Abstract
An increasing number of patients undergoing radiotherapy (RT) have cardiac implantable electronic devices [CIEDs, cardiac pacemakers (PMs) and implanted cardioverters/defibrillators (ICDs)]. Ionizing radiation can cause latent and permanent damage to CIEDs, which may result in loss of function in patients with asystole or ventricular fibrillation. Reviewing the current literature, the interdisciplinary German guideline (DEGRO/DGK) was developed reflecting patient risk according to type of CIED, cardiac condition, and estimated radiation dose to the CIED. Planning for RT should consider the CIED specifications as well as patient-related characteristics (pacing-dependent, previous ventricular tachycardia/fibrillation). Antitachyarrhythmia therapy should be suspended in patients with ICDs, who should be under electrocardiographic monitoring with an external defibrillator on stand-by. The beam energy should be limited to 6 (to 10) MV CIEDs should never be located in the beam, and the cumulative scatter radiation dose should be limited to 2 Gy. Personnel must be able to respond adequately in the case of a cardiac emergency and initiate basic life support, while an emergency team capable of advanced life support should be available within 5 min. CIEDs need to be interrogated 1, 3, and 6 months after the last RT due to the risk of latent damage.
Zusammenfassung
Strahlentherapie (RT) ist zunehmend häufig bei Patienten mit kardialen implantierten elektronischen Geräten (CIED; Herzschrittmacher [SM] und Kardioverter-Defibrillatoren [ICD]) indiziert. Durch ionisierende Strahlen können Schäden und Fehlfunktionen des CIED auftreten, die einen permanenten Funktionsverlust beim Gerät und eine Asystolie oder Kammerflimmern beim Patienten auslösen. Deshalb wurde vor dem Hintergrund der bisher verfügbaren Daten eine interdisziplinäre Leitlinie (DEGRO/DGK) erarbeitet, die sich an der zu erwartenden Strahlendosis am CIED sowie dem kardialen Risiko des Patienten orientiert. In die Planung zur Strahlentherapie sollten sowohl CIED-Spezifika als auch Charakteristika der kardialen Erkrankung (SM-Abhängigkeit, stattgehabte ventrikuläre Tachykardie/Kammerflimmern) einfließen. In implantierten ICDs sollte die antitachyarrhythmische Therapie zur RT pausiert werden. Diese Patienten sollten dann zwingend mittels Elektrokradioghramm überwacht werden und ein externer Defibrillator sollte unmittelbar verfügbar sein. Bei allen CIEDs sollte die Strahlenenergie auf 6(− 10) MV limitiert werden. Der CIED sollte niemals im direkten Strahlengang liegen. Eine Gesamtstreustrahlendosis sollte 2 Gy nicht überschreiten. Das Personal sollte in der Lage sein, adäquat auf kardiale Notfälle nach „Basic-life-support“-Kriterien zu reagieren. Ein Reanimationsteam muss innerhalb von 5 min präsent sein. Nach der letzten RT sollten die CIED innerhalb von 1, 3 und 6 Monaten erneut abgefragt werden, da ein Risiko für verspätet auftretende CIED-Schäden besteht.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In Germany, there is a rising coincidence of patients aged over 65 years undergoing radiotherapy for malignancies (approx. 480,000 newly diagnosed cases per year according to the Robert Koch Institute) and implantation of cardiac pacemakers (PMs) and cardioverter-defibrillators (ICDs, 150,000 newly implanted devices per year [63]). Radiotherapy (RT) is applied in up to 70 % of cancer cases [35] and it can be assumed that the number of patients in need of both therapies is growing due to demographic changes.
In the field of cardiology and radiation oncology there is a significant lack of knowledge regarding the safe handling of cardiac implantable electronic devices (CIEDs) during RT. This has been substantiated by a recent poll among British radiation oncologists, which showed that most clinicians still use the 1994 American Association of Physicists in Medicine (AAPM) guidelines, which are outdated today (Table 1) [32]. This is most likely true for many other countries and accounts for the need to review the available evidence. The goal of the national guidelines of the German Society of Radiation Oncology (DEGRO) and the German Society of Cardiology (DGK) is to provide information on the safe handling of CIEDs during RT and to develop practical guidelines that minimize risk for patients during their cancer treatment.
Effects of ionizing radiation on CIEDs
PMs detect the ventricular electric activity and are inhibited if the intrinsic heart rate is sufficient. PMs will stimulate the heart if the heart rate drops below a preprogrammed threshold rate. ICDs are implanted for treatment of symptomatic ventricular tachycardia (VT) and to prevent sudden cardiac death due to ventricular fibrillation (VFib). ICD functions include PM activity, antitachycardia pacing, and defibrillation therapy. The use of complementary metal oxide semiconductors (CMOS) in modern CIEDs results in less energy consumption, higher dependability, and smaller devices. In comparison with bipolar transistors that were used in older models, these modern CMOS are more sensitive to damaging events caused by ionizing radiation that might lead to electron-hole pairs resulting in electric leakage and short-cuts [29, 55, 60]. These events may occur in any part of the CMOS and even in more than one position at a time. The resulting damage can be temporary or permanent. In CIEDs, radiation tolerance may be limited due to the complex design in a small space, limited battery capacity, thinner housing with less shielding, and the usage of random access memory (RAM). RAM holds patient-related data by small amounts of highly volatile increments in energy. RAM damage can therefore lead to complete loss of function in a CIED.
The most critical defects comprise altered sensing (loss or inaccurate sensing), altered stimulation (change in stimulation frequency or amplitude), change of antitachyarrhythmia therapy (ATA therapy) settings in ICDs, premature battery depletion, loss of telemetry, and complete loss of function ([29, 34, 55, 60], Table 2). Clinical consequences of CIED failures depend on the patientʼs characteristics: For example, loss of stimulation in a patient with sick sinus syndrome may not be harmful but will lead to life-threatening cardiac pump deficiency in a patient with grade III atrioventricular blockade. The prevalence of PM dependency is unknown and can be caused by a variety of etiologies [58]. In pacing-dependent patients, failure of the PM may result in ineffective or missing stimulation and therefore cause symptomatic bradycardia or asystole making reanimation or temporary stimulation necessary. By contrast, loss of stimulation control may lead to fast stimulation (“runaway pacemaker” or “runaway ICD”) with loss of systolic blood pressure, cardiogenic shock, angina pectoris, and VT [45, 69]. Loss of sensing may lead to excessive and nonsynchronized ventricular stimulation occurring during the T wave. This can result in VFib with subsequent cardiac arrest and death. Additionally, loss of sensing can result in omission of ATA therapy in ICDs. It has been discussed that either electromagnetic interference or artificial sensing mimics high ventricular frequencies and results in inadequate shock therapy [53].
Data supporting evidenced-based guidelines
Table 3 shows all in vitro studies that have been published to date.
CIEDs were placed either directly in the beam or in close vicinity investigating scatter photon and secondary neutron radiation. In PMs, errors resulting in asystole > 10 s or even complete loss of stimulation were found at ≤ 1 Gy [43]. Other findings included changes in stimulatory impedance as first sign of failure and latent decrease in battery life after 1 week [21]. Device failure was not predictable by a threshold dose [56]. Electrical potentials up to 1.2 mV were detected in leads. This can result in oversensing with inhibition of PM stimulation leading to bradycardia, asystole, or fast pacing [7, 66]. In ICDs, ventricular oversensing was recorded after 0.5 Gy, which may be misread by the ICD as VFib or VT and can therefore result in inadequate defibrillation [20]. Complete device failure occurred even at < 1.5 Gy [20]. Errors happened only when the device itself but not the electrodes were within the beam [62].
Data from case series or case reports are presented in Table 4.
In three reported cases, PMs were directly located in the beam, which resulted in device failures [11, 61, 68]. One runaway PM occurred after 0.11 Gy. The PM was not in the beam and 18 MV photons were used, therefore secondary neutrons most likely contributed to the damage (personal communication; [69]). One PM reset happened during intensity-modulated RT (IMRT) for prostate cancer (15-MV photons) [54]. In several ICD failures, the devices were located out of the beam (scatter radiation < 0.5 Gy) and energies > 6–10 MV were applied (Gelblum, 15 MV; Lau, 23 MV; Thomas, 18 MV) [12, 31, 59]. Most reports describe ICD reset into a fallback or power-on-reset mode, with remaining basic diagnostic and therapeutic modalities. One runaway ICD is reported to have occurred during RT for lung cancer in the left hemithoracic region where the ICD was also located. In this case, reset of the ICD stimulatory frequency to 175/min induced polymorph VT of 230–370/min, which made resuscitation of the patient necessary [45].
Mechanisms leading to CIED failures
CIED failure caused by photon radiation occurred either when the device was directly irradiated or when the energy was > 6 MV (see Table 3). No proven threshold dose or linear relationship exists for radiation-induced damage to CIEDs [19]. However, risk for damage to the CIED is considered to increase with radiation dose. In this respect, it is necessary to understand that the amount of energy delivered to a CIED accumulates [30, 34, 55].
Energies > 6–10 MV cause excessive formation of secondary neutrons that harm the RAM or CMOS [10, 14, 15, 50, 65]. At 18 MV, PM defects occurred even at low radiation doses (15 cGy) [43]. On the other hand, irradiation of 20 ICDs with 6-MV photons up to 4 Gy did not result in any ionizing radiation-related effects [24]. In ICDs, placed either close to the central beam or 140 cm away, errors occurred in both locations about eight times more often at 18 MV than at 10 MV [14]. The neutron dose was 14–20 times higher with 18 MV than with 10 MV. No difference was observed in the photon scatter radiation dose (18.8 mSv/10 MV vs. 20.23 mSv/18 MV) [14]. Another study reported ICD failures with 18 MV while no failures were observed at 6 MV [8]. In CIEDS that were placed directly in the beam and irradiated up to a dose of 150 Gy with fractional doses of 2 Gy, one error was observed at 6 MV while 14 defects were noted with 18 MV [67]. Case series report CIED failures at 10- and 18-MV photon RT for tumors that were not located near the CIED. CIED radiation doses ranged from 84.4 ± 99.7 cGy (PM) to 92.1 ± 72.6 cGy (ICD) [8, 12, 31, 36,54]. Other case reports describe safe RT at 6 MV [12, 36, 52]. At our department, we observed CIED failures in five patients who received RT with 18–23 MV for breast, lung, and prostate cancer. No further incidents were observed under intensive surveillance after limiting the energy to 6 MV in more than 100 observed cases.
Dose rate effects were evaluated systematically in a study of 96 PMs [43]. While dose rates of 0.2 Gy/min did not result in any ionizing radiation-related effect, dose rates of up to 1 Gy/min yielded two defects and dose rates of 8 Gy/min resulted in failures in 70 % of the tested PMs. Sensitive were especially electronic parts of the CIEDs relevant for sensing and therefore failure would haveresulted in CIED reset, asystole, or inadequate defibrillation therapy [39]. Regularly used dose rates in the isocenter are between 1 and 10 Gy/min. Resulting dose rates at the CIED are about ten times lower (< 1 Gy/min) if the CIED is not placed within the RT field.
Electron radiation is less dangerous due to lower production of secondary neutrons at the same energy level. At 15 MeV, electron radiation produces only 5 % and at 25 MeV only 20 % of the amount of secondary neutrons that photon radiation produces at the same nominal energy.
Brachytherapy also exerts little influence on CIEDs due to the applied energy levels (20–380 keV) and steep dose gradient [26, 27]. To date, no brachytherapy-related incident of radiation-induced damage to CIEDs has been reported [5, 26, 27].
Radiological imaging techniques employing ionizing radiation also use less energy (kV) and smaller radiation doses (0.01–0.4 Gy) in comparison with RT [3]. Nevertheless, radiologic imaging may result in CIED failure if the device is subjected directly to radiation [16, 39].
Electromagnetic fields, produced by linear accelerators (LINAC) when the beam is switched on [47, 38], are well shielded and therefore do not significantly contribute to CIED failures in clinical routine.
Of greater concern are particles that are used increasingly for different tumor entities [51]. In four ICDs, subjected to scatter proton radiation, formation of secondary neutrons resulted in a total of 29 software failures during ten RT sessions with a cumulative dose of 107 Gy [15]. Several case series on particle radiation report severe failures (reset of stimulatory frequency to a rate of 180/min, runaway pacemaker), reset into fallback mode, and reprogramming of device settings that occurred at high rates in CIEDs that were located out of the field [13, 48, 49]. Therefore, no assumption can be made for safe strategies regarding particle radiation.
German Guideline for CIEDs
It is not possible to discern between different models because manufacturers provide heterogeneous recommendations (Table 5). It becomes apparent from the available data that placement of the CIED in the beam, energies > 6–10 MV, high radiation dose rate close to the CIED, as well as particle radiation may be positive predictors of CIED failure. In this respect, it is necessary to understand that CIED defects can occur with latency and may become manifest as total breakdown weeks or months after the end of RT [66]. This recommendation takes into account whether a patient is PM-dependent or has a history of previous VT as well as the cumulative dose at the CIED respecting the aforementioned precautions.
Risk assessment
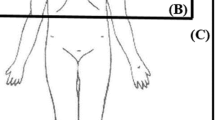
For practical reasons, RT dose in relative distance to the CIED is graded (Table 6, 7). Risk for CIED failure with RT doses close to the CIED < 2 Gy is considered low, between 2 and 10 Gy intermediate, and > 10 Gy high. The relative dose close to the CIED may be derived from Fig. 1 (according to Hurkmans [19]). This graph does not take into account that modern three-dimensional conformal radiation fields result in much smaller doses to the contralateral side or behind the penumbra. Modern IMRT in particular results in extremely low scatter radiation to the normal tissue close to the tumor. Therefore, even RT of thoracic tumors close to the CIED may be possible if precautions are followed (see Fig. 2, VMAT plan of RT for thoracic vertebral metastases at Th2–Th9). The exact dose over the CIED should be calculated using the treatment planning system (TPS) if the CIED was depicted in the planning CT [57]. Nevertheless, it is necessary to bear in mind that most TPS on regular workstations truncate scatter dose calculations behind the penumbra due to limited calculation time. Measuring RT dose with thermoluminescent dosimetry or optically stimulated dosimetry (TLD/OSLD) above the CIED during the first fraction adds more information.
Delineation of the estimated radiation dose to a CIED implanted in typical left pectoral location depending on the target volume in the patient (according to Hurkmans et al. [19]). If a tumor is located in the red area, then the radiation dose to the CIED is likely to be > 10 Gy, in the blue area between 2 and 10 Gy, and in the gray area < 2 Gy. This figure does not take into account the fact that modern three-dimensional conformal or stereotactic radiation fields result in much smaller doses to the CIED
VMAT (volumetric arc therapy) plan, a modern form of intensity-modulated radiotherapy for a patient suffering from hepatocellular carcinoma with vertebral metastasis in the thoracic vertebral bodies Th2–Th9. The patient had a right pectoral implanted pacemaker (PM) and was PM-dependent (heart frequency < 30/min). Left upper image shows the isodoses (yellow arrow indicating 100 % isodose for 30 Gy). Right upper images shows planning CT DICOM image with planning target volumes (PTV, yellow arrow indicating blue PTV Th1—Th10; red arrow indicating PM). Lower image shows dose–volume histograms (DVH, yellow arrow indicating DVH for Th1—Th0, mean dose 2,977.9 cGy; red arrow indicating DVH for PM, mean dose 43.8 cGy)
Patients at highest risk are PM-dependent and may experience a cardiac arrest due to severe bradycardia or asystole in case of device failure. Patients with ICD and a history of VT are also at high risk. These patients are endangered due to: (1) possible induction of VF resulting from fast pacing due to ICD failure; (2) imminent risk of VF during RT while the ATA therapy is deactivated; and (3) risk of sudden cardiac death in case the ATA therapy remains accidentally deactivated after RT or due to ICD failure that remains unrecognized after RT.
The risk assessment in the guideline presented here differs from other recent guidelines in the number or definition of risk categorizations [19, 57] and in the distinction between PM and ICD patients [19]. There is no clear evidence for discrete differentiation between 2 and 10 Gy that might serve as threshold radiation doses to distinguish between low and intermediate risk. Nevertheless, these RT doses have been used before in guidelines [38, 19]. Although ICDs are regarded to be more radiation-sensitive than PMs [8, 12, 14, 18, 36, 54, 55], this guideline proposes 2 Gy as a threshold dose for both ICD and PM due to lack of clear evidence from clinical studies.
Prerequisites for treatment of patients with CIEDs
All personnel treating CIED patients should be able to identify critical CIED complications immediately (asystole, VFib, cardiogenic shock) and initiate basic life support (BLS) [1]. This mandates regular training in BLS as well as CIED specific knowledge since the ATA therapy has to be deactivated in ICDs for RT. This is achieved either by reprogramming or magnet placement. Using a magnet may be safer because removal will immediately reactivate the ATA therapy in case of ventricular tachyarrhythmias. By contrast, external defibrillation can damage the ICD or leads [37]. Furthermore, ICDs with deactivated ATA therapy by reprogramming may accidentally stay in suspended ATA therapy mode and therefore leave the patient unnoticed at risk. A magnet still in place over the ICD is harder to overlook (Table 9). In this context, it is necessary to understand the difference between magnet functionality in ICDs and PMs: In a PM, a magnet will induce asynchronous stimulation while in an ICD it deactivates the ATA therapy and only reprogramming achieves asynchronous stimulation. Therefore, a magnet should be used only if the health-care provider has readily understood the underlying technical principles and if secure placement of the magnet over the CIED is ensured throughout the entire radiation treatment. Patients at high risk or with deactivated ATA therapy should be monitored more closely with an electrocardiogram (ECG) and pulse oxymetry. A defibrillator should be immediately available and competent personnel should be present. Contact via in-room camera and microphone needs to be maintained throughout every treatment session. One camera should always be directed toward the ECG monitor and camera quality should be sufficient to recognize a pathologic electric rhythm. An emergency protocol should be implemented and it should be ensured that a reanimation team is available in case of (suspected) emergency. Emergency equipment (monitoring ECG, blood pressure, blood oxygen saturation, external defibrillator, crash cart) should be available immediately in a cardiac emergency. In high-risk patients (Table 6 and 7), permanent presence of a team capable of advanced life support is warranted to avoid any delay in treatment and to assure the possibility of immediate defibrillation therapy [33]. A physician with qualification in CIED therapy should be available and present in case of CIED failure. Therefore, cooperation between the radiation oncology department and the cardiology department should be initiated early and all necessary steps for prevention as well as treatment of an emergency should be discussed and agreed on in an in-house standard operating procedure (SOP).
Specific considerations before initiation of RT are presented in Table 8.
Specific measures to be followed during radiotherapy are shown in Table 9.
The specific measures to be followed after RT are presented in Table 10.
Modifications that need to be made according to risk groups are listed in Table 11.
The feasibility and practicability of these guidelines are based on the structural conditions of the German health-care system. International recommendations in regard to the presence of manufacturer-affiliated technicians who interrogate or reprogram CIEDs in an outpatient setting are not applicable in Germany.
References
Berg RA, Hemphill R, Abella BS et al (2010) Part 5: adult basic life support: 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 122:685–705
Biotronik (2011) Strahlentherapie und Biotronik CRM-Implantate – Herzschrittmacher (IPG), Defibrillatoren (ICD) und CRT-Geräte. Berlin: Biotronik SE & Co. KG, Global Technical Service
Blamires NG, Myatt J (1982) X-ray effects on pacemaker type circuits. Pacing Clin Electrophysiol 5:151–155
Boston Scientific (2012) Therapeutic radiation and implantable device systems. http://www.bostonscientific.com/content/dam/bostonscientific/quality/educationresources/english/ACL_Therapeutic_Radiation_20120925.pdf
Croshaw R, Kim Y, Lappinen E et al (2011) Avoiding mastectomy: accelerated partial breast irradiation for breast cancer patients with pacemakers or defibrillators. Ann Surg Oncol 18:3500–3505
Dasgupta T, Barani IJ, Roach M 3rd (2011) Successful radiation treatment of anaplastic thyroid carcinoma metastatic to the right cardiac atrium and ventricle in a pacemaker-dependent patient. Radiat Oncol 6:16
Dorenkamp M, Roser M, Hamm B et al (2012) Magnetic resonance imaging and implantable cardiac devices. Current status and future perspectives of MR-compatible systems. Herz 37:136–145
Elders J, Kunze-Busch M, Jan Smeenk R et al (2013) High incidence of implantable cardioverter defibrillator malfunctions during radiation therapy: neutrons as a probable cause of soft errors. Europace 15:60–65
Ferrara T, Baiotto B, Malinverni G et al (2010) Irradiation of pacemakers and cardio-defibrillators in patients submitted to radiotherapy: a clinical experience. Tumori 96:76–83
Franco L, Gomez F, Iglesias A et al (2005) SEUs on commercial SRAM induced by low energy neutrons produced at a clinical linac facility. RADECS Proceedings, 2005
Frantz S, Wagner J, Langenfeld H (2003) Radiation-induced pacemaker malfunction. Z Kardiol 92:415–417
Gelblum DY, Amols H (2009) Implanted cardiac defibrillator care in radiation oncology patient population. Int J Radiat Oncol Biol Phys 73:1525–1531
Gomez DR, Poenisch F, Pinnix CC et al (2013) Malfunctions of implantable cardiac devices in patients receiving proton beam therapy: incidence and predictors. Int J Radiat Oncol Biol Phys 87:570–575
Hashii H, Hashimoto T, Okawa A et al (2013) Comparison of the effects of high-energy photon beam irradiation (10 and 18 MV) on 2 types of implantable cardioverter-defibrillators. Int J Radiat Oncol Biol Phys 85:840–845
Hashimoto T, Isobe T, Hashii H et al (2012) Influence of secondary neutrons induced by proton radiotherapy for cancer patients with implantable cardioverter defibrillators. Radiat Oncol 7:10
Hirose M, Tachikawa K, Ozaki M et al (2010) X-ray radiation causes electromagnetic interference in implantable cardiac pacemakers. Pacing Clin Electrophysiol 33:1174–1181
Hoecht S, Rosenthal P, Sancar D et al (2002) Implantable cardiac defibrillators may be damaged by radiation therapy. J Clin Oncol 20:2212–2213
Hudson F, Coulshed D, DʼSouza E et al (2010) Effect of radiation therapy on the latest generation of pacemakers and implantable cardioverter defibrillators: A systematic review. J Med Imaging Radiat Oncol 54:53–61
Hurkmans CW, Knegjens JL, Oei BS et al (2012) Management of radiation oncology patients with a pacemaker or ICD: a new comprehensive practical guideline in The Netherlands. Radiat Oncol 7:198
Hurkmans CW, Scheepers E, Springorum BG et al (2005) Influence of radiotherapy on the latest generation of implantable cardioverter-defibrillators. Int J Radiat Oncol Biol Phys 63:282–289
Hurkmans CW, Scheepers E, Springorum BG et al (2005) Influence of radiotherapy on the latest generation of pacemakers. Radiother Oncol 76:93–98
John J, Kaye GC (2004) Shock coil failure secondary to external irradiation in a patient with implantable cardioverter defibrillator. Pacing Clin Electrophysiol 27:690–691
St. Jude Medical (2014) http://www.sjm.de/media/2/D14051912/2410103808/Roentgenbestrahlung_therapeutisch.pdf
Kapa S, Fong L, Blackwell CR et al (2008) Effects of scatter radiation on ICD and CRT function. Pacing Clin Electrophysiol 31:727–732
Kesek M, Nyholm T, Asklund T (2012) Radiotherapy and pacemaker: 80 Gy to target close to the device may be feasible. Europace 14:1595
Keshtgar MR, Eaton DJ, Reynolds C et al (2012) Pacemaker and radiotherapy in breast cancer: is targeted intraoperative radiotherapy the answer in this setting? Radiat Oncol 7:128
Kim Y, Arshoun Y, Trombetta MG (2012) Pacemaker/implantable cardioverter-defibrillator dose in balloon high-dose-rate brachytherapy for breast cancer treatment. Brachytherapy 11:380–386
Kirova YM, Menard J, Chargari C et al (2012) Case study thoracic radiotherapy in an elderly patient with pacemaker: the issue of pacing leads. Med Dosim 37:192–194
Lambert P, Da Costa A, Marcy PY et al (2011) Pacemaker, implanted cardiac defibrillator and irradiation: management proposal in 2010 depending on the type of cardiac stimulator and prognosis and location of cancer. Cancer Radiother 15:238–249; quiz 57
Last A (1998) Radiotherapy in patients with cardiac pacemakers. Br J Radiol 71:4–10
Lau DH, Wilson L, Stiles MK et al (2008) Defibrillator reset by radiotherapy. Int J Cardiol 130:e37–38
Lester JF, Evans LM, Yousef Z et al (2014) A national audit of current cardiac device policies from radiotherapy centres across the UK. Clin Oncol (R Coll Radiol) 26:45–50
Link MS, Atkins DL, Passman RS et al (2010) Part 6: electrical therapies: automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 122:706–719
Little FA (1994) Pacemakers in radiotherapy. Clin Oncol (R Coll Radiol) 6:211–212
Lohr F, Baus W, Vorwerk H et al (2012) Rules and regulations applying to incidents in radiotherapy. Strahlenther Onkol 188:545–550
Makkar A, Prisciandaro J, Agarwal S et al (2012) Effect of radiation therapy on permanent pacemaker and implantable cardioverter-defibrillator function. Heart Rhythm 9:1964–1968
Manegold JC, Israel CW, Ehrlich JR et al (2007) External cardioversion of atrial fibrillation in patients with implanted pacemaker or cardioverter-defibrillator systems: a randomized comparison of monophasic and biphasic shock energy application. Eur Heart J 28:1731–1738
Marbach JR, Sontag MR, Van Dyk J et al (1994) Management of radiation oncology patients with implanted cardiac pacemakers: report of AAPM Task Group No. 34. American association of physicists in medicine. Med Phys 21:85–90
McCollough CH, Zhang J, Primak AN et al (2007) Effects of CT irradiation on implantable cardiac rhythm management devices. Radiology 243:766–774
Medtronic (2013) Therapeutic radiation. http://www.medtronic.com/wcm/groups/mdtcom_sg/@mdt/@corp/documents/documents/crdm_sl_radiation.pdf
Menard J, Campana F, Kirov KM et al (2011) Radiotherapy for breast cancer and pacemaker. Cancer Radiother 15:197–201
Mitra D, Ghosh K, Gupta P et al (2006) Radiation dose monitoring in a lung cancer patient with a pacemaker—a case report. Indian J Radiol Imaging 16:857–877
Mouton J, Haug R, Bridier A et al (2002) Influence of high-energy photon beam irradiation on pacemaker operation. Phys Med Biol 47:2879–2893
Munshi A, Wadasadawala T, Sharma PK et al (2008) Radiation therapy planning of a breast cancer patient with in situ pacemaker–challenges and lessons. Acta Oncol 47:255–260
Nemec J (2007) Runaway implantable defibrillator–a rare complication of radiation therapy. Pacing Clin Electrophysiol 30:716–718
Nibhanupudy JR, de Jesus MA, Fujita M et al (2001) Radiation dose monitoring in a breast cancer patient with a pacemaker: a case report. J Natl Med Assoc 93:278–281
Niehaus M, Tebbenjohanns J (2001) Electromagnetic interference in patients with implanted pacemakers or cardioverter-defibrillators. Heart 86:246–248
Oshiro Y, Sugahara S, Noma M et al (2008) Proton beam therapy interference with implanted cardiac pacemakers. Int J Radiat Oncol Biol Phys 72:723–727
Raitt MH, Stelzer KJ, Laramore GE et al (1994) Runaway pacemaker during high-energy neutron radiation therapy. Chest 106:955–957
Rothig H, Herrmann T, Kopcsek H (1995) Experience in dealing with artificial pacemaker patients during therapy with ionizing radiation. Strahlenther Onkol 171:398–402
Schlaff CD, Krauze A, Belard A et al (2014) Bringing the heavy: carbon ion therapy in the radiobiological and clinical context. Radiat Oncol 9:88
Sepe S, Schaffer P, Krimmel K et al (2007) Irradiation treatment of laryngeal cancer in a patient with an implantable cardioverter-defibrillator (ICD). Onkologie 30:378–380
Snow JS, Kalenderian D, Colasacco JA et al (1995) Implanted devices and electromagnetic interference: case presentations and review. J Invasive Cardiol 7:25–32
Soejima T, Yoden E, Y NI et al (2011) Radiation therapy in patients with implanted cardiac pacemakers and implantable cardioverter defibrillators: a prospective survey in Japan. J Radiat Res 52:516–521
Solan AN, Solan MJ, Bednarz G et al (2004) Treatment of patients with cardiac pacemakers and implantable cardioverter-defibrillators during radiotherapy. Int J Radiat Oncol Biol Phys 59:897–904
Souliman SK, Christie J (1994) Pacemaker failure induced by radiotherapy. Pacing Clin Electrophysiol 17:270–273
Sundar S, Symonds RP, Deehan C (2005) Radiotherapy to patients with artificial cardiac pacemakers. Cancer Treat Rev 31:474–486
TheTask Force on cardiac pacing, resynchronization therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association, Brignole M et al (2014) 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Rev Esp Cardiol 67:58
Thomas D, Becker R, Katus HA et al (2004) Radiation therapy-induced electrical reset of an implantable cardioverter defibrillator device located outside the irradiation field. J Electrocardiol 37:73–74
Tondato F, Ng DW, Srivathsan K et al (2009) Radiotherapy-induced pacemaker and implantable cardioverter defibrillator malfunction. Expert Rev Med Devices 6:243–249
Tsekos A, Momm F, Brunner M et al (2000) The cardiac pacemaker patient–might the pacer be directly irradiated? Acta Oncol 39:881–883
Uiterwaal GJ, Springorum BGF, Scheepers E et al (2006) Interference detection in implantable defibrillators induced by therapeutic radiation therapy. Neth Heart J 14:330–334
Kuck KH, Hindricks G, Padeletti L, et al (2014) The EHRA white book. The current status of cardiac electrophysiology in ESC Member countries. http://www.escardio.org/communities/EHRA/publications/Documents/ehra-white-book-2014.pdf
Wadasadawala T, Pandey A, Agarwal JP et al (2011) Radiation therapy with implanted cardiac pacemaker devices: a clinical and dosimetric analysis of patients and proposed precautions. Clin Oncol (R Coll Radiol) 23:79–85
Wilkinson JD, Bounds C, Brown T et al (2005) Cancer-Radiotherapy equipment as a cause of soft errors in electronic equipment. IEEE transactions device and materials reliability 5:449–451
Wilm M, Kronholz HL, Schutz J et al (1994) The modification of programmable pacemakers by therapeutic irradiation. Strahlenther Onkol 170:225–231
Zaremba T, Jakobsen AR, Thogersen AM et al (2014) The effect of radiotherapy beam energy on modern cardiac devices: an in vitro study. Europace 16:612–616
Zaremba T, Thogersen AM, Eschen O et al (2010) High-dose radiotherapy exposure to cardiac pacemakers may be safe in selected patients. Radiother Oncol 95:133–134
Zweng A, Schuster R, Hawlicek R et al (2009) Life-threatening pacemaker dysfunction associated with therapeutic radiation: a case report. Angiology 60:509–512
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Roser has received speaker’s fees from the companies Medtronic, Biotronik, and St. Jude Medical. He has also received counseling fees from Biotronik. R. Schimpf has received speaker’s fees from Medtronic and St. Jude Medical. B. Gauter-Fleckenstein, C.W. Israel, M. Dorenkamp, J. Dunst, V. Steil, J. Schäfer, U. Höller, and F. Wenz state that there are no conflicts of interest.
Additional information
Carsten W. Israel and Rainer Schimpf, Representatives of the German Society for Cardiology (Deutschen Gesellschaft für Kardiologie; DGK)
Jürgen Dunst and Frederik Wenz, Representatives of the German Society for Radiation Oncology (Deutschen Gesellschaft für Radioonkologie; DEGRO)
Rights and permissions
About this article
Cite this article
Gauter-Fleckenstein, B., Israel, C., Dorenkamp, M. et al. DEGRO/DGK guideline for radiotherapy in patients with cardiac implantable electronic devices. Strahlenther Onkol 191, 393–404 (2015). https://doi.org/10.1007/s00066-015-0817-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-015-0817-3
Keywords
- Radiation therapy
- Cardiac pacemaker
- Implanted cardioverter/defibrillator
- Cardiac implantable electronic devices
- Ionizing radiation