehen. Bei den mit ZnO- und MgO beschleunigten PUF-Harzen kann die Anlagerung von Formaldehyd an die freien phenolischen OH-Gruppen eine Nebenreaktion darstellen. Die Aktivierungsenergien (E a ) von mit Härtungsbeschleunigern versetzen PUF-Harzen waren, außer bei CaO, viel niedriger als die des Kontrollharzes. Der Einfluss der Katalysatoren und der Heißpresstemperatur auf das Klebstoffverhalten von PUF-Harzen wurde auch an Sperrholz geprüft. PUF-Harze mit zugesetztem Na2CO3, ZnO und MgO wiesen eine höhere Nassscherfestigkeit als das Kontrollharz auf. Die Heißpresstemperatur hatte ebenfalls einen starken Einfluss auf die Nassscherfestigkeit. Unter allen Katalysatoren hatte MgO den größten positiven Einfluss auf sowohl das Aushärten als auch die Nassscherfestigkeit des PUF-Harzes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phenol-formaldehyde (PF) resin has been extensively used as an adhesive for the manufacture of wood-based composite materials such as plywood, fiberboard, and oriented strand board (Pizzi 1994a). Over a decade, liquid and solid resins with different formulations have been developed to meet production requirements of these wood products (Pizzi 1994b; Pizzi et al. 1997; Park et al. 1999). In recent years, the increasing cost of phenol due to the increase of oil cost has prompted considerable amount of research in decreasing the cost of PF resins whereas maintaining or even improving their performance (Klasnja and Kopitovic 1992; Tomita and Hse 1993; Zhao et al. 2000; Vázquez et al. 2004; Effendi et al. 2008). Urea as a substitute for phenol has been successfully introduced into PF resins to form phenol-urea-formaldehyde (PUF) resins for reducing the cost of resins (Tomita and Hse 1993, 1998; Zhao et al. 1999; He and Riedl 2003). The added urea can also improve both the curing behavior and strength performance of PF resin. This new type of PUF resin has been extensively used as exterior-grade adhesive in the wood industry, especially in panels manufacture industry. The resol-type PUF resin was first prepared by the reaction of methylolphenol with urea under acidic conditions, in which the reaction rate of cocondensation was much faster than that of self-condensation (Tomita and Hse 1992, 1998). This resin presented good heat-resistance after full cure reaction. PUF resins can also be synthesized under alkaline conditions. Zhao et al. (1999) reported that low-condensation PF resins can coreact with up to 42% molar urea on phenol during resin preparation to yield PUF resins capable of shortening hardening times and presenting better performance than equivalent pure PF resins prepared under identical conditions. Recently, low-cost PUF cocondensed resins with 89.4 wt% urea on phenol were prepared by reacting a mixture of methylolureas, phenol, and formaldehyde under alkaline conditions in a previous paper (Fan et al. 2009a, 2009b). These resins of high urea content presented good water-resistance and wet strength after full cure reaction, however, they needed higher cure temperature and cure time than low-urea content PUF resins.
The through-put of the hot press significantly influences the productivity and cost for the manufacture of wood-based composite panels. Therefore, a reduced hot pressing time and temperature can result in savings for manufacturers. In a previous work, cure-accelerated PUF resins of high urea content were prepared with different catalysts and their structures were also characterized by 13C-NMR spectroscopy (Fan et al. 2009c). However, the cure process and adhesive performances that were very important for the manufacture of wood-based composite panels were not reported. The aim of this study was to investigate cure properties and adhesive performances of cure-accelerated PUF resins. Understanding those properties will give valuable information on the use of cure-accelerated PUF resins as adhesives for plywood. In this study, the cure properties of these PUF resins were investigated by gelation test and differential scanning calorimetry (DSC) analysis. The effects of catalysts and hot press temperature on the adhesive performances of PUF resins were also discussed with a cyclic 4-hour boiling test of plywood.
2 Experiment
2.1 Materials
In the following experiments, formaldehyde aqueous solution (37%), solid phenol, CaO, Na2CO3, ZnO, MgO, and solid urea were of industrial grade from Zhong’an Chemical Industries, China and were used directly as raw materials without further purification. Sodium hydroxide and all the other chemicals used were of AR grade from Beijing Chemical Industries, China.
2.2 Resins preparation
The control and cure-accelerated PUF resins were prepared with a F:P:U:NaOH molar ratio of 3.3:1:1.4:0.26. First, a mixture of methylolureas was prepared by a simple procedure. About 97 g of formaldehyde was charged into a stirred reactor and adjusted at the target pH value between 7.0 and 8.0 with 30% sodium hydroxide solution. After stirring for 5 min, 45 g of urea was gradually added over a period of 10 min. Subsequently, the reaction mixture was heated to 90°C within 40 min and maintained at this temperature for 30 min. The reaction product was then slowly cooled to room temperature, named as MMU.
The control PUF resin (traditional NaOH-catalyzed resin) was prepared according to the following procedure: About 71 g of phenol, 26 g of 40% sodium hydroxide solution and 83 g of formaldehyde were charged into a stirred reactor. The reaction mixture was then heated to 70°C over a period of 30 min. Subsequently, the MMU was added into the reaction system, and the temperature was gradually increased to 90°C within 20 min and then maintained for 30 min more. About 21 g of formaldehyde and an appropriate amount of distilled water were gradually added to the reactor and the reaction was continued for further 20 min. Then, 19 g of urea was added and the temperature was kept at 85°C until the Gardner-Holdt viscosity (measured at 20°C) of the resin has reached between 300 and 500 mPa.s.
The cure-accelerated PUF resins with different catalysts were based on this preparation procedure. The catalysts used were CaO, Na2CO3, ZnO, and MgO. All the catalysts were added with 26 g of 40% sodium hydroxide solution together at the beginning of the resin reaction and the addition amount was 2% of the total resin. The characteristics of the control and cure-accelerated PUF resins were pH=10.5±0.1, solid content=50±1%.
2.3 Liquid 13 C-NMR measurement
All the resins were characterized by quantitative 13C-NMR spectroscopy with a VARIAN INOUR-300 (USA) spectrometer with a frequency of 75.51 MHz and with the inverse-gated decoupling method. All the spectra were recorded at room temperature with a delay time of 8 s, a 13 h acquisition time, and a 15.4 μs pulse width (90°). About 8000 scans were accumulated to obtain spectra for each spectrum. The chemical shifts of each spectrum were accurate to 0.1 ppm and all the resin samples were directly used for 13C-NMR measurement.
2.4 Gel time determination
Gelation was defined as the point at which the resin ceased to be a viscous liquid and became a soft, elastic, and rubbery solid. Resin sample (about 5 g) was weighed in a 20 mm×150 mm test tube and a thin wire spring was then placed in the sample. The test tube was then immersed in a constant temperature oil-bath with stirring and the wire spring was manually moved rapidly up and down until gelation occurred. The gelation test was done at 140°C and the gel time was recorded using a stopwatch. Each value of gel time in Table 2 is an average of five test results.
2.5 DSC measurement
All DSC measurements were made on a DSC-60/60A (SHIMADZU, Japan) instrument with TA60 thermal analysis software. Around 10 mg of resin samples were sealed in high pressure stainless steel crucible that was capable of resisting up to 5 MPa pressure. Dynamic scans were carried out with heating rates of 3, 10, and 15°C/min, respectively. Scanning temperature ranged from 30 to 250°C.
2.6 Preparation and test of plywood
Three-layer laboratory plywood panels of 400×400×4.8 mm3 were prepared using control and cure-accelerated PUF adhesives, and poplar (Populus) veneers. To each PUF glue-mix 20% wheat flour by weight on the liquid resin was added. The adhesive-coated veneer was stacked between two uncoated veneers with the grain directions of two adjacent veneers perpendicular to each other. Three-layer plywood panels were prepared under the following conditions: moisture content of veneer 8–10%; glue spread 360–380 g/m2(double line); cold press under 1.0 MPa for 20 min; hot press temperature 120–150°C; hot press pressure 1.0 MPa; hot press time 5 min.
Twenty test specimens as shown in Fig. 1 were cut from the plywood panels along the face-grain axis. The wet sheer strength of plywood was determined according to China Industry Standard (GB/T 17657-4.15-1999). This standard is described as follows: at least fifteen specimens were soaked in boiling water for 4 h, then dried at about 63°C for 20 h, boiled again for 4 h, and then cooled down at room temperature for about 10 min; after that the shear strength was tested in a wet state. GB/T 17657-4.15-1999 requires that the minimum value of the wet shear strength for plywood is 0.7 MPa after the above 4-hour cyclic boil test.
In this experiment, the wet shear strengths of PUF adhesives were measured with a tension testing machine (Jinan Shijin Co. Ltd, China) under a crosshead speed of 5 mm/min. Each value of wet shear strength in Table 4 is an average of twenty specimens.
3 Results and discussion
3.1 Nonisothermal DSC scans analysis
Nonisothermal DSC is a very useful method for estimating the activation energy of reactions from relationships between the heating rate and the temperature to reach a constant conversion. This technique makes an evaluation of kinetic parameters requiring at least three dynamic DSC scans at different heating rates. Kissinger (1957) developed a useful method, from which an equation related to the heating rate and peak exotherm was obtained as follows:
where φ is the heating rate (°C/min), T P is the peak temperature (K), E a is the Arrhenius activation energy (kJ/mol), R is the universal gas constant, and A is the pre-exponential factor. A plot of \(\ln(\frac{\varphi}{T_{P}^{2}})\) versus \(\frac{1}{T_{P}}\) is a straight line, from which E a can be calculated.
3.2 Cure properties
The quantitative determination of structural groups of the cure-accelerated PUF resins was done by 13C-NMR spectroscopy, and the analysis results are collected in Table 1. Table 2 shows the gel time of cure-accelerated PUF resins compared with that of the control resin. As expected, the catalysts other than CaO were remarkably effective in increasing the curing rate of PUF resins. The gel time decreased rapidly when MgO was used in the synthesis of PUF resin. Both Na2CO3 and ZnO resulted in a moderate decrease in the gel time. The PUF resin with CaO had nearly similar gel time to the control resin. Fraser et al. (1957) showed that the bivalent metallic salts accelerated both the reaction of phenolic nuclei with formaldehyde and the condensation of methylolphenols with other phenolic nuclei. In addition, a previous paper by the authors indicated that the catalysts such as Na2CO3, ZnO, and MgO promoted both the self-condensation reaction of phenolic methylol groups and the cocondensation reaction of phenolic components with urea units of PUF resins (Fan et al. 2009c). Thus, cure-accelerated PUF resins with Na2CO3, MgO, and ZnO can cure faster than the control resin. The cure-acceleration effect of Na2CO3 possibly resulted from that the carbonic acid ions functioned as a catalyst of the reaction of crosslinking (Pizzi et al. 1997). In general, the para position of phenolic ring has slightly higher relative reactivity. From Table 1, it can be seen that the ZnO- and MgO-accelerated PUF resins had much higher para/ortho ratio of methylol group. This resulted in the fast curing rate of the resins with MgO and ZnO. It is also noted from Table 2 that MgO-accelerated PUF resin showed much shorter gel time than ZnO-accelerated resin. This was possibly related to the ionic radii and nature of cations. In conclusion, the catalysts such as Na2CO3, MgO, and ZnO in the PUF resins can increase curing rate. Among these catalysts, MgO had the most significant accelerating effect on the gelation of PUF resins.
In this study, the DSC curves were analyzed for peak temperature (T P ), activation energy (E a ) and reaction heat (ΔH). The calculation of peak temperature and ΔH were performed using the TA60 thermal analysis software. The E a values for the cure reactions of different resins were calculated from the plot of \(\ln(\frac{\varphi}{T_{P}^{2}})\) versus \(\frac{1}{T_{P}}\) .
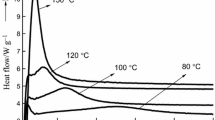
Figure 2 shows the DSC curves of the cure-accelerated and control PUF resins at a heating rate of 10°C/min. The results obtained are summarized in Table 3 containing T P at different heating rates, E a values and their corresponding regression coefficients (R 2), and ΔH at the heating rate of 10°C/min. As Fig. 2 shows, the DSC curves consist of either a single or two observable exothermic peaks indicating the occurrence of multiple reactions possibly in sequence or parallel to the main reaction. The DSC curves of the control, Na2CO3-accelerated, and CaO-accelerated PUF resins showed a broad exothermic peak with a maximum between 142 and 155°C (see Fig. 2 and Table 3). This peak was caused by the condensation of methylol groups with phenolic units to form methylene bridges (Turunen et al. 2003; Vázquez et al. 2005). The cure-accelerated resins with ZnO and MgO displayed two well-separated exothermic peaks. The first obvious peaks appeared at a temperature between 94 and 108°C. Christiansen and Gollob (2003) related the peak at 130–138°C to the methylolation reaction of free formaldehyde with free phenol. Also, the bivalent metallic salts can accelerate the reaction of phenolic nuclei with formaldehyde (Pizzi 1979a). But, if the signal is attributed to the addition reaction of free formaldehyde with free phenol due to the accelerating effect of ZnO and MgO, this conclusion contradicted the result that there was no form of free formaldehyde (Table 1) present in cure-accelerated PUF resins. Therefore, one reasonable interpretation was that methylol groups carried on the urea components under alkaline conditions are unstable and susceptibly decomposed to form free formaldehyde. The free formaldehyde can react with free phenolic site to form phenolic methylols in the presence of ZnO and MgO. As a result, the first peak at 94–108°C was associated with the addition reaction of formaldehyde with free phenolic site. This also indicated that ZnO and MgO can accelerate the addition reaction proceeding at a relative temperature. Compared with the number of peaks, it is known that the reaction of free formaldehyde with free phenolic site was difficult to take place. The second peaks (139–142°C) of the ZnO- and MgO-accelerated PUF resins were associated with the formation of methylene bridges, which was similar to the corresponding peak in the control, Na2CO3-accelerated, and CaO-accelerated PUF resins. From the discussion above it can be concluded that the formation of methylene bridges was considered to be the main reaction during curing because of similar characteristic peak exotherms at 139–154°C in all PUF resins. The other peak of the ZnO- and MgO-accelerated PUF resins was at 94–108°C, indicating the addition reaction of formaldehyde with free phenolic site may act as subsidiary reaction in the two resins. Compared with ZnO-accelerated PUF resin, MgO appeared to have more significant accelerating effect on both the formation of methylene bridges and the addition reaction of formaldehyde with free phenolic sites.
As Fig. 2 and Table 3 shows, the cure-accelerated PUF resins with Na2CO3, ZnO, and MgO had lower curing temperature than the control resin; whereas the CaO-accelerated resin had higher curing temperature. This indicated that Na2CO3, ZnO, and MgO can make PUF resins cure at a relative low temperature.
The ΔH values in Table 3 are based on the mass of liquid resin. As can be seen, the ΔH values of cure-accelerated PUF resins are much larger than that of the control resin. Considering proportionality betweenΔH and the crosslink created (number of links formed), the lower the heat released the lower the crosslinking, leading to the lower mechanical properties (Vázquez et al. 2005). The ΔH values in Table 3 suggested that MgO-accelerated PUF resin reacted more strongly than the other resins due to the larger ΔH released during curing.
The activation energy as an important cure kinetic parameter is usually used to describe the effect of curing temperature on the cure reaction. The E a values in Table 3 are calculated by Kissinger method which was very widely used for calculating the activation energies of PUF resins (He and Riedl 2003; Vázquez et al. 2005). There were two E a values at both ZnO- and MgO-accelerated PUF resins because of their two peaks. As Table 3 shows, R 2 ranged from 0.9991 to 0.9999, which is high for each regression analysis, that is, the E a values calculated by the Kissinger method are credible. The E a values of cure-accelerated PUF resins other than CaO-acceleration were much lower than the control resin, while the CaO-accelerated PUF resin presented higher E a value. In general, the reactions of urea or methylolurea with methylolphenol or phenolic nuclei of phenol and of PF polymer need higher activation energy values (He and Yan 2005). However, these reactions can take place at a relatively low temperature due to the cure- acceleration effect of Na2CO3, ZnO, and MgO. Thus, the PUF resins with these catalysts had lower E a values. The cure of PUF resin is a complicated process that contains many kinds of individual reactions including self-condensation and cocondensation reaction of phenolic ring and urea unit. Each kind of individual reaction between the different functional groups has different activation energy, making different contributions to the apparent activation energy. Among cure-accelerated resins, MgO-accelerate resin had the lowest E a value. This was due to the most significant accelerating effect of MgO.
3.3 Adhesive performances
The adhesive performances of PUF resins without and with the addition of catalysts were evaluated according to China Industry Standard (GB/T 17657-4.15-1999). The wet shear strength and wood failure of PUF resins are summarized in Table 4, where the effects of hot press temperature are investigated as variable. The cure-accelerated PUF resins with catalysts (Na2CO3, ZnO, and MgO) had higher wet shear strength than the control resin, regardless of the hot press temperature. However, the CaO-accelerated resin showed the reverse results. Pizzi (1979b) reported that when limited hot press time was involved, the plywood glued with tannin/formaldehyde adhesives containing metal ions were simply more cured and consequently present higher strength, which was due to faster cure promoted by the presence of metal ions. In addition, the 13C-NMR analysis results in Table 1 show that the cure-accelerated PUF resins with catalysts such as Na2CO3, ZnO, and MgO had much higher para/ortho ratio of phenolic methylol groups and lower proportion of unreacted urea and monosubstituted urea than the control PUF resin, while the CaO-accelerated PUF resin presented the reverse results. Unreacted urea and monosubstituted urea needed relatively high curing temperature, and further more decreased the cross-linking density of cured resins (Fan et al. 2009b). Therefore, Na2CO3-, ZnO-, and MgO-accelerated PUF resins were easy to adequately react to form high cross-linking network during curing. In Table 4 it can be seen that MgO-accelerated resin had the biggest wet shear strength under the same hot press temperature, indicating that the improving effect of MgO on the strength of cured PUF resin was more significant than that of the other catalysts.
As Table 4 shows, the effect of hot press temperature was obvious and a low hot press temperature of 120°C did not give relatively high wet shear strength after soaking in boiling water. This was because the hot press temperature was much lower than the curing temperature of PUF resins (139–150°C). When the hot press temperature was increased to 130°C, only both ZnO- and MgO-accelerated PUF resins can pass the GB/T 17657-4.15-1999 requirement (≥0.7 MPa). The higher hot press temperature resulted in higher wet shear strength. When the hot press temperature for the control and Na2CO3-accelerated resins increased from 130°C to 140°C, the wet shear strength significantly ranged from lower values up to the GB/T 17657-4.15-1999 requirement. But the CaO-accelerated PUF resin had a satisfactory strength (≥0.7 MPa) only if the hot press temperature was increased to 150°C (Table 4). This result was caused by the higher curing temperature of CaO-accelerated PUF resin. When the hot press temperature increased from 140°C to 150°C, the wet shear strength of cure-accelerated PUF resins other than CaO-acceleration changed slightly. This indicated that these resins can adequately cure to form good network density when the hot press temperature was 140°C.
4 Conclusion
The cure-accelerated PUF resins with Na2CO3, MgO, and ZnO can fast cure at a relative low temperature when compared with the control resin. The DSC curves indicated several reactions as observed by the presence of one or two peaks. The formation of methylene bridges was considered to be the main reaction during curing. The addition reaction of formaldehyde with free phenolic site may act as subsidiary reaction in the ZnO -and MgO-accelerated PUF resins.
The E a values of cure-accelerated PUF resins other than CaO-acceleration were much lower than the control resin; whereas the CaO-accelerated PUF resin presented higher E a value. The results of wet shear strength indicated cure-accelerated PUF resins with catalysts (Na2CO3, ZnO, and MgO) had higher wet shear strength than the control resin, while the CaO-accelerated resin showed the reverse results. Hot press temperature had a strong influence on the wet shear strength of PUF resins as well as the types of catalysts.
In one word, the catalysts such as Na2CO3, ZnO, and MgO were capable of increasing both the cure rate and the wet shear strength of PUF resins, and decreasing the cure temperature of the resins. But the CaO inhibited the cure reaction and decreased wet shear strength. Among all the catalysts, MgO had more significant improving effect on both the curing process and the wet strength of PUF resin.
References
Christiansen AW, Gollob L (2003) Differential scanning calorimetry of phenol-formaldehyde resols. J Appl Polym Sci 30(6):2279–2289
Effendi A, Gerhauser H, Bridgwater AV (2008) Production of renewable phenolic resins by thermochemical conversion of biomass: a review. Renew Sustain Energy Rev 12(8):2092–2116
Fan DB, Chang JM, Li JZH, Mao A, Zhang LT (2009a) 13C-NMR Study on the Structure of Phenol-Urea-Formaldehyde Resins Prepared by Methylolureas and Phenol. J Appl Polym Sci 112(4):2195–2202
Fan DB, Li JZH, Chang JM, Gou JSH, Jiang JX (2009b) Chemical structure and curing behavior of phenol-urea-formaldehyde cocondensed resins of high urea content. J Adhes Sci Technol 23(13–14):1787–1797
Fan DB, Li JZH, Chang JM (2009c) On the structure and cure acceleration of phenol-urea-formaldehyde resins with different catalysts. Eur Polym J 45(10):2849–2857
Fraser DA, Hall RW, Raum AL (1957) Preparation of ‘high-ortho’ novolak resins I. Metal ion catalysis and orientation effect. J Appl Chem 7(12):676–689
He GB, Riedl B (2003) Phenol-urea-formaldehyde cocondensed resol resins: their synthesis, curing kinetics, and network properties. J Polym Sci, Part B 41(16):1929–1938
He GB, Yan N (2005) Influence of the synthesis conditions on the curing behavior of phenol–urea–formaldehyde resol resins. J Appl Polym Sci 95(6):1368–1375
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706
Klasnja B, Kopitovic S (1992) Lignin-phenol-formaldehyde resins as adhesives in the production of plywood. Holz Roh- Werkst 50(7–8):282–285
Park BD, Riedl B, Hsu E, Shields J (1999) Differential scanning calorimetry of phenol–formaldehyde resins cure-accelerated by carbonates. Polymer 40(7):1689–1699
Pizzi A (1979a) Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. I. Phenolic chelates. J Appl Polym Sci 24(5):1247–1255
Pizzi A (1979b) Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. II. Tannin adhesive preparation, characteristics, and application. J Appl Polym Sci 24(5):1257–1268
Pizzi A (1994a) Advanced wood adhesives technology. Dekker, New York
Pizzi A (1994b) Handbook of adhesive technology. Dekker, New York
Pizzi A, Garcia R, Wang S (1997) On the networking mechanisms of additives-accelerated phenol–formaldehyde polycondensates. J Appl Polym Sci 66(2):255–266
Tomita B, Hse CY (1992) Cocondensation of urea with methylolphenols in acidic conditions. J Polym Sci, Part A 30(8):1615–1624
Tomita B, Hse CY (1993) Synthesis and structural analysis of cocondensed resins from urea and methylolphenols. Mokuzai Gakkaishi 39:1276–1284
Tomita B, Hse CY (1998) Phenol-urea-formaldehyde (PUF) co-condensed wood adhesives. Int J Adhes Adhes 18(2):69–79
Turunen M, Alvila L, Pakkanen TT, Rainio J (2003) Modification of phenol–formaldehyde resol resins by lignin, starch, and urea. J Appl Polym Sci 88(2):582–588
Vázquez G, López-Suevos F, Villar-Garea A, González-Alvarez J, Antorrena G (2004) 13C-NMR analysis of phenol-urea-formaldehyde prepolymers and phenol-urea-formaldehyde-tannin adhesives. J Adhes Sci Technol 18(13):1529–1543
Vázquez G, López-Suevos F, González-Alvarez J, Antorrena G (2005) Curing process of phenol-urea-formaldehyde-tannin, (PUFT) adhesives: kinetic studies by DSC and DMA. J Therm Anal Calorim 82(1):143–149
Zhao C, Pizzi A, Garnier S (1999) Fast advancement and hardening acceleration of low condensation alkaline PF resins by ester and copolymerized urea. J Appl Polym Sci 74(2):359–378
Zhao C, Pizzi A, Kuhn A, Garnier S (2000) Fast advancement and hardening acceleration of low condensation alkaline phenol-formaldehyde resins by esters and copolymerized urea. II. Esters during resin reaction and effect of guanidine salts. J Appl Polym Sci 77(2):249–259
Acknowledgements
The authors are very grateful for financial support from Chinese National Science and Technology planning (Project 2006BAD07A07-10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, DB., Chang, JM., Li, JZ. et al. Cure properties and adhesive performances of cure-accelerated phenol-urea-formaldehyde resins. Eur. J. Wood Prod. 69, 213–220 (2011). https://doi.org/10.1007/s00107-010-0414-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-010-0414-1