Abstract
Purpose
The purpose of this work was to evaluate the results of high-dose radiation treatment using carbon ion therapy, alone or combined with intensity-modulated radiation treatment (IMRT), in patients with sacral chordoma.
Materials and methods
Between 2009 and 2012, 56 patients with sacral chordoma were treated in our center. The tumor was located above S3 in 33 patients and in S3 or below in 23 patients. In all, 41 patients received radiation therapy for the primary tumor, while 15 patients were treated for the recurrent tumor. Toxicity was measured using NCI CTCAE v.4.03. Local control (LC) and overall survival (OS) were evaluated with the Kaplan–Meier method.
Results
A total of 23 patients were irradiated with carbon ions in combination with photon IMRT, while 33 received carbon ion therapy only. Forty-three patients had a macroscopic tumor at treatment start with a median tumor size (GTV) of 244 ml (range 5–1188 ml). The median total dose was 66 Gy (range 60–74 Gy; RBE). After a median follow-up time of 25 months, the 2- and 3-year local control probability was 76 % and 53 %, respectively. The overall survival rate was 100 %. Treatment for primary tumor and male patients resulted in significant better local control. No higher toxicity occurred within the follow-up time.
Conclusion
High-dose photon/carbon ion beam radiation therapy is safe and, especially for primary sacral chordomas, highly effective. A randomized trial is required to evaluate the role of primary definitive hypofractionated particle therapy compared with surgery with or without adjuvant radiotherapy.
Zusammenfassung
Zielsetzung
Evaluierung der Ergebnisse nach hochdosierter Kohlenstoffionentherapie, allein oder in Kombination mit einer intensitätsmodulierten Photonenbestrahlung (IMRT), bei Patienten mit einem sakralen Chordom.
Material und Methoden
Zwischen 2009 und 2012 wurden 56 Patienten mit sakralen Chordomen in unserem Zentrum behandelt. Der Tumor war bei 33 Patienten oberhalb von S3 und bei 23 Patienten auf Höhe von S3 oder unterhalb davon lokalisiert. Insgesamt wurden 41 Patienten innerhalb der Primärtherapie und 15 Patienten innerhalb einer Rezidivtherapie behandelt. Die Erfassung der Toxizität erfolgte gemäß NCI CTCAE v.4.03. Lokale Kontrolle (LC) und Gesamtüberleben (OS) wurden mithilfe der Kaplan-Meier-Methode bestimmt.
Ergebnisse
Insgesamt 23 Patienten wurden mit Kohlenstoffionen in Kombination mit Photonen-IMRT bestrahlt, während 33 eine alleinige Kohlenstoffionentherapie erhielten. Zu Beginn der Therapie hatten 43 Patienten einen makroskopischen Tumor mit einer medianen Tumorgröße (GTV) von 244 ml (5–1188 ml). Die applizierte mediane Gesamtdosis betrug 66 Gy (60–74 Gy; RBE). Nach einer medianen Nachbeobachtungszeit von 25 Monaten lag die 2- und 3-Jahres-LC bei 76% bzw. 53%. Die OS-Rate betrug 100%. Die Behandlung innerhalb der Primärtherapie und männlichen Patienten zeigten eine signifikant bessere LC. Die Behandlung führte zu keiner höhergradigen Toxizität.
Schlussfolgherung
Eine hochdosierte Strahlentherapie mit Photonen bzw. Kohlenstoffionen ist sicher durchführbar und ist, vor allem innerhalb der Primärtherapie eines sakralen Chordoms, sehr effektiv. Eine randomisierte Studie ist erforderlich, um die Rolle der primären definitiven hypofraktionierten Partikeltherapie im Vergleich zu einer operativen Therapie mit oder ohne adjuvante Strahlentherapie zu evaluieren.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Accounting for 1–4 % of all malignant bone tumors, chordomas are very rare. This slowly growing, locally aggressive tumor arises from the embryonic remnants of the notochord [1]. The overall incidence of chordoma is 8.4 per 10 million [2]. The typical location of the tumor is alongside the neuroaxis due to the origin of the tumor. Surgical resection is still the therapy of choice with a sacral chordoma. The quality of the surgical margin is the most important factor for local control and survival [3–6].
Since the 1970s, en bloc resection with a safety margin has been the treatment of choice [7]. Surgical resection of a sacral chordoma that is localized above S3 is associated with high morbidity. Radical resection of both S2 roots leads to complete urinary and bowel incontinence [4, 8]. However, 50 % normal bladder and bowel function has been reported after sparing both S2 roots and 100 % after the additional sparing one S3 nerve root [7, 9]. Chronic neuropathic pain, wound complications, and paresis are further possible side effects of sacrectomy [10, 11]. Furthermore, it was reported that an adequate safety margin was achieved with surgery in only 50 % of the patients with sacrococcygeal chordomas [5, 6, 12]. Finally, after R1/R2 resection the local recurrence rate is about 100 %.
Due to the relatively poor radiosensitivity of chordomas, highly conformal treatment techniques are needed for safe dose escalation. Owing to their physical properties, carbon ions and protons are superior compared with photons. Thus, a dose escalation to the target volume while sparing adjacent normal tissues could be simultaneously achieved [13–15]. Compared to protons, carbon ions seem to have a biological advantage due to their increased relative biological effectiveness [16]. Heavy charged particle therapy seems to be a good treatment option for tumors with poor radiosensitivity and critical locations. Indications include, among others, chordomas, low-grade chondrosarcomas, and adenoid cystic carcinomas [17–21]. Adjuvant treatment with particle therapy is able to increase the local control rate of patients with sacral chordoma after surgery [22]. Because of the rareness and the slow growth of chordomas, very little data from other sarcoma/particle centers have been published. In order to improve the management of this rare disease, the publication of more information is essential. Therefore, we present the data of 56 patients with sacrococcygeal chordoma who were treated with carbon ions in active raster scanning technique only or combined with photon intensity-modulated radiation treatment (IMRT) in our center.
Patients and methods
Patients
Between November 2009 and December 2012, 56 patients with histologically confirmed sacral chordomas were treated in our department. The tumor was located above S3 in 33 patients and in S3 or below in 23 patients. The median age at treatment was 60 years (range 37–84 years). Thirty-six of the patients were male and 20 female. Forty-one patients received radiation therapy within the primary treatment after biopsy (n = 20), R2 resection (n = 11) or R0/1 resection (n = 10), while 15 patients were treated due to recurrent disease after resection in the past. Four of them were treated after an additional biopsy, 8 after additional R2 resection, and 3 after R0/1 resection. The patients' properties are listed in Table 1.
Treatment planning
Immobilization was performed using individual vacuum bags or a combination of knee and foot rests. A planning CT with and without contrast enhancement was done in treatment position (3 mm slice thickness). Contrast-enhanced T1w and T2stir MRI scans were carried out for 3D image correlation. The treatment volumes and organs at risk were contoured using the Siemens Oncologist software tools (Siemens, Erlangen, Germany). The gross tumor volume (GTV) includes the visible tumor in MRI. If a boost plan was performed, the clinical target volume for the boost (CTV1) includes the GTV and/or the tumor bed with an additional safety margin of 3–5 mm. The clinical target volume for the primary plan (CTV2) includes the CTV1 with safety margins and typical ways of spread (mostly the whole sacrum). The planning target volumes (PTV1 and PTV2) include CTV1 or CTV2 plus a safety margin between 3–7 mm.
Follow-up
Follow-up examinations with MRI scans were carried out every 3 months in the first year after completion of irradiation. In the following years, every 6 months a follow-up examination with an MRI scan was performed. In addition, an annual restaging was done using chest and abdominal imaging. Recurrence was defined at the time of tumor regrowth in MRI. The RECIST criteria were not used. Toxicity was evaluated and compared with the baseline examination using NCI CTCAE v.4.03 criteria. Therefore, the symptoms at baseline are listed descriptively in the table ahead of the toxicity assessment of the therapy.
Statistics
The Kaplan–Meier method was used to evaluate the rates for local control (LC) and overall survival (OS). Age, tumor volume, sex, dose, tumor location, and time of treatment were tested for their prognostic significance using the log rank test (SPSS Version 20).
Results
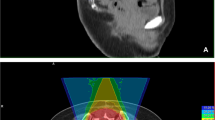
Forty-three patients had a macroscopic tumor with a median size (GTV) of 244 ml (range 5–1188 ml) at treatment start. The median corresponding CTV1 was 522 ml (range 64–1743 ml). The median volume of the CTV2 in all patients was 937.5 ml (range 60–2404 ml). The median prescribed total dose was 66 Gy (range 60–74 Gy; RBE). The total dose was applied using a median weekly fractionation of 5–6 fractions of 3 Gy (RBE) for carbon ions in active beam application using the raster scanning method and 5 fractions of 2 Gy for photon IMRT. IMRT was delivered with step-and-shoot IMRT or tomotherapy. Consequently, the corresponding median equivalent dose calculated for an α/βof 2 Gy for chordoma cells and a fraction dose of 2 Gy (ED2 Gy) was 75 Gy (RBE; range 68.8–82.5 Gy). Twenty-three patients were irradiated with carbon ions in combination with photon IMRT (Fig. 1), while 33 patients received carbon ion treatment only (Fig. 2, Table 1). The median follow-up time after treatment was 25 months. Nineteen patients (34 %) developed a local relapse within the follow-up time. The 2- and 3-year local control probability was 76 % and 53 %, respectively (Fig. 3). The overall survival rate was 100 %. Primary treatment resulted with 85 % in a significantly higher 2-year LC rate compared with 47 % in patients with recurrent tumors (p = 0.001; Fig. 4). Men had with 85 % a better 2-year local control rate than women (60 %; p = 0.03; Fig. 5). All other factors (dose, age, resection status, tumor volume, tumor location) that were evaluated with the log rank test showed no significant differences in LC. Only one patient presented with pulmonal metastasis. No patient developed a new ≥ °III toxicity. All toxicity results including the baseline symptoms are listed in Table 2.
Combined photon/carbon ion treatment and follow-up MRI of a patient with sacral chordoma: a Pretreatment MRI (T2stir). b Dose distribution of primary IMRT plan with a photon total dose of 50 Gy; isodoses in Gray. c Dose distribution of a boost plan with a carbon ion total dose of 24 Gy (RBE); isodoses in percent. d Follow-up MRI (T2stir) 39 months after treatment
Discussion
Tumor control
The treatment of sacral chordomas remains a challenge. In most cases, the tumor is very large at the time of the first diagnosis. Therefore, radical resection with safety margins is difficult to achieve. Data from Massachusetts General Hospital (MGH) in Boston indicate after a mean follow-up time of 104 months (range 30–261 months), a high 5- and 10-year local control rate of 91 % for primary surgical and radiation treatment of sacral chordomas. The resection status after surgery was R0/1. The results were worse for treatment with surgery and radiation of recurrent sacral chordomas (5-/10-year LC: 57 %/19 %) [22]. In contrast with MGH, 77 % of our patients had macroscopic tumors (median 244 ml) at radiation treatment start. Furthermore, 27 % were treated due to recurrent chordoma after previous surgery. Therefore, the 2-year local control rate of 85 % after primary therapy with surgery and radiation is remarkable, while our follow-up time was only a median of 25 months.
As shown at MGH, patients with recurrent chordoma had a significantly lower local control rate (47 % 2-year LC rate in our analysis). However, at least a complete resection with adjuvant high-dose radiation treatment should be attempted for the best outcome in patients with sacral chordoma [22]. Invasion into the surrounding muscle, incomplete excision, inadequate surgical margins, and tumor involvement above S3 seem to be poor prognostic factors after surgery [3]. In some patients, complete resection with an adequate safety margin is not possible or only possible with high morbidity. Hence, alternative treatments are required. MGH presented data of definitive high-dose proton therapy in 24 patients with unresected mobile spine (n = 5) and sacral chordoma (n = 19). The median tumor volume was 189 ml and the median follow-up time was 56 months. The resulting local progression-free survival was 90 % at 3 years and 80 % at 5 years. The applied median dose was 77.4 Gy (RBE) [23].
The Hyogo Ion Beam Medical Center (HIMBC) in Japan presented retrospective data of 23 patients with primary sacral chordoma treated either with carbon ions (n = 16) or protons (n = 7). The 3-year local control rate was 94 % for all patients without differences between proton and carbon ion treatments. The total dose was 70.4 Gy (RBE) given in 16 or 32 fractions; the median tumor volume was 264 ml and the median follow-up time was 38 months [24].
Data from the National Institute of Radiological Sciences (NIRS) in Chiba, Japan showed 5-year control rates of 88 % after primary hypofractionated treatment with carbon ions in 95 patients with unresected sacral chordomas. The median dose of 70.4 Gy (RBE) was given in 16 fixed fractions over 4 weeks [25]. Their patients had with 370 ml a smaller CTV compared with our patients with a median CTV1 of 522 ml [25]. Another factor that might be responsible for this outstanding result is the hypofractionated treatment with heavy ions.
In 2013 we started a prospective randomized phase II trial for patients with macroscopic saccrococygeal chordoma in order to improve the results of our patients and to verify these theses. In this trial, patients are randomized between hypofractionated proton therapy (16 fractions of 4 Gy; RBE) and hypofractionated carbon ion therapy (16 fractions of 4 Gy; RBE). The dose of 64 Gy (RBE) is given over 3 weeks [19].
Toxicity
Pain is the most often reported symptom in patients with sacral chordoma. Unfortunately, surgical and radiotherapeutic treatments can also result in pain or aggravation of motor and sensory functions. Chronic neuropathic pain, wound complications, and paresis are further reported side effects after sacrectomy [10, 11]. Sciatic nerve injury after carbon ion treatment is reported by NIRS after more than 70.4 Gy (RBE) hypofractionated in 16 fractions. [25]. Delaney et al. [26] reported about 3 patients with neural injuries after normofractionated photon/proton treatment with 77.4 Gy (RBE). Doses less than 70.4 Gy (RBE) seem to be safe. In another study [24], results of 23 patients with sacral chordomas who were treated at HIMBC with proton/carbon ion either hypofractionated 70.4 Gy (RBE) in 16 fractions or normofractionated with 70.4 Gy (RBE) in 32 fractions showed grade 3 neuropathy in 17 %. None of our patients showed new toxicity ≥ grade 3 after radiation treatment. At the start of treatment, 16 % of the patients had no pain, whereas 57 % reported mild pain and 27 % moderate pain. No grade 3 or higher toxicity occurred after radiation treatment in our patients. Five patients showed a decrease in pain after treatment. The short follow-up time and our limited dose to the cauda equina could be the reason for the low toxicity.
There was no significant change regarding urinary and bowel incontinence during follow-up: At baseline, 5 patients had an enterostoma and 2 patients needed manual help to defecate. All of them had a sacral chordoma of S3 or above and previous surgery. Placement of urinary, suprapubic or intermittent catheter placement was indicated at treatment start in 12 patients and in 11 of them as a result of surgical treatment. None required new colostomy or urinary diversion after radiation treatment. Similar results were published by NIRS, HIMBC, and MGH [22, 24–26]. The toxicity of radiation treatment is relatively low compared with expected toxicity after radical resection with complete urinary and bowel incontinence in at least 80 % of the patients if the chordomas were localized above S3/2.
An evaluation of skin toxicity after carbon ion treatment at NIRS showed grade 3 and 4 skin toxicity. There was no severe skin reaction if the prescribed dose was 64 Gy (RBE) or lower, as with our patients [27]. DeLaney et al. [26] recommend a skin dose of less than 66 Gy (RBE). Due to the mild hypofractionation, combination of photons and carbon ions or the use of opposing beams, only second degree skin reactions developed in our patients. Furthermore, active raster scanning application results in a lower skin dose compared with the passive application techniques.
The low rates of toxicity in our evaluation have to be considered carefully, since most neuropathic side effects occurred not only after 3–5 years, but also up to 10 years after radiation. However, the results of other particle centers showed a correlation between acute toxicity and long-term toxicity of skin and rectum after treatment.
Prognostic factors
Taking into account all the weaknesses of a retrospective analysis, we could not find any significant differences of LC and OS in consideration of resection status, tumor location, tumor volume, age of the patient, and the applied dose. Female patients and recurrent treatment showed a significantly worse LC in the log rank test. The HIMBC results showed a longer progression-free survival in male patients. A tumor volume < 400 ml was only associated with a tendency of better OS [24]. Staab et al. [28] also reported better OS of male patients. Furthermore, they found macroscopic residual tumors in patients with chordoma of the C-, T-, L-spine, and sacrum as a significant factor for LC and OS. However, in the same work, a significant disadvantage if the extended resection was associated with a surgical stabilization [28] was discussed. Researchers at MGH [22] reported a significantly better LC and OS rate in patients who were treated after surgery for primary versus recurrent tumors. The resection status was not a significant prognostic factor in their patients with spinal chordomas [26]. Data from 34 patients with sacral chordoma demonstrate a higher LC after IMRT in patients treated for primary tumors and using doses greater than 60 Gy [29].
Conclusion
High-dose radiation seems to be superior for primary tumors compared to radiation of recurrent tumors. Furthermore, men with sacral chordomas seem to have a better prognosis compared with women. The role of resection status, dose, and tumor volume is still unclear. Hypofractionated particle therapy should be performed in prospective trials only to detect the toxicity. After evaluating the safety in phase II trials, a randomized trial evaluating the role of primary definitive radiotherapy compared with surgery combined with or without adjuvant radiotherapy should be performed. Therefore, we started a prospective randomized phase II trial for patients with macroscopic saccrococygeal chordoma in 2013. In this trial, patients are randomized between hypofractionated proton therapy (16 fractions of 4 Gy RBE) and hypofractionated carbon ion therapy (16 fractions of 4 Gy RBE), applied over 3 weeks [19].
References
Salisbury JR, Deverell MH, Cookson MJ et al (1993) Three-dimensional reconstruction of human embryonic notochords: clue to the pathogenesis of chordoma. J Pathol 171:59–62
Smoll NR, Gautschi OP, Radovanovic et al (2013) Incidence and relative survival of chordomas: the standardized mortality ratio and the impact of chordomas on a population. Cancer 119:2029–2037
Chen KW, Yang HL, Lu J et al (2010) Prognostic factors of sacral chordoma after surgical therapy: a study of 36 patients. Spinal Cord 48:166–171
Cheng EY, Ozerdemoglu RA, Transfeldt EE et al (1999) Lumbosacral chordoma. Prognostic factors and treatment. Spine (Phila Pa 1976) 24:1639–1645
Fuchs B, Dickey ID, Yaszemski MJ et al (2005) Operative management of sacral chordoma. J Bone Joint Surg Am 87:2211–2216
Osaka S, Kodoh O, Sugita H et al (2006) Clinical significance of a wide excision policy for sacrococcygeal chordoma. J Cancer Res Clin Oncol 132:213–218
Stener B, Gunterberg B (1978) High amputation of the sacrum for extirpation of tumors. Principles and technique. Spine (Phila Pa 1976) 3:351–366
Devin C, Chong PY, Holt GE et al (2006) Level-adjusted perioperative risk of sacral amputations. J Surg Oncol 94:203–211
Hsieh PC, Xu R, Sciubba DM et al (2009) Long-term clinical outcomes following en bloc resections for sacral chordomas and chondrosarcomas: a series of twenty consecutive patients. Spine (Phila Pa 1976) 34:2233–2239
Davidge KM, Eskicioglu C, Lipa J et al (2010) Qualitative assessment of patient experiences following sacrectomy. J Surg Oncol 101:447–450
Hulen CA, Temple HT, Fox WP et al (2006) Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am 88:1532–1539
York JE, Kaczaraj A, Abi-Said D et al (1999) Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery 44:74–79; discussion 9–80
Lee SU, Park JW, Kim TH et al (2014) Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol 190:806–814
Demizu Y, Fujii O, Terashima K et al (2014) Particle therapy for mucosal melanoma of the head and neck. A single-institution retrospective comparison of proton and carbon ion therapy. Strahlenther Onkol 190:186–191
Mizumoto M, Okumura T, Ishikawa E et al (2013) Reirradiation for recurrent malignant brain tumor with radiotherapy or proton beam therapy. Technical considerations based on experience at a single institution. Strahlenther Onkol 189:656–663
Durante M, Loeffler JS (2010) Charged particles in radiation oncology. Nat Rev Clin Oncol 7:37–43
Uhl M, Mattke M, Welzel T et al (2014) High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results. Cancer 120:1579–1585
Uhl M, Mattke M, Welzel T et al (2014) Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer 120:3410–3417
Uhl M, Edler L, Jensen AD et al (2014) Randomized phase II trial of hypofractionated proton versus carbon ion radiation therapy in patients with sacrococcygeal chordoma—the ISAC trial protocol. Radiat Oncol 9:100
Schulz-Ertner D, Nikoghosyan A, Didinger B et al (2005) Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer 104:338–344
Uhl M, Welzel T, Oelmann J et al (2014) Active raster scanning with carbon ions: reirradiation in patients with recurrent skull base chordomas and chondrosarcomas. Strahlenther Onkol 190:686–691
Park L, Delaney TF, Liebsch NJ et al (2006) Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys 65:1514–1521
Chen YL, Liebsch N, Kobayashi W et al (2013) Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine (Phila Pa 1976) 38:E930–E936
Mima M, Demizu Y, Jin D et al (2014) Particle therapy using carbon ions or protons as a definitive therapy for patients with primary sacral chordoma. Br J Radiol 87:20130512
Imai R, Kamada T, Sugahara S et al (2011) Carbon ion radiotherapy for sacral chordoma. Br J Radiol 84:Spec No 1:S48–S54
DeLaney TF, Liebsch NJ, Pedlow FX et al (2009) Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys 74:732–739
Yanagi T, Kamada T, Tsuji H et al (2010) Dose-volume histogram and dose-surface histogram analysis for skin reactions to carbon ion radiotherapy for bone and soft tissue sarcoma. Radiother Oncol 95:60–65
Staab A, Rutz HP, Ares C et al (2011) Spot-scanning-based proton therapy for extracranial chordoma. Int J Radiat Oncol Biol Phys 81:e489–e496
Zabel-du Bois A, Nikoghosyan A, Schwahofer A et al (2010) Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol 97:408–412
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Debus states that he is CEO of Heidelberg Ion Therapy Center (HIT). M. Uhl, T. Welzel, A. Jensen, M. Ellerbrock, T. Haberer, O. Jäkel, and K. Herfarth state that there are no conflicts of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (in its most recently amended version). Informed consent was obtained from all patients included in the study
Rights and permissions
About this article
Cite this article
Uhl, M., Welzel, T., Jensen, A. et al. Carbon ion beam treatment in patients with primary and recurrent sacrococcygeal chordoma. Strahlenther Onkol 191, 597–603 (2015). https://doi.org/10.1007/s00066-015-0825-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-015-0825-3