Abstract
Aim
Primary hypothyroidism is one of the late complications that can occur after radiation therapy for malignant tumors in the head and neck region. The aim of this retrospective study was to show the validity of the Lyman–Kutcher–Burman (LKB) normal tissue complication model for thyroid gland based on clinical results.
Methods
Thyroid function was evaluated by measuring thyroid-stimulating hormone and free thyroxine serum levels before radiation therapy, 3 months after the beginning of radiation therapy, and afterwards at each follow-up visit. Cumulative incidence was calculated using the Kaplan–Meier method. Dose–volume histogram, total dose, fractionation schedule, total duration of the treatment, and other parameters were used for normal tissue complication probability calculation based on the LKB model. The model was evaluated after fitting with the three sets of parameters for grade 2 hypothyroidism: 1) “Emami,” where n = 0.22; m = 0.26, and D50 = 80 Gy; 2) “mean dose,” where n = 1; m = 0.27, and D50 = 60 Gy; and 3) “Lyman EUD,” where n = 0.49; m = 0.24, and D50 = 60 Gy. A value 3.0 Gy was used for α/β ratio
Results
Eighty-three patients treated with volumetric modulated arc therapy for head and neck cancers at the University Hospital Martin, Slovakia, from January 2014 to July 2017, were included in the retrospective study. Median follow-up was 1.2 years. Cumulative incidence of hypothyroidism grade 2 or higher after 12 and 24 months was 9.6 and 22.0%, respectively. Normal tissue complication probability values calculated with mean dose and Lyman EUD parameters showed the best correlation with our clinical findings.
Conclusion
Empirically based modelling of normal tissue complication probability was valid for our cohort of patients. With carefully chosen parameters, the LKB model can be used for predicting the normal tissue complication probability value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer (HNC) patients represent approximately 6% of all cancer cases worldwide [1]. After radiotherapy, these patients can develop serious acute and late undesired effects, which reduce patients’ quality of life. Among these, the best well-known are xerostomia, dysphagia, or dysgeusia. Also, primary hypothyroidism is one of the adverse effects that may result from treatment of patients with HNC. This medical condition can be diagnosed by laboratory tests for thyroid-stimulating hormone (TSH) and free thyroxin (fT4) levels in serum. Clinical hypothyroidism, defined as a reduction in fT4 with an increase in TSH, manifests as tiredness, cold intolerance, dryness of the skin, weight gain, cognitive dysfunction, constipation, hoarseness, edema, reduced hearing, myalgia and paresthesia, depression, menorrhagia, and arthralgia. Subclinical hypothyroidism is characterized by elevated TSH with the presence of normal fT4 and is generally asymptomatic, although it may present with clinical symptoms such as hypercholesterolemia and accelerated atherosclerosis. Hypothyroidism is treated by replacement of thyroxine [2,3,4].
A systematic review of the literature on radiation-induced hypothyroidism revealed that various studies showed different incidences of hypothyroidism after irradiation of the thyroid gland, and these varied from 23 to 53% for subclinical hypothyroidism and from 11 to 33% for clinical hypothyroidism in individual studies [5]. The median follow-up period in these studies was 2.4–6.1 years.
Concerning tolerance doses, the VGray value (the volume receiving a given dose in Gy) that would predict hypothyroidism in HNC patients after radiotherapy was found to be V40 > 85% [6].
In recent years, biologically based models have been developed to translate the dose–volume information into a single value that estimates the probability of biologic response, i.e., tumor control probability (TCP) or normal tissue complication probability (NTCP) [7]. The dose–volume histogram (DVH), corresponding to the percentage of organ volume covered by a specific dose, and the parameters of the model are needed for the calculation. The models may be used to supplement the dosimetric quantities in treatment planning [8]. According to ICRU 83 guidelines, DVHs are mandatory in reporting intensity-modulated radiation therapy (IMRT) treatment plans, while NTCP and equivalent uniform dose (EUD) are included in level 3 reporting (i.e., still investigative) [9]. TCP/NTCP values are usually used to compare several plans and, as such, to assess plan quality. At present, their absolute values are not used clinically to predict the outcome [10].
In the present study, the empirical Lyman–Kutcher–Burman (LKB) model [11, 12] was used, which is the most frequently used NTCP model. For calculation of NTCP, this model uses the DVH from the treatment plan. The model has several parameters which can be obtained by fitting the patients’ data. The set of parameters for clinical hypothyroidism as an endpoint was first published by Burman, based on Emami’s data: a volume parameter n = 0.22; a steepness parameter m = 0.26, and D50 = 80 Gy [13]. Bakhshandeh [14], whose study was aimed at a patient cohort irradiated by 3D conformal radiation therapy, published the following values for the parameters: n = 0.49; m = 0.24, and D50 = 60 Gy, which was named by these authors “Lyman EUD.” The simplified “mean dose” model with n = 1; m = 0.27, and D50 = 60 Gy was also proposed in the same study.
The aim of this retrospective study was to evaluate the cumulative incidence of hypothyroidism after radiotherapy in the studied group of patients. NTCP values based on the LKB model were calculated for individual patients and these values were compared with clinical findings. To the best of our knowledge, no publication has yet confirmed the LKB model parameters for hypothyroidism in patients treated by volumetric modulated arc therapy (VMAT).
Methods and materials
Patients
A total of 245 HNC patients were treated at the University Hospital Martin, Slovakia, from January 2014 to July 2017. Patients with both primary and postoperative radiotherapy to the head and neck region were included in the study. The patients who had known thyroid disease before treatment (7 patients), previous radiotherapy to the head and neck region, or early termination of treatment (57 patients) were excluded from the analysis. An additional group of 98 patients was excluded because they had no TSH and/or fT4 measurements during follow-up. These were mostly patients residing outside the Martin district who were followed-up by their referring physicians. Finally, 83 patients were included in the retrospective study. The cut-off date for analysis of data was November 30, 2017.
Assessment of thyroid gland function
The function of the thyroid gland was evaluated based on laboratory values of TSH and fT4 levels in serum before the beginning of radiotherapy, 3 months after the beginning of radiotherapy, and at each follow-up visit. All patients treated for HNC take part in a follow-up program which includes a visit to the otorhinolaryngologist every 3–4 months for the first 2 years, then every 6 months until 5 years after treatment, and after 5 years, once a year.
Thyroid gland hormones in serum samples were evaluated with the UniCel DxI 800 immunoassay system (Beckman Coulter, Brea, CA, USA). The normal range defined by the laboratory was 0.380–5.330 mlU/l for TSH (until June 30, 2017, the normal TSH range was 0.340–3.600 mlU/l) and 7.86–14.41 pmol/l for fT4.
Thyroid toxicity assessments were performed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 [15]. Thyroid gland late toxicity was defined as an adverse event first occurring 90 days or more after initiation of radiation therapy [16]. According to CTCAE, thyroid toxicity grade 1 is defined as subclinical hypothyroidism with normal fT4 and high serum levels of TSH. Toxicity grade 2 is defined as symptoms requiring thyroid replacement and is characterized as clinical hypothyroidism by low fT4 and high TSH levels. Toxicity grade 3 is characterized by severe symptoms, limiting self-care activities of daily living, requiring hospitalization.
Treatment
Patients were treated using VMAT radiotherapy, with or without concurrent chemotherapy. Planning computer tomography (CT) scans were acquired using a 128-slice CT scanner (Ingenuity; Philips Healthcare, Best, the Netherlands) in a supine position maintained with thermoplastic masks, with a slice thickness of 3 mm. If not contraindicated, a contrast-enhanced CT scan was performed. Structure delineation and treatment planning were performed on the Eclipse treatment planning system, and the treatment was administered using 6‑MV photons on a Clinac iX linear accelerator (both Varian Medical System, Palo Alto, CA, USA) with a dynamic multileaf collimator consisting of 60 pairs of leaves with 0.5 and 1.0 cm width projection at the isocenter. Patients were treated with a simultaneous integrated boost, which allowed treatment of multiple planning target volumes (PTVs) in a single plan. The total dose was 60 Gy, 66 Gy, or 69.96 Gy in 30–33 fractions, with 2 or 2.12 Gy per fraction, 5 days a week. Elective lymph nodes were generally treated with a dose of 59.4 Gy (ipsilateral lymph nodes, intermediate-risk PTV) and 54.12 Gy (contralateral lymph nodes, low-risk PTV). Planning objectives were used to decrease the dose to thyroid without compromising PTV coverage. Treatment was delivered as image-guided radiotherapy, which comprised two orthogonal kilovoltage images before every treatment session. In all patients, an adaptive radiotherapy approach was employed, which meant that after 19–21 fractions of radiotherapy, a new CT scan was obtained and patients were re-planned in order to deliver the dose to the PTV as planned and to spare organs at risk. From the DVH, the V40, V45, V50, and V60 doses to the thyroid gland were calculated for the analysis.
Endpoints
The primary endpoint of this study was the incidence of clinical hypothyroidism, i.e., CTCAE grade 2 or higher toxicity occurring 12 and 24 months from the beginning of radiation therapy. The secondary endpoint was cumulative incidence of hypothyroidism grade 1 or higher during the first 2 years from the beginning of radiation therapy. Patients without hypothyroidism were censored at the date of last follow-up. The Kaplan–Meier method was employed to calculate the cumulative incidence at a given time from the beginning of radiation therapy. Median follow-up was calculated using the reverse Kaplan–Meier method. Clinical risk factors were evaluated using the log-rank (Mantel–Cox) test. Statistical software SPSS [17] was used for the survival analysis (IMB Corp., Armonk, NY, USA).
Normal tissue complication probability
Normal tissue complication probability calculation was based on a linear-quadratic model using concept of biological effective dose (BED) [18, 19] and an empiric model introduced by Lyman for modelling complication probability in a uniformly irradiated organ [11, 20, 21], which is a function of dose (D) and volume (ν):
D is the physical total dose to a uniformly irradiated fractional reference volume (the total volume of the organ of interest, Vref), ν is the ratio of the irradiated volume and a reference volume Vref, D50(ν) is the dose at which probability of complication becomes 50% in 5 years for uniform partial-organ irradiation, and m is the tissue-specific parameter inversely proportional to the slope of the response curve. Tolerance dose–volume dependence is characterized by the following relationship:
D50 is the tolerance dose for 50% complications for uniform whole-organ irradiation, n is the parameter to find the EUD in inhomogeneous irradiation using the DVH reduction method proposed by Kutcher and Burman [12, 13], and \(0<n<1\). The parameters used in the calculation of thyroid gland NTCP grade 2 and higher are shown in Table 1.
Calculation of NTCP based on the LKB model assumes a dose per fraction of approximately 2 Gy. Instead of physical total dose, BED was used for calculation in order to account for a different dose per fraction and duration of the radiotherapy course. A value of 3.0 Gy was used for α/β ratio of the thyroid gland. BED was calculated by the following formula, which includes reparation and repopulation [22]:
df is dose per fraction, α/β is a tissue-specific parameter of the organ according to the linear-quadratic model, T is the total duration of the treatment in days, Tk is the time of onset of repopulation from the start of radiotherapy, K is a repopulation factor expressed by K = ln2/(α/Tpot), where α is a specific constant describing the cell’s radiosensitivity and Tpot is a potential doubling time. From fitted parameters, for thyroid gland: K = 0.3, Tk = 49 days [23].
NTCP values were calculated using BioGray software (Košice, Slovakia) [24]. Correlation of calculated values with clinical results of a group of HNC patients was calculated with the corrplot package of R software [25].
Results
The study group consisted of 83 patients. The summary of patient characteristics is shown in Table 2.
Median follow-up was 1.2 years as calculated by the reverse Kaplan–Meier method (range 98–1205 days). 43 patients had a follow-up longer than 12 months and 12 out of these patients had a follow-up longer than 24 months. The number of patients with complications is summarized in Table 3. Toxicities grade 2 (clinical hypothyroidism) occurred in 10 (12.0%) patients. No patients had grade 3 thyroid complications.
The average mean radiation dose to the thyroid gland calculated from DVH was 52.8 Gy (standard deviation ±11.4 Gy, range 5.8–69.6 Gy). Three patients with nasopharyngeal tumors were included in the study. These patients received a radiation dose to the pituitary gland. The mean radiation doses to the pituitary gland in these patients were 12.8 Gy, 17.7 Gy, and 59.8 Gy, but none of them developed pituitary hypothyroidism (these patients did not have decreased TSH levels).
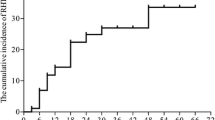
Clinical endpoint of this study was hypothyroidism grade 2 or higher according to CTCAE (clinical hypothyroidism). The cumulative incidence of clinical hypothyroidism after 12 and 24 months as assessed by Kaplan–Meier time-to-event analysis was 9.6% and 22.0%, respectively. The graphical representation of the analysis is shown in Fig. 1.
The cumulative incidence of hypothyroidism grade 1 or higher (either clinical or subclinical) after 12 and 24 months was 14.1% and 50.3%, respectively. The analysis of clinical risk factors is presented in Table 4. Baseline thyroid volume <12.9 cm3 and V40 > 85% were shown to be statistically significant for development of hypothyroidism grade 1 or higher. Concurrent chemotherapy and prior head and neck surgery did not show a statistically significant influence on the development of hypothyroidism.
The BioGray software allows for modification of model parameters. In the empirical LKB model, three parameters were optimized: D50 (BED50), n, and m. Parameters for grade 2 hypothyroidism were optimized as shown in Table 1.
Fig. 2 shows the sigmoid curve of NTCP vs. dose, which was calculated for an individual patient from the LKB model. In the particular case, the probability of complications is highest when using “mean dose” parameters of the model and lowest with “Emami” parameters. The results are shown in Fig. 3, combining a correlation matrix with the significance test, at the confidence level 0.95. Correlations with p-value > 0.05 were considered as insignificant, in which case the crosses were added to correlation coefficient values. All the displayed correlations were positive. NTCP values calculated with “mean dose” and “Lyman EUD” parameters showed significant correlation with clinical findings in individual patients. The correlation matrix shows that V40, V45, V50, and V60 values did not correlate with clinical findings.
Example of BioGray user interface: Sigmoid NTCP curves were calculated by BioGray software for an individual patient [24]. In this example, NTCP was calculated with parameters of Emami (a curve with squares), mean dose (triangles) and Lyman EUD (dots)
Correlation matrix with the significance test plotted using the corrplot R package [25]. Positive correlations are displayed in blue circles. If a correlation was negative, it would be displayed in a red circle. Color intensity and the size of the circle are proportional to the correlation coefficients. Values of correlation coefficients are shown in the color intensity bar. Crosses are added to correlations which are considered insignificant on confidence level 0.95. HT hypothyroidism, v40, v45, v50 and v60 the volume of thyroid gland receiving 40, 45, 50 and 60 Gy, respectively, ntcp1, ntcp2, ntcp3 NTCP calculated with Emami, mean dose and Lyman EUD parameters, respectively
Discussion
This study was aimed at proving the validity of the LKB NTCP model for radiation-induced hypothyroidism grade 2. It was the first study to use the model on a group of patients irradiated with highly conformal VMAT radiotherapy. Our parameters for the LKB model are: BED50 = 100 Gy, n = 0.43, and m = 0.24 (Lyman EUD), and BED50 = 100, n = 1, and m = 0.27 (mean dose). The parameters are consistent with the [14] best-fit parameters of the model for patients treated by 3D conformal radiation therapy.
The cumulative incidence of clinical hypothyroidism 1 year and 2 years after radiotherapy was 9.6% and 22.0%, respectively. It should be pointed out that patients with either clinical or subclinical hypothyroidism were referred to the endocrinologist. This may have underestimated the number of patients who would otherwise have progressed from subclinical to clinical hypothyroidism.
The current study showed that the cumulative incidence of any-grade hypothyroidism (subclinical or clinical) after 12 and 24 months was 14.1% and 50.3%, respectively. This result was somewhat different to the reported incidence rate of subclinical or clinical hypothyroidism 1 year and 2 years after radiotherapy in the head and neck region delivered by the IMRT method, which was 33% and 44.6%, respectively [6].
Tolerance limits of VGy values for thyroid gland were summarized in the publication of Emami [26]. The dose–volume relationship for thyroid gland was not included in QUANTEC (Quantitative Analyses of Normal Tissue Effects in the Clinic) [27]. NTCP values from Emami’s data do not correlate with clinical findings, because fitting of parameters n and m was based on insufficient data [26]. Several authors employed models other than LKB to calculate NTCP values for hypothyroidism. The NTCP model of Boomsma et al. [28] was composed of the mean thyroid dose and the thyroid gland volume as two prognostic variables. The probability of hypothyroidism rose with higher mean thyroid dose and it decreased with higher thyroid gland volume. Sommat et al. [6] worked with data of nasopharyngeal carcinoma patients who were treated by IMRT and were given 69.96 Gy in 33 fractions. The results showed that thyroid V40 > 85% (percentage of thyroid volume receiving more than 40 Gy not exceeding 85%) significantly increased risk of hypothyroidism in these patients. V50 predicts the risk of developing hypothyroidism, as suggested based on previous findings. The recommended dose constraint is V50 < 60% [29].
The objective of model-based calculation is to replace the currently used evaluation of radiotherapy plans based on discrete values from dose–volume histograms by a TCP/NTCP probability value. Currently, most TCP/NTCP models, including the LKB model, are based on dose–volume histograms. Because of this, one of their limitations is a loss of spatial information. There have been attempts to include spatial information in NTCP models for different organs at risk [30], which is beyond the scope of this work. Because dose–volume histograms and NTCP models do not take functional and structural heterogeneities into account, these should be used with care [31]. According to ICRU Report 83, biologic assumptions, parameters used in the models, and the models themselves must be unambiguously specified when reporting and should be used clinically only once their relevancy has been established for well-defined clinical conditions [9].
In the current group of studied patients, the NTCP model of thyroid gland provided valid predictions despite the fact that the dose delivery method was different compared to [14], i.e., VMAT vs. 3D conformal radiation therapy. The results also show that the thyroid gland can be described as tissue composed of functional units arranged in parallel architecture, which is shown by an n value close to 1 [32].
Limitations
This study has several limitations. The study was retrospective. Patients had a short median follow-up 1.2 years, whereas according to published studies, the median time to the onset of hypothyroidism after radiation therapy is 1.4–1.8 years [5]. Because of a low number of patients, it was not possible to perform a thorough analysis of clinical risk factors which were found to influence the risk of radiation-induced hypothyroidism [33].
Conclusion
VMAT is an advanced form of intensity-modulated radiotherapy that delivers a precisely conformal 3D dose distribution during gantry rotation in a single or multiple arcs. This method allows highly conformal delivery of radiotherapy, which, with a properly defined target and when used together with image-guided radiotherapy and adaptive radiotherapy approaches, can improve treatment outcomes of cancer patients, i.e., reduce toxicity and potentially improve loco-regional control by increasing the target dose [34].
Radiobiological models based on a linear-quadratic approach can be used effectively for calculating NTCP values of thyroid gland complications after VMAT radiotherapy, provided the referenced α/β, D50 (or BED50 in the case that biological effective dose is used instead of physical dose), n, and m values are used.
We suggest that assessment of thyroid hormones should be a routine part of follow-up examinations in patients after radiotherapy in the head and neck region. The reason is that clinical symptoms of hypothyroidism often arise several months after radiotherapy. If a patient is not examined for hypothyroidism, the clinical symptoms of hypothyroidism may be misinterpreted as general cancer-specific symptoms which are reported by patients after highly conformal radiotherapy [35].
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Zulewski H, Muller B, Exer P, Miserez AR, Staub JJ (1997) Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 82:771–776. https://doi.org/10.1210/jcem.82.3.3810
Jereczek-Fossa BA, Alterio D, Jassem J et al (2004) Radiotherapy induced treatment disorders. Cancer Treat Rev 30:369–384. https://doi.org/10.1016/j.ctrv.2003.12.003
Miller M, Agrawal A (2009) Hypothyroidism in post radiation head and neck cancer patients: Incidence, complications and management. Curr Opin Otolaryngol Head Neck Surg 2009(17):111–115. https://doi.org/10.1097/MOO.0b013e328325a538
Boomsma MJ, Bijl HP, Langendijk JA (2011) Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol 99(1):1–5. https://doi.org/10.1016/j.radonc.2011.03.002
Sommat K, Ong WS, Hussain A, Soong YL, Tan T, Wee J, Fong KW (2017) Thyroid V40 predicts primary hypothyroidism following intensity modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 98(3):574–580. https://doi.org/10.1016/j.ijrobp.2017.03.007
Marks LB, Ten Haken RK, Martel MK (2010) Guest editor’s introduction to QUANTEC: a users guide. Int J Radiat Oncol Biol Phys 76(3 Suppl):S1–S2. https://doi.org/10.1016/j.ijrobp.2009.08.075
Luxton G, Hancock SL, Boyer AL (2004) Dosimetry and radiobiologic model comparison of IMRT and 3D conformal RT in treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys 59(1):267–284. https://doi.org/10.1016/j.ijrobp.2004.01.024
ICRU (International Commission on Radiation Units and Measurements) (2010) Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). ICRU Report 83. J ICRU. https://doi.org/10.1093/jicru/ndq002
Halperin EC, Wazer DE, Perez CA, Brady LW (eds) (2018) Perez and Brady’s principles and practice of radiation oncology, 7th edn. Wolters Kluwer, Philadelphia, p 809 (eISBN: 9781496386823)
Lyman JT (1985) Complication probability as assessed from dose-volume histograms. Radiat Res 104(Suppl. 8):S13–S19. https://doi.org/10.2307/3576626
Kutcher GJ, Burman C (1989) Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys 16(6):1623–1630. https://doi.org/10.1016/0360-3016(89)90972-3
Burman C, Kutcher GJ, Emami B, Goitein M (1991) Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 21(1):123–135. https://doi.org/10.1016/0360-3016(91)90172-Z
Bakhshandeh M, Hashemi B, Mahdavi SRM, Nikoofar A, Vasheghani M, Kazemnejad A (2013) Normal tissue complication probability modeling of radiation-induced hypothyroidism after head-and-neck radiation therapy. Int J Radiat Oncol Biol Phys 85(2):514–521. https://doi.org/10.1016/j.ijrobp.2012.03.034
National Cancer Institute Common terminology criteria for adverse events v5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed 15 Jan 2018
National Cancer Institute Common toxicity criteria manual. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcmanual_v4_10-4-99.pdf. Accessed 15 Jan 2018
IBM Corp (2015) IBM SPSS statistics for Windows, version 23.0. IBM Corp, Armonk
Barendsen GW (1982) Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys 8(11):1981–1997. https://doi.org/10.1016/0360-3016(82)90459-x
Fowler JF (1983) Dose response curves for organ function or cell survival. Br J Radiol 56(667):497–500. https://doi.org/10.1259/0007-1285-56-667-497
Lyman JT, Wolbarst AB (1987) Optimization of radiation therapy. III. A method of assessing complication probabilities from dose-volume histograms. Int J Radiat Oncol Biol Phys 8(7):90266–90265. https://doi.org/10.1016/0360-3016
Lyman JT, Wolbarst AB (1989) Optimization of radiation therapy. IV. A dose-volume histogram reduction algorithms. Int J Radiat Oncol Biol Phys 17(2):433–436. https://doi.org/10.1016/0360-3016(89)90462-8
Fowler JF, Harari PM, Leborgne F, Leborgne JH (2003) Acute radiation reactions in oral and pharyngeal mucosa: tolerable levels in altered fractionation schedules. Radiother Oncol 69(2):161–168. https://doi.org/10.1016/S0167-8140(03)00231-7
Matula P, Koncik J (2018) Key to radiobiological modelling effects in radiation oncology. LAP LAMBERT Academic, Beau Basin, p 15
BioGray http://www.q4space.netkosice.sk/. Accessed 15 Jan 2019
Wei T, Simko V (2017) R package “corrplot”: visualization of a correlation matrix (version 0.84). https://github.com/taiyun/corrplot. Accessed 15 Jan 2019
Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21(1):109–122. https://doi.org/10.1016/0360-3016(91)90171-Y
Bentzen SM, Constine LS, Deasy JO et al (2010) Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 76(3, Suppl 1):S3–S9. https://doi.org/10.1016/j.ijrobp.2009.09.040
Boomsma MJ, Bijl HP, Christianen ME, Beetz I, Chouvalova O, Steenbakkers RJ, van der Laan BF, Wolffenbuttel BH, Oosting SF, Schilstra C, Langendijk JA (2012) A prospective cohort study on radiation-induced hypothyroidism: development of an NTCP model. Int J Radiat Oncol Biol Phys 84(3):e351–e356. https://doi.org/10.1016/j.ijrobp.2012.05.020
Sachdev S, Refaat T, Bacchus ID, Sathiaseelan V, Mittal BB (2014) Thyroid V50 predicts hypothyroidism in head and neck cancer patients treated with IMRT. Int J Radiat Oncol Biol Phys 90(1):S549. https://doi.org/10.1016/j.ijrobp.2014.05.1666
Dean JA, Wong KH, Welsh LC, Jones AJ, Schick U, Newbold KL, Bhide SA, Harrington KJ, Nutting CM, Gulliford S (2016) Normal tissue complication probability (NTCP) modelling using spatial dose metrics and machine learning methods for severe acute oral mucositis resulting from head and neck radiotherapy. Radiother Oncol 120(1):21–27. https://doi.org/10.1016/j.radonc.2016.05.015
Marks LB (1996) The impact of organ structure on radiation response. Int J Radiat Oncol Biol Phys 34:1165–1171. https://doi.org/10.1016/0360-3016(95)02186-8
Marks LB, Yorke ED, Jackson A et al (2010) Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 76(3 Suppl):S10–S19. https://doi.org/10.1016/j.ijrobp.2009.07.1754
Vogelius IR, Bentzen SM, Maraldo MV, Petersen PM, Specht L (2011) Risk factors for radiation-induced hypothyroidism: a literature-based meta-analysis. Cancer 117(23):5250–5260. https://doi.org/10.1002/cncr.26186
Feng M, Eisbruch A (2007) Future issues in highly conformal radiotherapy for head and neck cancer. J Clin Oncol 25(8):1009–1013. https://doi.org/10.1200/JCO.2006.10.4638
Tribius S, Meyer MS, Pflug C et al (2018) Socioeconomic status and quality of life in patients with locally advanced head and neck cancer. Strahlenther Onkol 194:737–749. https://doi.org/10.1007/s00066-018-1305-3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I. Kinclová, E. Hajtmanová, P. Matula, S. Balentová, P. Muríň, M. Ďuroška, and K. Kozlíková declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kinclová, I., Hajtmanová, E., Matula, P. et al. Model-based calculation of thyroid gland normal tissue complication probability in head and neck cancer patients after radiation therapy. Strahlenther Onkol 196, 561–568 (2020). https://doi.org/10.1007/s00066-020-01579-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01579-y