Abstract
Objective
Previous studies demonstrated that prophylactic cranial irradiation (PCI) significantly reduced the incidence of brain metastases in patients with extensive disease small cell lung cancer (ED-SCLC). However, the appropriate timing for PCI in treating ED-SCLC is still unclear. This study aimed to compare the effect and safety of early versus late PCI.
Methods
Between November 2011 and July 2016, 103 patients with ED-SCLC were reviewed, receiving appropriate imaging tests to exclude brain metastases prior to cranial irradiation. Of these 103 patients, early PCI was performed in 47 patients and the other 56 patients received late PCI. The primary endpoint was the incidence of brain metastases. The progression-free survival (PFS), overall survival (OS), and adverse events (AEs) were also assessed.
Results
Early PCI significantly lowered the risk of brain metastases, as compared to late PCI (p = 0.024). Additionally, multivariate analyses demonstrated that early PCI was a favorable independent predictor of the incidence of brain metastases. The PFS and OS of patients in the early and late PCI groups were comparable (PFS: 8.4 months vs. 7.5 months, p = 0.234; OS: 16.1 months vs. 15.2 months, p = 0.753). The AEs were generally acceptable in both groups.
Conclusion
To reduce the incidence of brain metastases, early PCI is more effective than late PCI for ED-SCLC patients.
Zusammenfassung
Zusammenfassung
In früheren Studien wurde gezeigt, dass eine prophylaktische Ganzhirnbestrahlung („prophylactic cranial irradiation“, PCI) die Häufigkeit zerebraler Metastasen im Gehirn bei Patienten mit metastasiertem kleinzelligem Lungenkarzinom („extensive disease small cell lung cancer“, ED-SCLC) signifikant reduzieren kann. Der richtige Zeitpunkt einer PCI bei ED-SCLC ist unklar. Ziel dieser Studie ist es, die Wirkung und Sicherheit von früher vs. später PCI zu vergleichen.
Methode
Zwischen November 2011 und Juli 2016 wurden 103 Patienten mit ED-SCLC untersucht. Mittels schnittbildgebender Untersuchung wurden Hirnmetastasen vor der Ganzhirnbestrahlung ausgeschlossen. Bei 47 Patienten wurde eine frühe PCI durchgeführt, bei den anderen 56 Patienten eine späte PCI. Primärer Endpunkt war das Auftreten von Hirnmetastasen. Das progressionsfreie Überleben („progression-free survival“, PFS), Gesamtüberleben („overall survival“, OS) und unerwünschte Ereignisse („adverse events“, AE) wurden ebenfalls ausgewertet.
Ergebnisse
Eine frühe PCI senkt das Risiko für Hirnmetastasen im Vergleich zur späten PCI (p = 0,024). Zusätzlich zeigen multivariate Analysen, dass eine frühe PCI ein unabhängiger Prädiktor für das seltenere Auftreten von zerebralen Filiae war. PFS und OS waren bei Patienten mit früher und später PCI vergleichbar (PFS: 8,4 vs. 7,5 Monate; p = 0,234; OS: 16,1 vs. 15,2 Monate; p = 0,753). Die AE waren in beiden Gruppen tolerabel.
Schlussfolgerung
Um die Auftretenswahrscheinlichkeit von Metastasen im Gehirn zu reduzieren, ist eine frühe PCI effektiver als eine späte PCI bei Patienten mit ED-SCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, lung cancer has become the leading killer of cancer patients [1]. Small cell lung cancer (SCLC), the most aggressive subtype, accounts for approximately 14% of all newly diagnosed primary lung cancers and about 60% of patients present with extensive disease small cell lung cancer (ED-SCLC) at diagnosis because of its high growth fraction, rapid doubling time, and early wide dissemination [2,3,4,5]. SCLC is sensitive to cytotoxic agents and radiation therapy. Therefore, the combination of chemotherapy and thoracic radiotherapy is able to improve the response rate and prolong the short survival of SCLC patients [3, 5,6,7,8,9,10], but their long-term survival is still unfavorable [2]. One of the reasons is the frequent incidence of brain metastases, which are present in at least 18% of patients at diagnosis [11], with the incidence increasing to approximately 58% within 2 years [12]. Unfortunately, most patients do not survive after a diagnosis of brain metastasis [13].
Previous studies have demonstrated that prophylactic cranial irradiation (PCI) can reduce the incidence of brain metastases in patients with ED-SCLC [12, 14,15,16,17,18,19]. However, the appropriate timing for PCI in treating ED-SCLC is still unclear. Lifting the veil from this puzzle will help doctors choose an appropriate time to administer PCI to their ED-SCLC patients. The purpose of this study was to compare the effects and adverse events (AEs) of early PCI versus late PCI.
Patients and methods
Patients
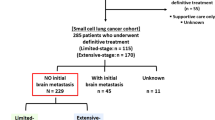
A retrospective study on 103 patients (21 patients were from our previous study [17]) diagnosed with ED-SCLC was initiated and all candidates were treated between November 2011 and July 2016, showing complete or partial response to the initial chemotherapy. Of 103 patients, the absence of brain metastases was either confirmed using cranial magnetic resonance imaging (MRI; 91 patients) or cranial computed tomography (CT; 12 patients). This study was approved by the Ethics Committee of the Chinese PLA General Hospital and all methods were performed in accordance with relevant guidelines and regulations. Written informed consent was provided by each patient before the treatment.
Inclusion and exclusion criteria
The inclusion criteria were: (1) pathologically diagnosed ED-SCLC; (2) Eastern Cooperative Oncology Group Performance Status (ECOG-PS) ≤ 2; (3) age ≥ 18 years; (4) any response to initial chemotherapy; (5) without evidence of brain metastases before PCI; (6) complete follow-up data. The exclusion criteria were: (1) any active concomitant cancer; (2) previous radiotherapy within the head and neck region; (3) any mental disorder or somatic comorbidities of clinical concern.
Performance status, diagnosis, and staging
Performance status was assessed according to ECOG-PS scale. The diagnosis of SCLC was determined by the results of bronchofibroscopy, mediastinoscopy, percutaneous lung biopsy, or lymph node biopsy. The staging of the patients was based on the U.S. Veterans Administration Lung Cancer Group. ED-SCLC referred to disease beyond the ipsilateral hemithorax, including contralateral supraclavicular nodes, malignant pericardial or pleural effusion, and distant hematogenous metastasis. ED-SCLC also included patients with locally advanced disease not encompassed in a reasonable radiation portal. All initial staging investigations included CT, MRI, ultrasonography of abdomen, bone scan, and sometimes positron-emission tomography (PET-CT).
Treatment and treatment response
All involved patients had received four to eight cycles of initial chemotherapy and showed positive responses, either complete response (CR) or partial response (PR), according to the criteria of RECIST (Response Evaluation Criteria In Solid Tumors) version 1.1. Concurrent or sequential thoracic radiotherapy (TRT) was performed with chemotherapy and delivered with an intensity-modulated radiotherapy technique (IMRT) and the prescription dose was 40–70 Gy in 20–35 fractions.
PCI was carried out subsequent to the end of initial chemotherapy or chemoradiotherapy. Early or late PCI were determined by the interval between the start of initial chemotherapy and the initiation of PCI. Early PCI means the interval was less than or equal to 6 months and late PCI means the interval was greater than 6 months. The target volume was the whole brain volume with at least normal tissue sparing of the lens. Each PCI was delivered by a 6-MV photon linear accelerator (Elekta Synergy, Elekta, Sweden), with two opposed lateral portals. Most patients received a total prescribed dose of 25 Gy in 10 fractions.
Follow-up
The selected patients were followed-up every 6 weeks during initial chemotherapy and every 3 months after recovery from primary therapy in our hospital.
Definition of endpoints
The primary endpoint of this study was the incidence of brain metastases. Time to brain metastasis was calculated from the start date of PCI until the date of diagnosis of brain metastasis (Fig. 1). The secondary endpoints were: (1) Progression-free survival (PFS), defined as the interval from the date of diagnosis to the date of intra-cranial and/or extra-cranial progression, or death, or patient censorship at the last follow-up; (2) Overall survival (OS), defined as the interval from the date of diagnosis to the date of death or patient censorship at the last follow-up; and (3) AEs, assessed by National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 [20]. Cognitive function was assessed by mini mental state examination (MMSE) score at baseline and every 3 months after PCI, which was classified as degree 0 (none, score from 27 to 30), degree I (mild, score from 21 to 26), degree II (moderate, score from 10 to 20), degree III (severe, score ≤ 9). The MMSE was chosen as it was commonly used in our clinical practice due to its convenience.
Statistical analysis
The patients’ characteristics were described by continuous variables and/or categorical variables. Continuous variables were compared by t‑test or rank-sum test, and categorical variables were compared by chi-square or Fisher’s exact test. PFS and OS were estimated by the Kaplan–Meier method and analyzed by the log-rank test. Multivariate analyses of incidence of brain metastases were performed by logistic regression analysis and multivariate analyses of PFS and OS were performed by Cox proportional hazard regression model. P < 0.05 was considered as statistically significant. SPSS 21.0 software (IBM; Armonk, NY, USA) was used for statistical analysis.
Results
Patient characteristics
The study reviewed 103 patients including 89 males and 14 females. The median age was 59 years (interquartile range [IQR]: 53–65 years). Early PCI was delivered to 47 patients and 56 received late PCI. The median follow-up period was 12 months (range: 3–36 months). The general characteristics of the cohort stratified by early and late PCI groups are shown in Table 1. The baseline characteristics of the two groups were well balanced.
Patients’ treatment
Of the 47 patients treated with early PCI, 45 patients received 25 Gy in 10 fractions, 1 patient received 30 Gy in 10 fractions, and 1 patient received 22.5 Gy in 9 fractions. For the remaining 56 patients treated with late PCI, 54 received 25 Gy in 10 fractions and 2 patients received 22.5 Gy in 9 fractions. The median time to PCI in the early PCI group was 5 months (4.5 months to 6.0 months) after commencement of chemotherapy and in the late PCI group this was 8 months (6.3 months to 9.1 months). During the period of observation, 10 out of 47 patients receiving early PCI developed brain metastases, of whom 2 (20.0%) were treated with whole brain radiation therapy (WBRT) just after the confirmation of brain metastases by MRI. One patient received 20 Gy in 10 fractions and the other received 22 Gy in 11 fractions. Out of the 56 cases receiving late PCI, 23 patients developed brain metastases, of whom 5 (21.7%) were treated with WBRT. All these 5 patients received 20 Gy in 10 fractions. Additionally, symptom palliation was achieved in 1 of the patients treated with re-irradiation in the early PCI group and in 2 of the patients in the late PCI group.
Incidence of brain metastases and timing of PCI
Fig. 1 shows the cumulative incidence of brain metastases in the early and late PCI groups. The incidence of brain metastases was significantly lower in the early PCI group than in the late PCI group (HR, 0.45; 95% CI, 0.23 to 0.89; p = 0.024). The 1‑year incidence of brain metastases was 22.5% and 45.4% for the early and late PCI groups, respectively.
Progression-free survival
PFS was comparable between the two groups. Median PFS was 8.4 months in the early PCI group versus 7.5 months in the late PCI group (HR, 0.755; 95% CI, 0.474 to 1.232; p = 0.234; Fig. 2).
Overall survival
During the period of follow-up, 27 out of 47 patients died in the early PCI group and 31 out of 56 patients died in the late PCI group. Fig. 3 shows that there was no significant difference in the OS between the early and late PCI groups (16.1 months vs. 15.2 months, p = 0.753). The HR for death was 0.91 (95% CI, 0.54 to 1.55) in the patients receiving early PCI.
Multivariate analyses of incidence of brain metastases
Table 2 shows the results of multivariate analyses, suggesting that timing of PCI and tumor load were significantly associated with the incidence of brain metastases. Patients receiving early PCI and with locally advanced disease had a lower risk of developing brain metastases.
Multivariate analyses of PFS and OS
Multivariate analyses were conducted on PFS and OS. It was found that tumor load was significantly associated with the survival of patients, which indicated that patients with distant metastases had a higher risk of progression and death compared with locally advanced disease. Furthermore, thoracic radiotherapy (TRT) was significantly associated with the PFS, which suggested that TRT was a favorable independent prognostic factor for PFS. However, the OS was not impacted by TRT in this study. Similarly, timing of PCI was not found to be significantly correlated with survival (Table 3).
Adverse events
The early AEs often occurred within 3 months after PCI (Table 4), including headache, anorexia, alopecia, nausea, vomiting, fatigue and lethargy, skin reaction, and leg weakness. The most frequent grade ≥ 3 AEs in the early and late PCI groups were anorexia (4% vs. 2%), nausea (2% vs. 2%), fatigue and lethargy (2% vs. 0%).
Cognitive impairment was assessed in the long-term survivors without brain metastases. During the follow-up period, 5 patients in the early PCI group had a mild cognitive disturbance at 6 months, 9 months, 12 months, 18 months, and 24 months, respectively, after the PCI. Three patients in the late PCI group had mild cognitive dysfunction at 9 months, 15 months, and 18 months, respectively, after the PCI. Additionally, patients aged ≥ 60 years were more likely to develop cognitive impairment in both groups. Out of the 5 patients developing cognitive impairment in the early PCI group, 4 patients (80%) were aged ≥ 60 years. Similarly, all the patients (100%) developing cognitive impairment in late PCI group were aged 60 years old or older.
Discussion
Previous studies and meta-analyses have demonstrated that PCI significantly reduced the incidence of brain metastases and improved the OS in ED-SCLC patients [12, 14,15,16,17,18]. The same results have been confirmed by our previous study [17]. However, the latest phase III randomized trial [19] performed by Takahashi et al. reported that PCI significantly reduced the incidence of brain metastases, but that the OS in the PCI group and the observation group were comparable (p = 0.094). The results regarding the effect of PCI on OS in the Japanese trial were inconsistent with the EORTC trial [18] and our previous study [17], and the following causes may account for this: First, the patients in the Japanese trial were older than those in the EORTC trial and our previous study. Several publications [21, 22] suggested that the efficacy of PCI and therapeutic WBRT on OS in older patients is reduced. Second, subsequent chemotherapies were less frequently used in the PCI group compared with the observation group in the Japanese trial, while the use of second-line or third-line chemotherapies were more frequent in the PCI group in the EORTC trial, and were comparable in both groups in our previous study. These aforementioned differences may have led to an absence of survival benefit in the PCI group in the Japanese trial. It is worth noticing that routine brain imaging after chemotherapy was not performed in the EORTC study, which might partly contribute to the beneficial effect of PCI. However, Slotman et al. did an analysis of the EORTC study excluding the patients with possible asymptomatic brain metastases and the effect of PCI on survival was still statistically significant [23]. Collectively, up to now, current international guidelines [24] still recommend PCI for patients with ED-SCLC who respond to initial chemotherapy. However, limited data exist regarding the timing of PCI in treating ED-SCLC patients. Our study observed that early PCI significantly reduced the incidence of brain metastases compared to late PCI, with a 1-year incidence of brain metastases decreasing from 45.4% down to 22.5%. In addition, multivariate analyses indicated that early PCI was a favorable independent predictor of the incidence of brain metastases in ED-SCLC patients. These favorable results are consistent with previous studies. A meta-analysis conducted by Auperin et al. [15] reported that PCI had a significantly greater effect on the incidence of brain metastasis in patients who received PCI within 6 months following induction therapy compared with patients who received PCI after 6 months (p = 0.01). Suwinski et al. [25, 26] reported that the incidence of brain metastases in patients with SCLC was significantly reduced by PCI performed at the initiation of chemoradiotherapy (p < 0.05). Similar findings were reconfirmed by Sas-Korczynska et al. [27] in a retrospective study involving 86 LD-SCLC patients, in which the incidence of brain metastases in the early PCI group was significantly lower than that in the late PCI group (p = 0.009). In previous publications on this issue, which included patients with LD-SCLC or ED-SCLC, the sample size was quite significant. Nevertheless, the conclusions of previous studies [15, 25,26,27] were mainly based on LD-SCLC patients, and thus were not completely applicable to ED-SCLC patients. Until now, as far as we know, there has been no study designed or conducted specifically to explore the timing for administering PCI in patients with ED-SCLC. Our study echoed the aforementioned studies and extended the evidence in favor of applying early PCI to ED-SCLC patients for better control of brain metastases. Moreover, it was found that patients with distant metastases were also more likely to develop brain metastases compared to patients with locally advanced disease.
PFS and OS were comparable between the early and late PCI groups. Further multivariate analyses showed that the timing of PCI was not significantly associated with survival. Intriguingly, less tumor load was proven to be a significantly favorable independent prognostic factor for survival, which suggested that the patients with locally advanced disease had a significantly lower risk of developing progression and death. Additionally, TRT was also found to be a positive independent prognostic factor for PFS.
As is known, progression of brain metastases after PCI commonly occurs, hence the survival of patients with intracranial progression generally remains poor [18, 28]. Salvage treatment in this setting is often confined to re-irradiation or best supportive care. In this study, 10 patients in the early PCI group and 23 in the late PCI group developed brain metastases. Two of the 10 patients in the early PCI group and 5 of the 23 patients in the late PCI group received therapeutic re-irradiation after PCI. Subgroup analyses showed that re-irradiation didn’t improved the OS of the patients in early and late PCI groups compared to the best supportive care (13.3 vs. 12.1 months in the early PCI group, p = 0.611; 13.7 vs. 14.8 months in the late PCI group, p = 0.634).
Regarding AEs, headache, fatigue, and lethargy seemed more severe in the early PCI group, but this didn’t reach a statistically significant difference. Other AEs were similar between the two groups. Of note, no adverse event-related deaths were recorded in our study. Moreover, the AEs observed in this work were in line with previous studies [16, 18, 19] and were generally well tolerated and acceptable in both groups.
In terms of cognitive function, the patients receiving early PCI had a trend toward more severe cognitive disturbance, but the difference was not significant. Interestingly, subgroup analyses indicated that among patients treated with early or late PCI, older individuals (≥60 years) were significantly more likely to develop cognitive impairment. A retrospective study reported similar findings in LD-SCLC [29]. Furthermore, this was reconfirmed by a prospective study reporting that a trend toward a stronger reduction of fractional anisotropy in the white matter was shown in the patients aged 65 years or older after PCI [30]. Another study also concurred with these results, reporting that older age was associated with chronic neurotoxicity [31].
The limitations of the current study include a relatively small sample size of 103 patients and the retrospective nature of the analysis. Better-designed randomized phase III studies are recommended to determine if either early or late PCI is more favorable for treating ED-SCLC patients. However, it is worth considering that this may be best addressed in those patients with locally advanced disease, rather than metastatic disease.
Conclusion
This study indicated that early PCI significantly reduced the incidence of brain metastases in ED-SCLC patients with any response to the initial chemotherapy compared with late PCI. Additionally, tumor load was a significantly independent factor of incidence of brain metastases and survival. Futhermore, TRT was also found to be a positive independent prognostic factor for PFS. However, the conclusions should be interpreted with caution because of the limited sample size and nature of the retrospective analysis.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24:4539–4544
Feld R, Pringle JF, Evans WK, Keen CW, Quirt IC, Curtis JE, Baker MA, Yeoh JL, Deboer G, Brown TC (1981) Combined modality treatment of small cell carcinoma of the lung. Arch Intern Med 141:469–473
Oberg K, Hellman P, Ferolla P, Papotti M (2012) Neuroendocrine bronchial and thymic tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):i120–i123
Simon M, Argiris A, Murren JR (2004) Progress in the therapy of small cell lung cancer. Crit Rev Oncol Hematol 49:119–133
Chua YJ, Steer C, Yip D (2004) Recent advances in management of small-cell lung cancer. Cancer Treat Rev 30:521–543
Kurup A, Hanna NH (2004) Treatment of small cell lung cancer. Crit Rev Oncol Hematol 52:117–126
Schnabel T, Schmitt G (1993) The role of radiotherapy in the management of small cell lung cancer (SCLC). Strahlenther Onkol 169:329–338
Socinski MA, Bogart JA (2007) Limited-stage small-cell lung cancer: the current status of combined-modality therapy. J Clin Oncol 25:4137–4145
Stupp R, Monnerat C, Turrisi AR, Perry MC, Leyvraz S (2004) Small cell lung cancer: state of the art and future perspectives. Lung Cancer 45:105–117
Seute T, Leffers P, Ten VG, Twijnstra A (2004) Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer 100:801–806
Komaki R, Cox JD, Whitson W (1981) Risk of brain metastasis from small cell carcinoma of the lung related to length of survival and prophylactic irradiation. Cancer Treat Rep 65:811–814
Hardy J, Smith I, Cherryman G, Vincent M, Judson I, Perren T, Williams M (1990) The value of computed tomographic (CT) scan surveillance in the detection and management of brain metastases in patients with small cell lung cancer. Br J Cancer 62:684–686
Arriagada R, Le Chevalier T, Borie F, Riviere A, Chomy P, Monnet I, Tardivon A, Viader F, Tarayre M, Benhamou S (1995) Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 87:183–190
Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H, Aisner J (1999) Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N Engl J Med 341:476–484
Bernhardt D, Adeberg S, Bozorgmehr F, Opfermann N, Hoerner-Rieber J, Repka MC, Kappes J, Thomas M, Bischoff H, Herth F, Heussel CP, Debus J, Steins M, Rieken S (2017) Nine-year experience: prophylactic cranial irradiation in extensive disease small-cell lung cancer. Clin Lung Cancer 18:e267–e271
Chen Y, Li J, Hu Y, Zhang Y, Lin Z, Zhao Z, Jiao S (2016) Prophylactic cranial irradiation could improve overall survival in patients with extensive small cell lung cancer: a retrospective study. Strahlenther Onkol 192:905–912
Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, Postmus P, Collette L, Musat E, Senan S (2007) Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 357:664–672
Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, Nishio M, Kaneda H, Takayama K, Ishimoto O, Takeda K, Yoshioka H, Tachihara M, Sakai H, Goto K, Yamamoto N (2017) Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 18:663–671
National Cancer Institute (2006) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 7 June 2017
Windsor AA, Koh ES, Allen S, Gabriel GS, Yeo AE, Allison R, van der Linden YM, Barton MB (2013) Poor outcomes after whole brain radiotherapy in patients with brain metastases: results from an international multicentre cohort study. Clin Oncol (R Coll Radiol) 25:674–680
Farooqi AS, Holliday EB, Allen PK, Wei X, Cox JD, Komaki R (2017) Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: Do all patients benefit? Radiother Oncol 122:307–312
Slotman BJ (2015) Prophylactic cranial irradiation postchemotherapy response. J Thorac Oncol 10(suppl 2):S171 (abstract)
Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E (2013) Small-Cell Lung Cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):i99–i105
Suwinski R, Lee SP, Withers HR (1998) Dose-response relationship for prophylactic cranial irradiation in small cell lung cancer. Int J Radiat Oncol Biol Phys 40:797–806
Suwinski R, Withers HR (2003) Time factor and treatment strategies in subclinical disease. Int J Radiat Biol 79:495–502
Sas-Korczynska B, Korzeniowski S, Wojcik E (2010) Comparison of the effectiveness of “late” and “early” prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. Strahlenther Onkol 186:315–319
Ramlov A, Tietze A, Khalil AA, Knap MM (2012) Prophylactic cranial irradiation in patients with small cell lung cancer. A retrospective study of recurrence, survival and morbidity. Lung Cancer 77:561–566
Grosshans DR, Meyers CA, Allen PK, Davenport SD, Komaki R (2008) Neurocognitive function in patients with small cell lung cancer: effect of prophylactic cranial irradiation. Cancer 112:589–595
Welzel T, Niethammer A, Mende U, Heiland S, Wenz F, Debus J, Krempien R (2008) Diffusion tensor imaging screening of radiation-induced changes in the white matter after prophylactic cranial irradiation of patients with small cell lung cancer: first results of a prospective study. AJNR Am J Neuroradiol 29:379–383
Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, Werner-Wasik M, Videtic GM, Garces YI, Choy H (2011) Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys 81:77–84
Acknowledgements
This work was jointly supported by National Natural Science Foundation of China (Grants No. 11505012), Beijing Natural Science Foundation (Grants No. 7172048, 1174016 and 1184014), and Capital’s Funds for Health Improvement and Research (2018-4-1027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Chen, J. Li, Y. Zhang, Y. Hu, G. Zhang, X. Yan, Z. Lin, Z. Zhao, and S. Jiao declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Chen, Y., Li, J., Zhang, Y. et al. Early versus late prophylactic cranial irradiation in patients with extensive small cell lung cancer. Strahlenther Onkol 194, 876–885 (2018). https://doi.org/10.1007/s00066-018-1307-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1307-1