Abstract
Purpose
To investigate the outcomes of transcatheter arterial embolization (TAE) for the treatment of peptic ulcer bleeding (PUB).
Materials and Methods
This is a retrospective, multicenter study, which investigated all patients who underwent TAE for the treatment of severe upper gastrointestinal hemorrhage from peptic ulcers in five European centers, between January 1, 2012 and May 1, 2017. All patients had undergone failed endoscopic hemostasis. Forty-four patients (male; mean age 74.0 ± 11.1 years, range 49–94), with bleeding from duodenum (36/44; 81.8%) or gastric ulcer (8/44; 18.2%) were followed up to 3.5 years (range 2–1354 days). In 42/44 cases, bleeding was confirmed by pre-procedural CT angiography. In 50% of the cases, coils were deployed, while in the remaining glue, microparticles, gel foam and combinations of the above were used. The study’s outcome measures were 30-day survival technical success (occlusion of feeding vessel and/or no extravasation at completion DSA), overall survival, bleeding relapse and complication rates.
Results
The technical success was 100%. The 30-day survival rate was 79.5% (35/44 cases). No patients died due to ongoing or recurrent hemorrhage. Re-bleeding occurred in 2/44 cases (4.5%) and was successfully managed with repeat TAE (one) or surgery (one). The rate of major complications was 4.5% (2/44; one acute pancreatitis and one partial pancreatic ischemia), successfully managed conservatively. According to Kaplan–Meier analysis survival was 71.9% at 3.5 years.
Conclusions
TAE for the treatment of PUB was technically successful in all cases and resulted in high clinical success rate. Minimal re-bleeding rates further highlight the utility of TAE as the second line treatment of choice, after failed endoscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peptic ulcer bleeding (PUB) remains the main cause of acute non-variceal upper gastrointestinal hemorrhage. According to population-based estimates the annual incidence of bleeding from a peptic ulcer in the USA is approximately 60 per 100,000 people, with an associated mortality ranging from 5 to 10% and an estimated annual direct cost of in-hospital care reaching over $2 billion [1]. Endoscopy is the first-line treatment due to its high uncomplicated hemostasis success rate and the possibility of concomitant diagnosis. Although approximately 25,000 urgent operations for PUB were performed in the USA alone in 2006, recently, the incidence of emergency surgery rates for PUB has been decreased to 6.5–7.5% [2, 3]. Nevertheless, surgery remains the most common main treatment option after failed endoscopy [3]. Importantly, a rate of hemorrhage up to 20% has been reported following initial endoscopy, resulting in a 10% mortality rate [4].
Percutaneous transcatheter arterial embolization (TAE) is a minimally invasive alternative to emergency surgery for PUB, as several studies report excellent hemostatic outcomes and low complications rates. Considering, that in 68% of the cases PUB occur in patients older than 60 years and 27% in patients over 80 years of age, a population presenting various comorbidities and usually of high-surgical risk, TAE appears as an appealing alternative treatment option.[1]. However, according to data reported from US hospitals, TAE was performed in only 0.24% of PUB cases in 2006, while endoscopy to control bleeding demonstrated an increase of 58.9% between 1993 and 2006 [5]. In a European population-based study, performed in Sweden, between 2000 and 2014, in total 97 patients with PUB underwent TAE group and 185 surgery [6]. As TAE obtains results similar to surgery but with fewer complications, it is an appropriate treatment for PUB particularly in critically ill, high-surgical-risk patients [6,7,8,9]. The European Society of Gastrointestinal Endoscopy (ESGE) strongly recommends TAE or surgery for patients with recurrent bleeding following failure of a second endoscopic attempt and reports that this is supported by high level of evidence [7]. However, published data regarding of TAE for life-threatening PUB after failed endoscopic hemostasis remain limited to single-center and retrospective studies [3, 6,7,8,9]. We have investigated the outcomes of real-life TAE for the treatment of PUB, in the setting of five tertiary European Hospitals.
Materials and Methods
This is a retrospective, multicenter study, investigating all consecutive patients who underwent (TAE for the treatment of upper gastrointestinal bleeding due to PUB confirmed by endoscopy, in five European centers (Athens, London, Rome and Matera), between January 1, 2012 and May 1, 2017.
Electronic databases of the participating departments were meticulously searched as to identify all possible cases, using the following keywords: embolization, transcatheter embolization, gastrointestinal bleeding, peptic ulcer, duodenal ulcer, gastric ulcer, bleeding and hemorrhage. The medical records of patients identified in the search were retrieved and scrutinized retrospectively. Institutional review board approval was obtained. Patients who were able to consent had signed a procedural consent form regarding the risks and benefits of the procedure. In cases in which patients were not able to consent due to the severity of the clinical condition, consent was obtained from relatives where possible, or from the treating physicians.
Inclusion criteria treatment was an ongoing UGI acute bleeding from a duodenal or gastric ulcer after failed endoscopic hemostasis. Hemodynamically unstable patients, defined as those with < 90 mmHg and heart rate > 100–130 bpm at admission or after > 1–2 L initial fluids > 2–6 packed red blood cells/24 h, were judged fit for TAE only if they were stabilized following anesthetic drug/fluid support and remained under continuous anesthetic support and monitoring throughout the CTA and embolization procedure. Patients not stabilized despite hemodynamic resuscitation were not judged fit to undergo TAE and underwent emergency surgery [10]. Patients were excluded from the study if they had perforated ulcers and were treated surgically. In addition, patients with UGI bleeding related to cancer or causes other than PUB according to endoscopy or pre-procedural CT, were excluded from the analysis. Diagnosis of PUB was always confirmed by endoscopy. Patients with coagulation disorders, defined as an international normalized ratio (INR) > 1.5 and/or a blood platelet count < 50,000/ml, were treated in order to correct their coagulation profile using fresh frozen plasma, prothrombin complex concentrates, vitamin K administration and omission of antithrombotic therapy.
Demographic and procedural details were recorded. Statistical analysis was performed using the Graphpad Prism statistical software (Graphpad Prism, version 5.0; San Diego, CA).
Procedure
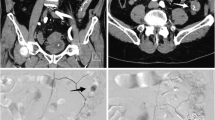
Pre-procedural CT angiography (CTA) was performed in all cases but two, in order to confirm the bleeding, detect the feeding vessel and/or the bleeding site and plan the embolization procedure. Two patients were transferred directly from the endoscopy room to the angiography suite, without a prior CTA. This was a team decision made between anesthesiology, endoscopy and interventional radiology physicians and was based on the fact that these two patients suffered from massive hematemesis due to bleeding duodenal ulcer evident during endoscopy, causing hemodynamic instability. Despite the fact that the patients were stabilized using fluids and inotropic therapy, multidisciplinary team decision was to proceed directly with TAE in order to minimize the risk of further hemodynamic compromise. In cases of hemodynamic compromise, prophylactic empiric proximal gastroduodenal artery (GDA) or left gastric artery (LGA) TAE was performed using coils and/or microcoils, even after a negative DSA, in order to decrease the risk of a new massive UGI hemorrhage. All procedures were performed by interventional radiologists with at least 5 years of experience in emergency endovascular procedures, with anesthesiology support. TAE was performed according to standard endovascular embolization techniques aiming in super-selective embolization of the bleeding vessel. In cases of GDA embolization the target was to position the microcatheter just beyond the bleeding site in order to perform the back-to-front door technique and minimize the risk of backflow filling. In outline, common femoral artery access was gained and a 5 or 6Fr sheath was inserted. The celiac axis was catheterized using standard angiographic catheters (SIM or COBRA angiographic catheters, CORDIS Endovascular, USA; Simmons or Cobra Glidecath angiographic catheters, TERUMO, Japan) and guide wires (Radiophocus hydrophilic guide wire, TERUMO, Japan). The choice between different angiographic catheters depends on the anatomic characteristics of the celiac axis and in the majority of the cases a SIM or Simmons catheter successfully catheterized the vessel. Subsequently to celiac axis catheterization, microcatheters were required to catheterize small diameter vessels as GDA branches or the left gastric artery and avoid spasm that will lead to failure to embolize the bleeding vessel. Once the celiac axis was catheterized, DSA was performed in order to depict the arterial anatomy and identify the bleeding site. Subsequently, the GDA or LGA was catheterized (most frequently using a microcatheter), and selective DSA was carried out to confirm the bleeding findings. The microcatheters that have been used were the Prograte 2.7Fr (TERUMO, Japan) and the ASAHI Masters 2.6Fr, (ASHAHI INTECC, CO, Japan). Embolization was performed using pushable coils or microcoils, N-butyl cyanoacrylate (NBCA), microspheres, gel foam pledgets or combinations of the above according to the operating physicians’ judgment. The type of coils and microcoils used were pushable Nestercoils, (Cook), Microplex coils or AZUR peripheral hydrocoils (Terumo, Japan) with diameters ranging from 2 to 6 mm. Microparticles used were the Embozene microspheres (CELONOVA, Germany), the Contour PVA (Boston Scientific, USA) and the PVA Foam embolization particles (COOK endovascular, USA) with diameters ranging from 250 to 700 μm. NBCA glue used was the Gluebran® (GEM SRL, Italy), in mixtures proportions with lipiodol ranging from 1:2 to 1:3. The superior mesenteric artery (SMA) was always catheterized in cases of GDA embolization, in order to check for backflow filling of the ulcer, which occurred frequently from the inferior pancreaticoduodenal artery (IPDA). If no signs of bleeding (active contrast extravasation, contrast blushing) were noted, empiric embolization of the proximal GDA or LGA was performed guided by the pre-procedural CTA findings, in order to diminish the arterial inflow to the ulcer and decrease the risk of recurrent hemorrhage.
Endpoints, Definitions and Follow-Up
The efficacy outcome measure was technical success, defined as occlusion of the feeding vessel and/or no signs of bleeding at completion DSA. The safety outcome measure was the incidence of procedure-related complications. Complications were classified according the six-grade CIRSE classification system (grade: 1 intra-procedural complication solved within the same session; no additional therapy, no post-procedure sequelae, no deviation from the normal post-therapeutic course; up to grade 6: death) [11]. Other outcome measures investigated included the 30-day and overall survival rates, and the incidence of recurrent hemorrhage. Telephone interviews were carried out by the physicians participating in the study and the findings were recorded in electronic databases and in the patients’ hospital records. The follow-up period ended on October 1, 2017.
Results
In total, 44 patients (male 20; 45.5%), with a mean age of 74.0 ± 11.1 years (range 49–94) and bleeding from a duodenal (36/44; 81.8%) or gastric ulcer (8/44; 18.2%), were included. All patients had previously undergone failed endoscopy and presented various comorbidities including coronary artery disease (25/44; 56.8), respiratory disease (10/44; 22.7%), malignancy (7/44; 15.9%; one case of hemicolectomy for colon cancer, one case of left hepatectomy due to cholangiocarcinoma, two cases of prostatic cancer with bone metastasis, one hepatocellular carcinoma, and two cases metastatic ovarian cancer, none related to the site of bleeding), recent trauma/surgery (13/44; 29.5%), and hereditary bleeding disorders (factor V and factor VIII disorders: 2/44; 4.5%). In three patients, coagulation disorders were corrected before TAE. In 100% of the cases (42/42 cases) in which pre-procedural CTA was performed, active bleeding of the ulcer was diagnosed, while in the two cases that were transferred directly in the angiography suite, DSA was positive for active bleeding and were embolized using microcoils. The most frequent clinical symptom was hemoglobin drop (100%) and recurrent (> 1) melena in 42/44 cases (95.5%), while 25/44 patients (56.8%) were hemodynamically unstable and were stabilized by the anesthesiology team at the time of embolization. General anesthesia was required in 3/44 (6.8%) procedures. In 22/44 cases (50.0%), coils or microcoils or both were deployed. However, glue, microparticles, gel foam and combinations of the above were also used. The most commonly used combination of embolic materials was coils and glue (5/44; 11.4%) and coils with microparticles (5/44; 11.4%). The demographic data and procedural details are shown in detail in Table 1. The rate of technical success was 100%. The mean hemoglobin (Hb) value increased significantly following TAE (mean pre-embolization value 7.3 ± 1.4 vs. 9.7 ± 1.7 post-embolization; p < 0.0001) (Fig. 1). Empiric embolization of the feeding vessel without evidence of bleeding at intra-procedural DSA was performed in 10/44 cases (22.7%) and was not associated with increased re-bleeding rate which was zero (0/10 cases) for the empiric TAE subgroup versus 5.9% (2/34 cases) for the targeted TAE subgroup (p = 0.21). In total, 9 patients died during their stay in hospital, resulting in a 30-day survival rate of 79.5% (35/44 cases). No patient died due to ongoing or recurrent hemorrhage. In total, two cases of re-bleeding were noted (2/44; 4.5%), all within 1 week following the initial embolization procedure using microparticles and glue in one case and glue in the second case and both successfully managed (during hospitalization)—one with repeat TAE (microcoil embolization) and the other with gastrectomy. Procedure-related complications (CIRSE classification grade 3) included one case of acute pancreatitis following embolization of the GDA with 500 μm microparticles and gel foam, and one case of ischemia of the pancreatic head following glue embolization of the GDA (2/44; 4.5%), diagnosed according to clinic-laboratory criteria. Both patients were managed successfully with standard conservative treatment. There was one case of non-occlusive bowel ischemia, which resulted in death 3 days following coil embolization of the GDA using 3 mm and 5 mm microcoils, but its relation to the procedure was considered highly improbable. According to Kaplan–Meier analysis, the estimated overall survival rate was 71.9% in up to 3.5-year follow-up (Fig. 2).
Discussion
According to this multicenter, retrospective, real-life study, TAE demonstrated very high technical and clinical success rates in the management of severe bleeding due to peptic ulcers that had not responded to endoscopic hemostasis, in patients with severe comorbidities, who would otherwise have had to undergo emergency surgery. Immediate hemostasis was achieved in all cases. The procedure-related complication rate was 4.5%, which is significantly lower than the rate of 46% reported following emergency surgery [3]. Recurrent hemorrhage was encountered only in two cases, both occurring within the first week following initial TAE, and managed successfully with repeat TAE or gastrectomy. Surgery remains a valid option after TAE failure. Interestingly, in cases of recurrent hemorrhage, initial embolization was performed using a combination of microparticles and glue in the first case and glue alone in the second case, although one might speculate that glue and microparticles is a more aggressive embolization. The use of glue was traditionally not popular in gastrointestinal bleeding. However, glue alone or in combination with coils or microparticles used in 11 cases in this study, did not incite major ischemic complications. The advantages of glue embolization include permanent and immediate total occlusion of the target lesion, which is not affected by future coagulation disarrangement, frequently noted in bleeders, or vasodilation noted following hemodynamic stabilization, as well as distal diffusion of the embolic material, particularly useful in cases of super-selective catheterization of the “crater” of the ulcer. The authors believe that all embolic agents should be available for the management of PUB, as the choice of embolic material is case sensitive. According to the authors’ opinion, the back-to-front door technique should be attempted in GDA cases in order to block retrograde lesion filling and prevent from recurrent bleeding episodes, which cannot easily be treated with repeat TAE if the proximal GDA is embolized. Following subgroup analysis for various embolization techniques (empiric versus targeted) and materials no difference in technical success and re-bleeding rate was noted. However, number of recurrent hemorrhages may have been too small to demonstrate a difference. Various embolic agents have been used by many different operators in this real-life study. Interestingly, the use of glue and/or microparticles did not incite ischemic events. The authors believe that this should be attributed to operator’s experience with the specific agents and the selection of the appropriate embolic agent for each case. According to our experience, small size microparticles (< 300 μm in diameter) is not of particular usefulness and should be avoided as to decrease the risk of ischemia, while glue dilution although case sensitive, in general should range between 1:2 and 1:4. Moreover, the authors recommend the use of glue and/or microparticles following super-selective catheterization of the bleeding vessel and not in less targeted embolization.
Focusing on empiric embolization, previous studies have reported that this technique is justified by the intermittent nature of PUB and by the fact that diagnosis of ongoing PUB can be missed during DSA, while its safety and efficacy in preventing re-bleeding is similar to that of targeted embolization [12, 13]. In this retrospective study, 10 patients underwent empiric embolization as they presented with massive PUB not controlled by endoscopy, while CTA few minutes before diagnostic DSA had demonstrated active contrast extravasation at the site of endoscopic findings. Moreover, the fact that hemodynamic compromise was still present at the time of DSA should indicate that ongoing PUB was probably missed during DSA. Nonetheless, the authors support the use of empiric embolization, in such lifesaving cases, even if active bleeding has stopped at the exact time of DSA, as a recurrent bleeding event should be awaited and could be fatal, while empiric embolization did not incite ischemic complications. On the other hand, one could advocate that in these cases, active bleeding had already stopped just before embolization and a relapse would not occur even without embolization. This study has not sufficient data to reject this notion, and further studies are required to assess the possibility of over-treatment. At this point, the authors would like to highlight the utility of pre-procedural CTA in performing a safe and effective empiric embolization, as it provides accurate pre-procedural detection of the bleeding site/vessel. Without pre-procedural CTA guidance, empiric embolization is based only on clinical signs and endoscopy findings, which in many cases can be misleading regarding the site of bleeding. Despite the 100% rate of hemostasis achieved, the 30-day survival rate was approximately 80%, as 9 patients died during their stay in hospital. This is probably attributable to the characteristics of the specific study population, which included high-surgical-risk patients, with hemodynamic compromise from acute blood loss and various severe comorbidities including metastatic cancer, recent trauma and coagulation disorders. Such patients have an increased risk of death following emergency surgery. None of the deaths was directly related to the procedure. In one case, bowel ischemia was diagnosed 2 days following GDA coil embolization for a bleeding duodenal ulcer, but there was no apparent correlation between the coil embolization of the GDA and the extensive ischemic colitis, especially as branches of superior mesenteric artery were not embolized and the superior and inferior mesenteric arteries were patent on the final DSA. Despite the fact that CTA is not the gold standard for the diagnosis of non-occlusive mesenteric ischemia (NOMI), clinical, laboratory and CTA findings (patent large vessels diminished diameter of peripheral arteries and irreversible signs of large bowel ischemia) led to the diagnosis of NOMI. Arteriography of the mesenteric artery and vasodilator therapy was not performed as the patient underwent emergency laparotomy for the inspection of bowel condition and died the day after. NOMI was attributed to general hypoperfusion aggravated by inotropic drug therapy required to treat hemodynamic instability. We believe that while technical success depends mainly on the experience of the performing physician, survival is governed by several factors in addition to age and co morbidities, which include the quality of the clinical and technical support available to the patient before and after the procedure.
The results achieved in this study are comparable to the best in previously published series. All previous series that reported on more than ten patients demonstrated 91–100% technical success rates and 30-day mortality rates ranging from 4 to 46% [8, 9, 12,13,14,15,16]. In this multicenter study, technical success was 100% and the 30-day mortality rate was 20.5%. The 4.5% re-bleeding rate was near the lower end of previously reported rates, which ranged from zero to 56% [16].
Endovascular therapy is recommended by the ESGE guidelines as a valid method of treatment following failed endoscopic therapy of primary or recurrent hemorrhage. However, the choice between TAE and surgical treatment is not clear, and decisions are taken on a case-by-case basis. Data suggesting that TAE is preferable to surgery are mounting [12]. A total of 451 patients who underwent TAE have been reported in 14 single-center, retrospective studies within a 20-year period (1992–2012), while the largest series reported the results on 97 patients [6, 12, 16]. Laffroy has suggested that “surgery is dead,” in an attempt to emphasize the undisputed utility of TAE, in cases refractory to endoscopic hemostasis [15]. However, perforation remains an absolute indication for surgery.
The main limitation of the current study is the retrospective, single-arm design and the limited number of patients. Some cases may have been missed, while the lack of a surgical control arm did not allow comparison of treatment options. Ideally, a prospective randomized trial comparing TAE with surgery would help to determine the optimal treatment of patients after failed endoscopic therapy. However, such trial would be difficult to organize as the number of PUB cases requiring TAE is low, and the number of patients eligible for both surgery and TAE is even lower. This is the first multicenter study reporting real-life outcomes from a large series of patients with PUB treated with TAE and indicating the reproducibility of these outcomes, as procedures were performed in five centers in the UK, Italy and Greece, by more than ten different interventional radiologists.
In summary, high clinical success and low complication rates were achieved using TAE for the treatment of non-variceal, upper gastrointestinal bleeding from peptic ulcers not responding to endoscopic hemostasis. Minimal re-bleeding rates further highlight the utility of TAE as the second line treatment of choice, after failed endoscopy.
References
Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359(9):928–37.
Lee CW, Sarosi GA Jr. Emergency ulcer surgery. Surg Clin North Am. 2011;91(5):1001–13.
Abe N, Takeuchi H, Yanagida O, Sugiyama M, Atomi Y. Surgical indications and procedures for bleeding peptic ulcer. Dig Endosc. 2010;22(Suppl 1):S35–7.
Kyaw M, Tse Y, Ang D, Ang TL, Lau J. Embolization versus surgery for peptic ulcer bleeding after failed endoscopic hemostasis: a meta-analysis. Endosc Int Open. 2014;2(1):E6–14.
Wang YR, Richter JE, Dempsey DT. Trends and outcomes of hospitalizations for peptic ulcer disease in the United States, 1993–2006. Ann Surg. 2010;251(1):51–8.
Sverdén E, Mattsson F, Lindström D, Sondén A, Lu Y, Lagergren J. Transcatheter arterial embolization compared with surgery for uncontrolled peptic ulcer bleeding: a population-based cohort study. Ann Surg. 2017. https://doi.org/10.1097/sla.0000000000002565.
Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47(10):a1–46.
Wong TCF, Wong TT, Chiu PWY, et al. A comparison of angiographic embolization with surgery after failed endoscopic hemostasis to bleeding peptic ulcers. Gastrointest Endosc. 2011;73:900–8.
Beggs AD, Dilworth MP, Powell SL, et al. A systematic review of transarterial embolization versus emergency surgery in treatment of major nonvariceal upper gastrointestinal bleeding. Clin Exp Gastroenterol. 2014;7:93–104.
Loggers SAI, Koedam TWA, Giannakopoulos GF, Vandewalle E, Erwteman M, Zuidema WP. Definition of hemodynamic stability in blunt trauma patients: a systematic review and assessment amongst Dutch trauma team members. Eur J Trauma Emerg Surg. 2017;43(6):823–33.
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Interv Radiol. 2017;40(8):1141–6.
Ichiro I, Shushi H, Akihiko I, Yasuhiko I, Yasuyuki Y. Empiric transcatheter arterial embolization for massive bleeding from duodenal ulcers: efficacy and complications. J Vasc Interv Radiol. 2011;22(7):911–6.
Laffroy Lin M, Thompson C, Harsha A, Rao P. A comparison of the results of arterial embolization for bleeding and non-bleeding gastroduodenal ulcers. Acta Radiol. 2011;152(10):1076–82.
Wang YL, Cheng YS, Liu LZ, He ZH, Ding KH. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18:4765–70.
Loffroy R. Management of duodenal ulcer bleeding resistant to endoscopy: surgery is dead! World J Gastroenterol. 2013;19(7):1150–1.
Loffroy R, Guiu B. Role of transcatheter arterial embolization for massive bleeding from gastroduodenal ulcers. World J Gastroenterol. 2009;15:5889–97.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Procedural informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Spiliopoulos, S., Inchingolo, R., Lucatelli, P. et al. Transcatheter Arterial Embolization for Bleeding Peptic Ulcers: A Multicenter Study. Cardiovasc Intervent Radiol 41, 1333–1339 (2018). https://doi.org/10.1007/s00270-018-1966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-1966-4