Abstract
Background
The objective of this study was to compare the results of transcatheter arterial embolization (TAE) with surgery in terms of efficacy in the context of bleeding duodenal ulcer (BDU) refractory to endoscopic treatment.
Materials and methods
From January 2006 to December 2016, all patients treated for a BDU refractory to endoscopic treatment were included in this observational, comparative, retrospective, single-center study. Primary endpoint was the overall success of treatment of BDU requiring surgical and/or TAE. The secondary endpoints were pre-interventional data, recurrence rates, feasibility of secondary treatment, morbidity and mortality of surgical and radiological treatment, intensive care unit and length of stay. A systematic review of the literature was performed to compare results of surgery and TAE.
Results
59 out of 396 patients (14.9%) treated for BDU required embolization and/or surgery: 15 patients underwent surgery (group S) including 7 patients after embolization failure and 44 patients underwent TAE (group TAE). The overall treatment success in intention to treat (85.7% vs 67.3%), per protocol (80% vs 79.5%) and bleeding recurrence rates (20% vs 15.9%) were also identical. Mortality (14.2% vs 15.3%) was similar between the two groups. Our study data were pooled with data from eight published studies and suggest that surgery have significant increased overall success (68.3% vs. 55.4%, p < 0.005).
Conclusion
The overall success rate was in favour of surgery according our meta-analysis. Our single-center study highlights the fact that predictive factors for recurrent bleeding after TAE must be identified to select good candidates for TAE and/or surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Duodenal ulcer is a very common disease, with between 50,000 and 100,000 new cases per year in France [1, 2]. This disease is particularly common in developed countries because of living conditions and dietary habits. Several risk factors have been identified, such as Helicobacter pylori infection that promotes acid production, nonsteroidal anti-inflammatory drugs and smoking [3,4,5].
Duodenal ulcer is associated with two main complications, perforated duodenal ulcer, which requires urgent surgical management, and bleeding duodenal ulcer (BDU), which may require endoscopic, radiological or surgical treatment [6]. Peptic ulcer bleeding is responsible for 50% of all cases of upper gastrointestinal bleeding with a mortality ranging from 3 to 14% according to various studies. The mortality rate increases proportionally with the patient’s age and has not decreased over the last 10 years [1].
First-line treatment of BDU requires upper gastrointestinal endoscopy (UGIE) to confirm the diagnosis, identify the site of bleeding and treat the majority of cases of BDU by injection of vasoconstrictors and/or the use of clips with a success rate ranging from 80 to 90% [7]. However, failures of endoscopic treatment may require the use of other techniques.
Historically, treatment after failure of endoscopy is based on surgery. Various conservative (ulcer suture with or without double ligation of the gastroduodenal artery) and radical (distal gastrectomy with partial duodenectomy) surgical techniques have been described. The morbidity and mortality of these emergency surgical procedures is high with a morbidity rate around 50% and a mortality rate around 30% [8, 9].
In view of this significant morbidity and mortality, other treatment options, therefore, need to be developed. As an alternative to surgery, Rösch et al. [10] reported in 1972 the results of the first series evaluating transcatheter arterial embolization (TAE) as management of acute nonvariceal upper gastrointestinal bleeding. Several series subsequently confirmed the feasibility of TAE for the management of upper gastrointestinal bleeding, including peptic ulcer disease, Mallory–Weiss tears, vascular ectasias and other diseases, with success rates ranging between 44 and 94% [11]. Finally, a few series focussed specifically on the results of TAE as management for BDU [11,12,13,14,15] and, to date, only one meta-analysis based on 6 series (423 patients) has compared TAE to surgery [16]. Recently, an article was published [17] comparing mortality between TAE and surgery in uncontrolled peptic ulcer bleeding (including duodenal and gastric ulcer) and showed a superiority of TAE for the length of stay (even if these criteria are difficult to analyze) and the complications even if the rate of rebleeding was higher.
The objective of this study was to compare the overall efficacy of these two techniques following failure of endoscopic treatment retrospectively in our center, and to perform a systematic review of the literature, with adding our results, to evaluate the results of these two techniques.
Materials and methods
Population
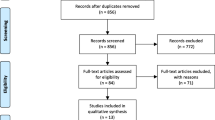
From January 2006 to December 2016, all patients managed for BDU were included in this single-center (University Hospital), retrospective, observational study comparing patients treated by surgery and patients treated by TAE after failure of endoscopic treatment for BDU (Fig. 1).
Inclusion criteria
Inclusion criteria were as follows: patients with BDU diagnosed on UGIE, BDU localized in the first part of the duodenum, failure of UGIE as treatment of BDU, patients requiring surgical and/or radiological management. Exclusion criteria were as follows: bleeding not localized in the duodenum (gastric location for example), bleeding due to upper gastrointestinal varices (esophageal varices, portal hypertension gastropathy), and upper gastrointestinal bleeding due to tumor or trauma.
Diagnosis of bleeding duodenal ulcer
All patients underwent first-line UGIE to identify the site of bleeding and, in most cases, to perform first-line treatment.
Endoscopic procedures
UGIE was performed within the first 24 h in the case of severe bleeding, active hematemesis and stable hemodynamics. Patients received an injection of erythromycin 250 mg over 30 min, 1 h before endoscopy to facilitate the procedure whenever compatible with hemodynamic parameters.
Endoscopic treatment was performed for duodenal ulcer with active bleeding or in the presence of a high risk of bleeding according to the FORREST classification [18]. Class I (spurting arterial bleeding, oozing arterial bleeding), IIa (signs of recent haemorrhage with visible vessel) and IIb (signs of recent haemorrhage with adherent clot) ulcers required endoscopic hemostatic therapy. Patients without active bleeding and a low risk of re-bleeding did not require endoscopic therapy.
Endoscopic hemostatic therapy consisted of infusion of saline with 1/10,000 adrenaline, a mechanical method with hemostatic clips, a thermal method with monopolar/bipolar cautery or hemostatic powder spray (Hemospray®). Endoscopic hemostatic therapy required a combination of two different procedures (injection of saline adrenaline and a mechanical procedure). In several cases of recurrence after the first endoscopic treatment, a second UGIE was performed to repeat endoscopic hemostatic therapy. Failure of endoscopic therapy was defined as recurrence of bleeding after two endoscopic hemostatic procedures.
TAE procedure
All TAE procedures were performed under general anesthesia by radiologists with an extensive experience of embolization [19]. The Seldinger technique was used with a unilateral femoral approach (using a 5F valved introducer sheath) with catheterization of the celiac axis, followed by the superior mesenteric artery (with a 5F Cobra catheter, Mallinckrodt Medical, Inc., Saint Louis, MI, USA). Superselective catheterization was then performed with a microcatheter (Progreat 2.7 or 2.4, Terumo Europe, Leuven, Belgium). After identifying the site of bleeding, the responsible artery was cannulated and arterial embolization with coils or glue was performed. Arteriography (using Iomeron 350 from Bracco Imaging, Evry, France) was then performed to verify perfusion of the celiac axis and the absence of bleeding at the bleeding site. If the bleeding artery was not identified, the gastroduodenal artery was always embolized. Clinical success was defined as the absence of a visible blush on the completion angiography (Fig. 2).
Surgical procedures
Surgical procedures were either conservative, consisting of duodenotomy with suture of the ulcer usually located on the posterior surface of the first duodenum, associated with double ligation of the GDA (on the superior and inferior part of the first part of the duodenum), such as Weinberg procedure (Fig. 3) [9, 20], or radical, consisting of distal gastrectomy including the first part of the duodenum (antrectomy) and reconstruction by Roux-en-Y or omega gastrojejunal anastomosis.
Weinberg procedure for a patient with failure of endoscopic treatment and recurrent bleeding after TAE. a Double ligation of the gastroduodenal artery (white arrow), the stomach is reclined upward, left and right with two valves; b suture of the ulcer after gastrotomy; white circle: location of the ulcer
Definition of post-operative/post-interventional events
Clinical success was defined by stop of bleeding during TAE or surgery performed for BDU refractory to endoscopic treatment. Recurrent bleeding was defined as any episode of upper gastrointestinal bleeding after TAE and/or surgery and intraperitoneal bleeding in case of surgery.
Endpoints and data collected
The primary endpoint was the overall success of treatment of BDU requiring surgical and/or radiological management following failure of endoscopic treatment (surgical or radiological haemostasis at the end of the procedure, with no recurrence after the procedure). The analysis was done in intention to treat and in per protocol analysis. The secondary endpoints were feasibility and clinical success (which means the technical success with no apparent bleeding after the completion of TAE or surgery), morbidity and mortality, length of hospital stay and recurrence rate.
The following parameters were recorded:
-
Demographic and clinical data: age, gender, risk factor of BDU (smoking, non-steroidal anti-inflammatory drug, oral anticoagulant/antiplatelet therapy, Helicobacter pylori infection, postoperative BDU), Charlson Comorbidity Index score [21]
-
Endoscopic data: date of first UGIE, interval between onset of symptoms and date of diagnosis, number of therapeutic UGIE, type of endoscopic treatment for BDU, number of red blood cell transfusions, initial haemorrhagic shock prior to surgery or TAE.
-
Surgical data: type of procedure, operative time, intraoperative events, feasibility rate, clinical success.
-
Radiological data: type of procedure (type of embolization procedure), feasibility rate and cause of failure, clinical success.
-
Outcomes: overall morbidity and mortality rate according Dindo–Clavien classification [22], cause of death, morbidity and mortality relative to surgery and/or radiological procedure.
-
Length of stay: in the intensive care unit (ICU) and in the hospital.
-
Recurrence of bleeding: recurrence rate after surgical and/or radiological procedure, time to recurrence (after surgical and/or radiological procedure), type of treatment for recurrent BDU.
Systematic review
To put our results in context with regard to other studies on this topic, we searched PubMed, the Embase databases, and the Cochrane Library for articles comparing results of surgery and transcatheter arterial embolization published between January 2004 and December 2017. The search terms were “bleeding ulcer”, “peptic ulcer”, “transcatheter arterial embolization”, “gastric surgery”, “duodenal surgery” and “gastrectomy”.
The reference list of each selected article was checked for studies not listed in the PubMed and Embase databases or not found in the computerized search.
All retrieved articles were screened for relevance. The methodologic quality of the diagnostic studies was evaluated independently by two reviewers, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria. The studies were graded according to items of relevance to the present review. Retrieved studies were divided into three groups as a function of the calculated PRISMA score: a score of 8 or 9 indicates good quality, a score of 6 or 7 indicates fair quality, and a score of 5 or less indicates poor quality.
Statistics
Patient baseline characteristics were expressed as mean ± standard deviation (SD) and median (interquartile range) for continuous data and number (frequency) for categorical data. Univariate analysis was based on Student's t test for quantitative variables. A Mann–Whitney U test was used for non-parametric variables. The limit for statistical significance was p < 0.05. All statistical tests were performed with SPSS software (version 15.0 for Windows, SPSS Inc., Chicago, IL, USA).
For systematic review, we first estimated the odds ratio (OR) and its standard error within each study. Data analyzed concerned a comparison of overall success, bleeding recurrence, major complication and mortality between surgery and TAE as treatment of BDU refractory to endoscopic treatment. The length of stay was not studied in the systematic review because of the inhomogeneity of the results which were in means or in medians without all data to analyse all the studies perfectly. Then, we performed a random effect (restricted maximum likelihood estimation) meta-analysis to take into account the heterogeneity between studies. The heterogeneity was assessed with inconsistency index I2 and the Q test [23]. The extent of heterogeneity was classified as follows: I2 < 25%, no heterogeneity; 25% ≤ I2 ≤ 50%, moderate heterogeneity; et I2 > 50%, large heterogeneity. Statistical heterogeneity was declared for a p value < 0.05 with the Q test. Forest plots were displayed to illustrate the study-level OR and the pooled OR with a 95% confidence interval. Publication bias or small-study effect was assessed graphically using funnel plots and tested using Egger’s method [24]. All statistical analyses were performed with RStudio software [Version 1.0.143—© 2009–2016 RStudio, Inc. (using the rmeta and metafor packages)].
Results
Demographic and clinical data
During the study period, 396 patients were managed for BDU: 59 of these patients (14.9%) required surgery and/or TAE.
Patients with failure of endoscopic treatment comprised 71.2% of males (n = 42) with a mean age of 72 years ± 17 (37–90). Comorbidities included hypertension in 42.4% of patients and a history of abdominal surgery in 16.9% of patients. Surgery and TAE groups were comparable in terms of demographic data and risk factors in intention to treat and per protocol population (Table 1). Mean Charlson Comorbidity Index score was 2.14 (1–4) in the surgery group and 3.17 (1–8) in the TAE group (p = 0.15). The two groups were also similar in terms of the endoscopic procedures performed prior to surgery or TAE and the mean number of red blood cell transfusions (Table 2).
Primary endpoint (Table 3)
The overall success rate for management of refractory BDU, in the intention-to-treat analysis, was 85.7% in the surgery group and 67.3% in TAE group (p = 0.27). The overall success in the per protocol analysis was 80% in the surgery group and 79.5% in the TAE group (p = 1).
Surgical and TAE data
The mean interval between the first UGIE and the surgical or TAE procedure (3.1 days vs. 5.4 days, respectively) and the rate of patients with hemorrhagic shock (57.1% vs. 82.6%, respectively; p = 0.25) were similar in the two groups (Table 3).
Radical surgery, consisting of antrectomy, was performed in six patients (40%) and a conservative procedure, consisting of duodenotomy with suture of the ulcer combined with double ligation of the GDA, was performed in nine patients (60%). The mean operating time for the surgery group was 135 ± 125 min (110–340). Surgery constituted first-line treatment after failure of UGIE in nine patients, while surgery was performed in a context of recurrent bleeding after TAE in seven patients and surgery was performed after failure of TAE in one patient.
An embolization procedure was performed in 51 patients but could not be performed in one case due to failure of femoral artery catheterization in a context of hemodynamic instability (4.5%). Gelfoam was used in 20 patients (46.5%), coils were used in 35 patients (81.3%), and glue was used in 12 cases (28%). Two combined embolization materials were used in 30 patients (69.7%). 13 patients had a “prophylactic” embolization of the gastroduodenal artery, because the radiologist did not find a bleeding (Fig. 1).
Secondary endpoints (Table 3)
Clinical success rates were 100% in the surgery group and 98% in the TAE group.
The morbidity rate was similar even if a higher rate of severe postoperative complications (Dindo–Clavien ≥ 3) was observed in the surgery group compared to TAE group (57.1% vs. 30.7%, respectively; p = 0.25). The mortality rate was similar in the two groups (14.2%, 1/7) in the surgery group vs. 15.3%, 8/52 in the TAE group [p = 0.94)]. However, three of the four patients (75%) who died after surgery in the per protocol analysis had previously undergone a TAE procedure but experienced recurrent bleeding after TAE and three of the four patients who died had undergone antrectomy. The main causes of death were haemorrhagic or septic shock (Table 3).
The length of stay (in ICU and in hospital) was similar in the two groups towards a longer ICU stay in surgery group (Table 3).
Recurrent bleeding was observed in 20% (n = 3) of cases in the surgery group and 15.9% (n = 7) of cases in the TAE group (p = 0.7). The TAE procedure failure rate was 4.5% (n = 1). In case of recurrence, the seven patients in the TAE group were treated surgically following recurrent bleeding. The three patients who developed recurrent bleeding in the surgery group were treated endoscopically with saline adrenaline and/or clips.
Systematic review (Fig. 4)
Our study data were pooled with data from 8 published studies (totalling 504 patients in the surgery group and 366 patients in the TAE group) [13,14,15, 17, 25,26,27,28]. The data showed that surgery for BDU refractory to endoscopic treatment were of benefit significantly compared to TAE (Fig. 4a, d) in terms of overall success and bleeding recurrence. On the other hand, TAE had significantly fewer major complications compared to surgery (Fig. 4b). No significant difference was found between surgery and TAE concerning mortality (Fig. 4c).
Discussion
This study was conducted in the context of simplification of the emergency care of complex patients designed to limit invasive surgical procedures. In this study, 59 out of 396 patients (14.9%) required complementary treatment for recurrent bleeding, corresponding to TAE in 44 patients and surgery in 15 patients (7 after failure of TAE). The two groups were similar in terms of demographic data, endoscopic data and criteria of severity (shock, red blood cell transfusions). In the intention-to-treat analysis, the overall success rate (85.7% vs 67.3%, p = 0.27), mortality rate (14.2% vs. 15.3%, p = 0.94) and recurrence rate (14.2% vs 28.8%, p = 0.37) were similar in the two groups. These results do not support the use of first-line TAE as 75% (3 out of 4) of the patients who died in the surgery group (in the per protocol analysis) had previously undergone a TAE procedure with recurrence of bleeding.
UGIB is a common reason for emergency admission. In a British cohort representing 74 centers, the presumed incidence of UGIB was estimated to be 103 cases per 100,000 adults per year [29]. In this study, the majority of cases of UGIB were due to peptic ulcers (46%), predominantly involving the duodenum (53%). UGIB constitutes a real public health problem, as the recurrent bleeding rate in a cohort of 627 patients was 11.2%, the same as that observed in our study, and the overall mortality was 5.74% [30]. The present study identified several risk factors for mortality, such as age > 60 years old, hypotension (systolic blood pressure < 100 mmHg), absent or delayed endoscopic diagnosis, presence of comorbidities, blood transfusion and recurrent bleeding. In their Canadian cohort study, Quan et al. [31] reported a similar mortality rate (8.5%) with 4.3% of cases requiring surgical management. Most cases of UGIB were related to peptic ulcer, which is why the Japanese Society of Gastroenterology recommends upper GI endoscopy within the first 24 h following admission to establish the diagnosis (site of bleeding and etiology) and to perform first-line treatment [32]. In these guidelines, surgical and/or radiological management is reserved for failure of endoscopic treatment, while no first-line technique after failure is recommended.
The place of surgery or radiological treatment for management of BDU is generally not described in detail. The “International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding” [2] do not describe the place and indication of surgery following failure of endoscopic treatment and radiological treatment is only described as a good alternative to surgical treatment but with no definition of a precise framework. This is probably related to the limited published data on this topic, as the majority of cases of BDU are successfully treated by endoscopy. As highlighted in our series, conducted over a 12-year period, only 59 (14.9%) of 396 patients with a diagnosis of BDU on endoscopy required surgical and/or radiological management. Several recent series have described the results of TAE [11,12,13,14,15, 27], while the majority of series on surgical management [33,34,35] correspond to old studies.
Our study compared the results of surgery and TAE following failure of endoscopic treatment. Analysis of preoperative data showed that the two groups were similar in terms of age and comorbidities, in contrast with the data in the literature, in which patients undergoing TAE are usually older with more comorbidities than patients treated surgically [16]. The present study also highlights the fact that TAE or surgery was performed with the same time interval with respect to the first UGIE and with a similar patient status, as the rate of preoperative shock was similar with even a higher rate of preoperative shock in the TAE group demonstrating the feasibility of TAE in this context. However, the type of endoscopic treatment is different, as more clips are placed in patients undergoing secondary surgery, suggesting that these patients may not present the same type of ulcers as in the TAE group (Table 2).
Our systematic review of the literature showed that overall success is in favour of surgery with an odds ratio of 2.19 (Fig. 4a). Also, despite surgery was significantly associated with increased rate of major complications, the bleeding recurrence was decreased compared to TAE. When analysing mortality, no significant differences were found between surgery and TAE even if there is a lower rate of major complications and mortality in case of TAE. An important point that would have been interesting to analyse, would be the evolution of patients who had failure of a primary interventional therapy (TAE or surgery). In our single-center series, recurrence of bleeding after TAE was associated with an increased risk of death as 75% of patients died in the surgery group had previous TAE. In these cases, delayed management due to failure of TAE may have led to overall delayed management and may be associated with a poorer prognosis. Our study highlights the need to identify predictive criteria for recurrent bleeding after TAE to propose first-line surgery in selected cases. Unfortunately, these data are not available as part of this review of the literature suggesting that these data would be interesting to analyse in the future.
Recently, Sverdén et al. [17] published a large cohort study of 282 patients, 97 in TAE group and 185 in surgery group. The main outcome was time to all-cause overall mortality. Their results showed a statistically significantly decreased of complications (8.3% vs 32.2%, p < 0.0001) and length of stay (9 days vs 18, p < 0.0001) in the TAE group, despite a higher rate of rebleeding. Our series and meta-analysis showed different results than those of Sverdén et al. [16] as shown in Table 4. Also, the overall success was not available and is an important point to take into account for comparing such two techniques.
Our study has a number of limitations, such as a limited number of surgical procedures and its retrospective nature that may have been responsible for missing data in some cases. The type of radiological procedure was also not standardized, as only glue or coils were used in the first patients treated by TAE, while it is now generally accepted that TAE requires the use of two embolization materials [11]. The success rate of TAE would probably be even higher with the use of two embolization materials.
Conclusion
Failure of endoscopic treatment for BDU is rare. In our study, the overall success rate was similar in patients undergoing surgery or TAE, while a review of the literature showed that surgery was more efficient compared to TAE. No significant differences were found between surgery and TAE concerning major complications, mortality and bleeding recurrence. Our single-center study highlights the fact that predictive factors for recurrent bleeding after TAE must be identified to select good candidates for TAE and/or surgery to decrease the overall mortality.
References
van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209–24.
Barkun AN, Bardou M, Kuipers EJ, et al. International consensus upper gastrointestinal bleeding conference group. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–13.
Magalhães Queiroz DM, Luzza F. Epidemiology of helicobacter pylori infection. Helicobacter. 2006;11(Suppl 1):1–5.
Komen NA, Bertleff MJ, van Doorn LJ, Lange JF, de Graaf PW. Helicobacter genotyping and detection in peroperative lavage fluid in patients with perforated peptic ulcer. J Gastrointest Surg. 2008;12:555–60.
Liu T, He W, Li Y. Helicobacter pylori infection of gastric epithelial cells affects NOTCH pathway in vitro. Dig Dis Sci. 2016;61:2516–21.
Bertleff MJ, Lange JF. Perforated peptic ulcer disease: a review of history and treatment. Dig Surg. 2010;27:161–9.
Michaud L. Interventional digestive endoscopy in pediatrics. Arch Pediatr. 2006;13:399–404.
Chevallier P, Novellas S, Vanbiervliet G, et al. Transcatheter embolization for endoscopically unmanageable acute nonvariceal upper gastrointestinal hemorrhage. J Radiol. 2007;88:251–8.
Brehant O, Fuks D, Sabbagh C, et al. Surgical management of duodenal ulcer with hemorrhage from the gastroduodenal artery: antrectomy versus conservative surgery? J Chir (Paris). 2008;145:234–7.
Rösch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303–6.
Loffroy R, Guiu B, D'Athis P, et al. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleeding. Clin Gastroenterol Hepatol. 2009;7:515–23.
Katano T, Mizoshita T, Senoo K, et al. The efficacy of transcatheter arterial embolization as the first-choice treatment after failure of endoscopic hemostasis and endoscopic treatment resistance factors. Dig Endosc. 2012;24:364–9.
Eriksson LG, Ljungdahl M, Sundbom M, Nyman R. Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol. 2008;19:1413–8.
Ripoll C, Bañares R, Beceiro I, et al. Comparison of transcatheter arterial embolization and surgery for treatment of bleeding peptic ulcer after endoscopic treatment failure. J Vasc Interv Radiol. 2004;15:447–50.
Venclauskas L, Bratlie SO, Zachrisson K, Maleckas A, Pundzius J, Jönson C. Is transcatheter arterial embolization a safer alternative than surgery when endoscopic therapy fails in bleeding duodenal ulcer? Scand J Gastroenterol. 2010;45:299–304.
Kyaw M, Tse Y, Ang D, Ang TL, Lau J. Embolization versus surgery for peptic ulcer bleeding after failed endoscopic hemostasis: a meta-analysis. Endosc Int Open. 2014;2:E6–E14.
Sverdén E, Mattsson F, Lindström D, Sondén A, Lu Y, Lagergren J. Transcatheter arterial embolization compared with surgery for uncontrolled peptic ulcer bleeding: a population-based cohort study. Ann Surg. 2019;269:304–9.
Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;17(2):394–7.
Chivot C, Rebibo L, Robert B, Regimbeau JM, Yzet T. Ruptured pancreaticoduodenal artery aneurysms associated with celiac stenosis caused by the median arcuate ligament: a poorly known etiology of acute abdominal pain. Eur J Vasc Endovasc Surg. 2016;51:295–301.
Weinberg JA. Treatment of the massively bleeding duodenal ulcer by ligation, pyloroplasty and vagotomy. Am J Surg. 1961;102:158–67.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Larssen L, Moger T, Bjørnbeth BA, Lygren I, Kløw NE. Transcatheter arterial embolization in the management of bleeding duodenal ulcers: a 5.5-year retrospective study of treatment and outcome. Scand J Gastroenterol. 2008;43:217–22.
Wong TC, Wong KT, Chiu PW, et al. A comparison of angiographic embolization with surgery after failed endoscopic hemostasis to bleeding peptic ulcers. Gastrointest Endosc. 2011;73:900–8.
Ang D, Teo EK, Tan A, et al. A comparison of surgery versus transcatheter angiographic embolization in the treatment of nonvariceal upper gastrointestinal bleeding uncontrolled by endoscopy. Eur J Gastroenterol Hepatol. 2012;24:929–38.
Nykänen T, Peltola E, Kylänpää L, Udd M. Bleeding gastric and duodenal ulcers: case-control study comparing angioembolization and surgery. Scand J Gastroenterol. 2017;52:523–30.
Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ. 1995;311:222–6.
Lu M, Sun G, Zhang XL, et al. Risk factors associated with mortality and increased drug costs in nonvariceal upper gastrointestinal bleeding. Hepatogastroenterology. 2015;62:907–12.
Quan S, Frolkis A, Milne K, et al. Upper-gastrointestinal bleeding secondary to peptic ulcer disease: incidence and outcomes. World J Gastroenterol. 2014;20:17568–77.
Satoh K, Yoshino J, Akamatsu T, et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2015. J Gastroenterol. 2016;51:177–94.
Dousset B, Suc B, Boudet MJ, et al. Surgical treatment of severe ulcerous hemorrhages: predictive factors of operative mortality. Gastroenterol Clin Biol. 1995;19:259–65.
Millat B, Hay JM, Valleur P, Fingerhut A, Fagniez PL. Emergency surgical treatment for bleeding duodenal ulcer: oversewing plus vagotomy versus gastric resection, a controlled randomized trial. French associations for surgical research. World J Surg. 1993;17:568–73 (discussion 574).
Pautrat K, Valleur P, Pocard M. Urgent surgery for bleeding duodenal ulcer. J Chir (Paris). 2007;144:231–5.
Funding
No funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest or sources of funding to declare.
Rights and permissions
About this article
Cite this article
Darmon, I., Rebibo, L., Diouf, M. et al. Management of bleeding peptic duodenal ulcer refractory to endoscopic treatment: surgery or transcatheter arterial embolization as first-line therapy? A retrospective single-center study and systematic review. Eur J Trauma Emerg Surg 46, 1025–1035 (2020). https://doi.org/10.1007/s00068-020-01356-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-020-01356-7