Abstract
Purpose
The purpose of this work was to investigate the prognostic value of response analysis using early 3’-deoxy-3’-[18F]-fluorothymidine (18F-FLT) PET/CT in esophageal squamous cancer patients and make a comparison with [18F]-fluorodeoxyglucose (18F-FDG) PET/CT.
Patients and materials
For 34 patients with esophageal squamous cell cancer, both 18F-FLT PET/CT and 18F-FDG PET/CT scans were performed at baseline (pre), 4 weeks after the start of radiotherapy or chemoradiotherapy (interim), and 2 weeks after therapy completion (final). SUVmax1, SUVmax2, and SUVmax3 represent SUVmax (SUV: standard uptake values) measured on the pre, interim, and final scans, respectively. GTVFLT-PET and GTVFDG-PET (GTV: gross tumor volume) were measured on the pre and interim scans. ΔSUV/ΔGTV represents the fractional changes of SUVmax/GTV between two different time points. PET parameters were evaluated for correlations with outcome.
Results
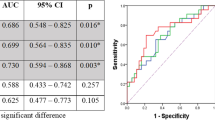
Regarding 18F-FLT PET/CT, according to receiver operating characteristic (ROC) curve analysis, parameters for predicting 2-year progression-free survival (PFS) and locoregional control (LRC) showed the highest area under curve (AUC) on interim 18F-FLT PET/CT scans (ΔSUV12, AUC of 0.812 for PFS, 0.775 for LRC, with a cutoff of 60 %; P = 0.008), compared with the parameters on pre and final scans. Patients with a ΔSUV12 greater than 60 %, who were defined as interim PET-negative group, were associated with better 2-year PFS and LRC than the interim PET-positive group (PFS: 70.6 % vs. 35.2 %, P = 0.025; LRC: 84.2 % vs 52.9, P = 0.046). In terms of 18F-FDG PET/CT, ΔSUV13 on the final 18F-FDG PET/CT scan demonstrated better prediction (AUC of 0.812 for PFS, 0.807 for LRC, with a cutoff of 75 %; P = 0.016) than the parameters on pre and interim scans. An SUVmax decrease ≥ 75 % on the final 18F-FDG PET/CT scan was associated with better clinical outcome (PFS: 73.3 % vs. 36.8 %, P = 0.022; LRC: 86.7 % vs 52.6, P = 0.029). These correlations were most prominent in the subgroup of patients treated with chemoradiotherapy.
Conclusion
Early interim 18F-FLT PET/CT is a significant predictor of 2-year PFS and LRC, which is correlated better with early responses and late outcomes than interim 18F-FDG PET/CT in esophageal squamous cancer patients.
Zusammenfassung
Ziel
Das Ziel der Arbeit war es, die Vorhersage der biologischen Tumorantwortmit einer frühen [18F]-Fluorothymidin(FLT)-PET/CT bei mit Radiochemotherapie behandelten Patienten mit einem Plattenepithelkarzinom des Ösophagus zu untersuchen und die Ergebnisse mit der [18F]-Fluorodeoxyglukose(FDG)-PET/CT zu vergleichen.
Patienten und Methodik
Bei 34 Patienten mit einem Plattenepithelkarzinom des Ösophagus, wurden beide [18F]-FLT-PET/CT- und [18F]-FDG-PET/CT-Untersuchungen jeweils zu Beginn, 4 Wochen nach Beginn der Strahlen- oder Radiochemotherapie und 2 Wochen nach Therapieabschluss durchgeführt. SUVmax1, SUVmax2 und SUVmax3 (SUV: „standard uptake values“) entsprechen der gemessenen SUVmax vor-, zwischen- und nach der Strahlen- oder Radiochemotherapie. Das makroskopische Tumorvolumen (GTV: „gross tumor volume“) GTVFLT-PET und GTVFDG-PET wurde anhand der PET/CT-Ergebnisse vor und während der Strahlentherapie vollzogen. ΔSUV/ΔGTV entspricht der Änderungsrate von SUVmax/GTV zwischen den beiden unterschiedlichen Zeitpunkten. Es wurde die Korrelation der PET/CT-Parameter mit dem Outcome analysiert.
Ergebnisse
Die Analyse der ROC-(Receiver-Operating-Characteristic-Kurve- zeigte, dass im Vergleich zu den Untersuchungen vor- und nach Strahlentherapie während der [18F]-FLT-PET/CT die höchste Fläche unter der Kurve („area under the curve“, AUC) für die Parameter zur Vorhersage des progressionsfreien 2-Jahres-Überlebens (PFS) und der lokalen Kontrolle (LRC) festgestellt wurden (ΔSUV12, AUC von 0,812 für PFS und 0,775 für LRC, bei einem Grenzwert von 60 %; P = 0,008) . Patienten mit einem ΔSUV12 größer als 60 %, die als PET-negative Patienten definiert wurden, hatten eine höhere LKC und ein besseres 2-Jahres-PFS als PET-positive Patienten (LRC: 84,2 % vs. 52,9; P = 0,046; PFS: 70,6 % vs. 35,2 %; P = 0,025). Die Analyse der [18F]-FDG-PET/CT ergab, dass ΔSUV13 bei der PET/CT nach Strahlentherapie eine bessere Vorhersage der Prognose ermöglicht, als die PET/CT vor- und während der Strahlentherapie (AUC 0,812 für PFS; 0,807 für LRC, bei einem Grenzwert von 75 %; P = 0,016). Eine Reduktion der SUVmax von mehr als 75 % warmit einem besseren klinischen Outcome der Patienten assoziiert (PFS: 73,3 % vs. 36,8 %; P = 0,022; LRC: 86,7 % vs. 52,6; P = 0,029). Für die Subgruppe der Patienten, die mit einer Chemoradiotherapie behandelt wurden, waren diese Korrelationen deutlicher.
Schlussfolgerungen
Die frühe [18F]-FLT-PET/CT während der Strahlentherapie ist ein signifikanter Prädiktor für das 2-Jahres-PFS und die LRC und korreliert bei Plattenepithelkarzinomen des Ösophagus im Vergleich zur [18F]-FDG-PET/CT besser mit einem frühem Ansprechen und einem späten Outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Esophageal cancer is one of the most lethal human malignancies and is the eighth leading cause of tumor-related death worldwide [1]. It is associated with a high mortality rate due to not being diagnosed until the cancer has reached an advanced stage in the majority of cases. Of the various local treatments, radiotherapy (RT) plays an important role in the treatment of esophageal cancer, especially for those who are unresectable or those with contraindications [2]. In cases without postoperative pathological finding, therapeutic monitoring and prognostic prediction based on AJCC staging are not appropriate; therefore, treatment evaluation of esophageal cancers with nonsurgical therapy mainly relies on diagnostic imaging methods. However, traditional anatomic imaging modalities, such as CT and endoscopic ultrasonography, are only capable of showing the gradual changes in volume and, therefore, have inherent limitations in evaluating therapeutic response during and after RT [3].
Positron emission tomography (PET) imaging with [18F]-fluorodeoxyglucose (18F-FDG), a molecular imaging technique that effectively provides information on the effect of RT on tumor metabolism, has widely been investigated in the management of patients during the course of radiotherapy [4, 5]. However, the interpretation of 18F-FDG PET/CT findings requires caution due to the uptake in nonmalignant tissues caused by peritumoral inflammation and physiologic changes, especially in the head and neck regions [6, 7]. Because radiation esophagitis is a common complication of radiation therapy in esophagus cancer patients, 18F-FDG-PET/CT may, thus, be limited in differentiating inflammatory tissues from tumor residual or recurrence [8]. Therefore, exploring the role of radiopharmaceuticals other than 18F-FDG is warranted.
3’-Deoxy-3’-[18F]-fluorothymidine (18F-FLT), being a pyrimidine nucleoside analogue, can be phosphorylated by thymidine kinase 1 (TK1) enzyme and changed into 18F-FLT monophosphate after uptake by the cell. Due to the relationship of 18F-FLT uptake with TK-1 activity and percentage of cells in the S-phase, cellular proliferation reflected by 18F-FLT may be regarded as an indicator of tumor-relevant biological behavior. 18F-FLT accumulation was reported in several tumor types and 18F-FLT PET has already been validated in a variety of studies for imaging proliferation and evaluating tumor therapeutic responses [9–11]. In these studies, there was negligible accumulation of 18F-FLT in granulocytes; the accumulation of 18F-FLT in inflamed tissues was not significantly different from that in noninflamed tissues. Therefore, 18F-FLT may be more tumor specific and might be better suited than 18F-FDG for depicting and differentiating inflammation and tumors. The primary objective of our research was to investigate the utility of 18F-FLT PET/CT for detecting esophageal cancer, to use serial 18F-FLT PET/CT to determine whether 18F-FLT PET/CT may be used for evaluating the prognostic value of treatment response in esophageal cancer patients, particularly the early interim PET/CT scan 4 weeks after the start of therapy, and to compare it with 18F-FDG PET/CT.
Patients and methods
Patients
Between February 2011 and December 2012, previously untreated patients with newly pathologically diagnosed esophageal squamous cell cancer (SCC) were enrolled into the study. Patients were staged II–IV according to tumor, node, metastasis (TNM) cancer staging manual of AJCC on the basis of multidetector CT, endoscopic ultrasonography, and ultrasound of the neck. Subjects were excluded if they had surgery as their primary tumor therapy, had palliative treatment, and/or were pregnant. In accordance with curative intent, 15 patients received RT only and 19 received concurrent chemoradiotherapy (CRT). Radiotherapy was delivered to a total dose of 60 Gy (2 Gy/daily fraction) in 30 fractions (5 fractions/week, 6 weeks in total), using 6 MV photons in three-dimensional conformal radiotherapy or conventional plans (anterior–posterior technique). Cisplatin-based chemotherapy concurrent with radiation was performed by using one of the following three regimens: weekly cisplatin 40 mg/m2 for 7–8 weeks, cisplatin 30 mg/m2/day on days 1–3 combined with fluorouracil 750 mg/m2/day on days 1–5 for two or three 28-day cycles, or paclitaxel 135 mg/m2/day on day 1 combined with cisplatin 30 mg/m2/day on days 2–4 for two or three 28-day cycles. All patients underwent whole-body 18F-FLT PET/CT and 18F-FDG PET/CT scans at baseline (pre), 4 weeks after the start of RT or CRT (interim), and 2 weeks after completion of therapy (final), respectively. 18F-FDG PET/CT and 18F-FLT PET/CT were conducted within 48 h of each other.
Follow-ups were conducted every 2 months during the first year, every 3 months during the second year, and every 4 months during the third year. Each follow-up assessment included a complete history and physical examination. Esophagoscopy was performed for those who were suspected of recurrence. Repeated chest and abdominal CT scans were performed every 3 months posttreatment. Follow-up was censored on 31 March 2014. Clinical outcomes were defined as the 2-year progression-free survival (PFS), locoregional control (LRC), and overall survival (OS) rates. The study protocol was discussed and agreed by the Medical Ethics Committee, and all patients were informed and provided written consent.
PET/CT acquisition protocol
18F-FDG was produced in accordance with method that described by our laboratory, using the coincidence 18F-FDG synthesis module (TracerLab FxFN, GE Healthcare). The preparation of 18F-FLT was referred to the method of Machulla et al. [12]. PET/CT acquisition (Discovery STE; GE Medical Systems, Milwaukee, WI, USA) was started 50–60 min after injection of 340–450 MBq 18F-FLT or 220–330 MBq 18F-FDG. Baseline scans were performed using the whole-body mode, and the partial-body mode was implemented in follow-up scans from the seventh cervical vertebra to the abdomen, with a speed of 1.5 min per bed position. The axial field of view covered 14.5 cm, with an axial sampling thickness of 3.75 mm per slice. Images were reconstructed by using normalized attenuation weighted, ordered subset-expectation maximization iterative processing, and were interpreted on Xeleris Workstation (GE Healthcare) by two experienced nuclear medicine radiologists.
PET/CT imaging analysis
After reconstructed, the lesion maximum standardized uptake value (SUVmax) was calculated by region of interest (ROI) analysis. The SUVmax was recorded on the pre, interim, and final scans, expressed as SUVmax1, SUVmax2, and SUVmax3, respectively. Gross tumor volume (GTV) contouring of 18F-FLT PET/CT (GTVFLT-PET) and 18F-FDG PET/CT (GTVFDG-PET) were performed by an experienced nuclear-medicine physician who did not know the treatment outcome. To delineate the GTV, ROIs were positioned around the tumors slice by slice in the volume file, using an isocontour of 40 % of the SUVmax of the whole lesion similarly for all slices. Lymph node metastases were also included in the GTV for the imaging analysis. Since minimal tracer uptake was visible on PET/CT images on the final scan, GTVFLT-PET and GTVFDG-PET were only calculated on the pre and interim scans. When significant activity on the interim scan could not be visually distinguished by the physician, the SUVmax was calculated in the region drawn on the pre scan. Fractional changes of SUVmax/GTV (ΔSUV/ΔGTV) between two different times of scans were calculated using the following formula: ΔSUV12 = [(SUVmax1−SUVmax2)/SUVmax1] × 100 %.
Statistical analysis
Statistical analysis was performed with SPSS software 13.0 (SPSS, Inc.). Differences between paired parameters were tested by Wilcoxon signed-rank or t-testing (2 groups) and the Friedman test (3 groups). The Mann–Whitney U test was used to compare SUVmax or ΔSUV between the subjects who were detected with progression (relapse, metastasis, or death) and those who were progress-free during follow-up. A receiver operating characteristic curve (ROC) analysis was performed to identify the optimal cutoff value of PET parameter before and after treatment for predicting outcome of esophageal SCC. The area under the curve (AUC) values of each modality were compared to identify the levels that afforded the most precise predictions. 18F-FLT PET or 18F-FDG PET positivity/negativity was defined when the parameters were higher/lower than the calculated cutoff value. Survival was calculated from the date of the first PET examination until the date of death or until censoring on the date of last follow-up. The 2-year PFS, LRC, and OS were analyzed using Kaplan–Meier with the log-rank test for univariate comparison. All statistical tests were two-sided, and P values less than 0.05 were considered statistically significant.
Results
Patients’ characteristics and outcomes
Informed consent was obtained from 39 newly diagnosed esophageal SCC cases during the study period. All patients (100 %) underwent pre scans at diagnosis. Due to two false negatives at pre 18F-FLT PET/CT and one follow-up loss, 36 interim scans (92.3 %) were subsequently obtained 4 weeks after the start of RT or CRT. Two weeks after therapy completion, all three paired PET/CT scans, including final scans, were available for 34 patients (87.2 %).
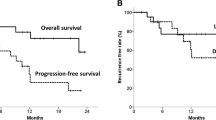
Data on these 34 patients were used in the response analysis. The characteristics of 34 patients are summarized in Table 1. After a median follow-up duration of 24 months (range 7–27 months), 11 patients were confirmed of having locoregional recurrence by taking esophageal pathology biopsies. Upon administrating of percutaneous biopsies, 5 patients were confirmed to have distant metastases (1 with left supraclavicular and cervical lymph node metastases, 2 with lung metastases, 1 suffered multiple hepatic metastasis, and 1 suffered from combined lung and bone metastasis), the other patient with systematic multiple metastasis was diagnosed through visual interpretation in a whole-body 18F-FDG PET/CT scan. A total of 13 patients died during the follow-up period; all of these patients died of progressive disease. Thus, the 2-year PFS, LRC, and OS rates of all enrolled patients were 52.9, 67.6, and 61.8 %, respectively.
Changes in SUVmax and GTV during and after treatment
Relative to the baseline scan, there was a significant decrease in 18F-FLT SUVmax in primary tumors on the interim scan (P < 0.001). Almost complete diminishment of 18F-FLT uptake was observed on the final scan. There were no significant differences in 18F-FLT SUVmax between the interim scan and final scan in primary lesions (P = 0.525). As for 18F-FDG PET/CT, in comparison with the baseline scan, significant SUVmax reductions in primary tumors were observed (P < 0.001), but most lesions (31/34) still depicted a relatively high tracer uptake on the interim scan (SUVmax2 > 2.5). Significant differences of SUVmax between the interim and final scan were also demonstrated (P = 0.014). Moreover, an overall reduction in the GTV was observed in all cases on the interim scan (GTVFLT-PET, 21.34 ± 7.86 vs. 5.40 ± 3.54, P < 0.001; GTVFDG-PET, 22.86 ± 9.17 vs. 13.33 ± 7.96, P < 0.001) (Figs. 1 and 2 and Table 2).
Box plots of a FDG SUVmax, b FLT SUVmax, c GTVFDG-PET, and d GTVFLT-PET on consecutive 18F-FDG PET/CT or 18F-FLT PET/CT scans. Bottom and top of each box are lower and upper quartiles. Black band near middle of box is median. Extremes of lower and higher whiskers represent range of minimum and maximum values. *P < 0.05, Wilcoxon signed-rank test
18F-FLT PET/CT and 18F-FDG PET/CT on the pre, interim, and final scans. The pre 18F-FLT PET shows moderate tracer uptake in primary tumors (arrow, SUVmax = 6.5) and corresponding 18F-FDG PET depicts relatively higher uptake in primary tumors (arrow, SUVmax = 19.7). The interim 18F-FDG PET depicts high “long-line tracer uptake” along the esophagus (arrow, SUVmax = 7.7), while the corresponding 18F-FLT PET/CT reveals greatly reduced tracer uptake in the same region (SUVmax = 2.1) and irradiated vertebrae. The final 18F-FLT PET/CT images depict nearly complete absence of tracer uptake in the primary tumor, while the irradiated vertebrae delineated higher uptake in contrast to interim scan. Corresponding 18F-FDG PET/CT reveals greatly reduced tracer uptake in primary lesions (arrow, SUVmax = 3.5)
Comparing 18F-FLT PET/CT with 18F-FDG PET/CT, statistical analysis demonstrated changes of 18F-FLT uptake displayed significant changes in both ΔSUV12 (64.36 % ± 17.51 % vs. 37.65 % ± 23.72 %; P < 0.001) and ΔSUV13 (73.76 % ± 15.03 % vs. 68.68 % ± 17.12 %; P = 0.049) in tumor lesions. In addition, tumor SUVmax in 18F-FLT PET/CT was much lower than that in 18F-FDG PET/CT on both the interim (SUVmax2, 8.21 ± 2.69 vs. 2.28 ± 1.12, P < 0.001) and final scans (SUVmax3, 4.70 ± 1.72 vs. 1.76 ± 0.48, P < 0.001; Fig. 1, Fig. 2 and Table 2).
Correlations between baseline PET parameters and clinical outcome
Patients were divided into two groups based on whether or not patients suffered from disease progression (relapse, metastasis, or death). The Mann–Whitney U test was used to compare the PET parameters between two groups during follow-up. The purpose of this step was to select suitable parameters as prognostic factors for survival analysis. In the baseline 18F-FLT PET/CT, no statistical significance was observed in FLT SUVmax1 between two groups, but the GTVFLT-PET of primary tumors was statistically significant (Table 3). ROC curve analysis was performed to calculate the most distinguishing cutoff value of these PET parameters in the prediction of 2-year PFS, LRC, and OS.
In the whole group, patients with a GTVFLT-PET less than 22.5 cm3, who were defined as the pre PET-negative group, were associated with better 2-year PFS rates (73.3 % vs. 36.8 %, P = 0.042) than those pre PET-positive patients. In patients receiving CRT, those who had a GTVFLT-PET less than 22.5 cm3 also showed better 2-year LRC and significantly better PFS rates (69.2 % vs. 33.3 %, p = 0.042). However, in patients treated with only RT, baseline GTVFLT-PET dichotomized at the optimal cut-off was not significantly predictive for outcome (Table 4).
In baseline 18F-FDG PET/CT, similar to 18F-FLT PET/CT, a significant difference in the GTVFDG-PET of primary tumors was demonstrated between the two groups, whereas FDG SUVmax1 was not significant (Table 3). Smaller tumors on FDG PET (< 20.8 cm3) showed significantly better 2-year PFS rates (66.7 % vs 37.5 %, P = 0.046). Regarding the CRT-treated group, patients with a GTVFDG-PET < 20.8 cm3 had better 2-year PFS and OS, although this difference was not statistically significant. As for the subgroup of RT, a baseline GTVFDG-PET less than 20.8 cm3 did not predict significantly better 2-year PFS, LRC, or OS (Table 4).
Correlations between follow-up PET parameters and clinical outcome
For all patients, interim 18F-FLT PET/CT showed a reduction of FLT SUVmax in primary tumors, delineating significant differences between progression and progression-free patients (P = 0.008); there was also a decrease in the GTVFET-PET (P = 0.033). Meanwhile, the final scan showed a borderline significant difference in ΔSUV13 between the two groups, whereas not in ΔSUV23 (Table 3). The most discriminative cutoff values of these parameters were sought using ROC methodology optimizing specificity and sensitivity to obtain the highest accuracy. A SUVmax decrease above 60 % between the pre and interim scan was significantly associated with better 2-year PFS and LRC rates than did a SUVmax decrease below 60 %, although the difference in 2-year LRC did not reach statistical significance in patients receiving only RT (Table 4). The association was most prominent in the group of patients receiving CRT (PFS: 75.0 % vs 28.6 %, P = 0.027; LRC: 91.7 % vs 42.9 %, P = 0.017; Fig. 3). On the final scan, SUVmax3 in all patients had reduced to such an extent that ΔSUV13 greater than the cutoff no longer discriminated between outcomes. In addition, a GTVFLT-PET decline ≥ 78 % between pre and interim scan was associated with significantly better 2-year PFS in the whole group and significantly better 2-year PFS and LRC in the subgroup of CRT. The AUC values of GTVFLT-PET, ΔGTV, and ΔSUV predicting 2-year PFS, LRC, and OS are also described in Table 4. The AUCs derived from the interim 18F-FLT PET/CT (ΔSUV12) was higher than those from the pre (GTVFLT-PET) and final (ΔSUV13) scans. Overall, in 18F-FLT PET/CT, a reduction in tumor SUVmax and GTVFLT-PET during RT or CRT for esophageal SCC were good predictors of clinical outcome; a SUVmax decrease ≥ 60 % during the first 4 weeks of treatment served as the optimal cut-off value in predicting 2-year PFS and LRC.
SUVmax above or below 60 % decrease between pre and interim 18F-FLT PET/CT for all patients, the radiotherapy group, and the chemoradiotherapy group. For progression-free survival, P = 0.025, 0.031, and 0.027, respectively; for locoregional control, P = 0.046, 0.100 and 0.017, respectively. mo months
As for 18F-FDG PET/CT, in patients with disease progression, FDG ΔSUV12 varied (range 15 %~ 55 %) on the interim FDG PET scan. There were no significant differences observed between progression and progression-free patients in terms of ΔGTVFDG-PET or ΔSUV12. However, on the final scan, ΔSUV23 and ΔSUV13 were significantly different between the two groups (Table 3). Contrary to 18F-FLT PET/CT, a statistically significant difference was not reached until the final scan. A final 18F-FDG PET negativity higher than the cutoff value (ΔSUV13 ≥ 71 %) was associated with significantly better 2-year RFS and LRC than did those with final 18F-FDG PET positivity (Table 4). These correlations were most pronounced in the subgroup of patients treated with CRT, with a PFS of 76.9 % and LRC of 84.6 % for those with a ΔSUV13 ≥ 71 % on final 18F-FDG PET/CT versus 16.7 % and 50.0 % for those with a ΔSUV13 < 71.0 % (P = 0.008 and 0.039). In all three groups, the AUCs extracted from the final 18F-FDG PET/CT (ΔSUV13) were significantly higher than those from the pre and interim 18F-FDG PET/CT (Table 4). Therefore, ΔSUV13 ≥ 71 % could be used as the most discriminative cutoff in predicting the 2-year PFS and LRC in 18F-FDG PET/CT.
Radiation inflammation/tumor residual differentiation
On the interim scan, the region of primary tumor demonstrated absence of 18F-FLT uptake but high 18F-FDG uptake (FDG PET-positive/FLT PET-negative) in 28 patients (Fig. 4a). Visual interpretation in 18F-FDG PET/CT was regarded in 28 of the 28 patients as radiation inflammation according to the characteristic “long line uptake” along the esophagus. In all, 12 of these FLT-negative but FDG-positive cases received subsequent histopathological examination in the irradiated esophageal tissues (3 days after the interim scan), and results showed the presence of inflammatory infiltrates and interstitial expansion with no evidence of residual tumor (Fig. 4a). On the other hand, PET/CT displayed both negative 18F-FLT and 18F-FDG uptake (FDG PET-negative/FLT PET-negative) in three histopathologically responding tumors (Fig. 4b). For another 3 patients whose primary regions had both moderate visible 18F-FLT and 18F-FDG uptake (FDG PET-positive/FLT PET-positive) on the interim scan, all of them received subsequent biopsy (3–6 days after the interim scan), which revealed residual malignant squamous cell cancer in the region with high 18F-FDG and 18F-FLT uptake (Fig. 4c).
a On the interim scan, 18F-FDG PET/CT demonstrates characteristic “long line uptake” along the esophagus (arrow , SUVmax = 8.1), while less tracer uptake is observed in the corresponding 18F-FLT PET/CT. Endoscopic biopsy in the region of irradiated esophageal tissues reveals inflammatory infiltrates and interstitial expansion. b Both interim 18F-FLT PET/CT and 18F-FDG PET/CT display negative tracer uptake in the region of the primary tumor. Endoscopic biopsy in the low uptake region reveal inflammatory infiltrates and interstitial expansion. c Interim 18F-FLT PET/CT still displays a visible accumulation of radioactivity in the region of the primary tumor (arrow, SUVmax = 7.8), the corresponding 18F-FDG PET/CT also indicates moderate tracer uptake in the region of the primary lesion (SUVmax = 6.3). Endoscopic biopsy in the high uptake region reveals residual malignant squamous cell cancer
Discussion
Accelerated tumor cell repopulation is an important cause of treatment failure in a variety of malignancies [13]. SCC of the esophagus is considered a rapidly proliferating tumor and is thought to exhibit the phenomenon of accelerated repopulation [14]. It is well known that RT and CRT rapidly decrease proliferation rates in responding tumors, therefore, visualization of the proliferative tumor cell compartment and early modification of therapy to counteract this resistance mechanism can help improve outcome. Decisions for more aggressive therapy, such as accelerated radiotherapy [15], concomitant chemoradiotherapy [16], or radiotherapy combined with cetuximab [17] to counteract proliferation, are currently driven by clinical and radiologic tumor characteristics and by patient factors. In individualized therapy strategies, evaluating the proliferative activity of tumor before, during, and after treatment might be of complementary value for better selection of patients and modification of treatment strategy according to the early assessment of each patient’s therapeutic response. The present study describes preliminary work on a comparative study of 18F-FDG and 18F-FLT PET/CT imaging for predicting response to therapy early during RT or CRT treatment for SCC of esophagus. We hypothesized that 18F-FLT uptake would reflect early cellular changes after the administration of RT or CRT.
In terms of prediction with the baseline scan, both baseline GTVFDG-PET and GTVFLT-PET showed statistically significant differences between progression and progression-free patients, indicating pretreatment GTVFDG-PET and GTVFLT-PET might be significant in predicting the patients’ prognoses. We found that in the group of patients who are routinely treated with RT alone, a GTVFLT-PET < 22.5 cm3 or GTVFDG-PET < 20.8 cm3 did not predict significantly better 2-year PFS, LRC, or OS (Table 4). However, in patients who are treated with concurrent CRT, both GTVFLT-PET < 22.5 cm3 and GTVFDG-PET < 20.8 cm3 were associated with significantly better outcome, suggesting that the additional chemotherapy might be particularly relevant for SCC of the esophagus. Although CRT most likely enhance the antiproliferative/metabolic effect of the accelerated irradiation schedule and ultimately result in improved treatment outcome, we still cannot conclude that CRT is superior to RT alone for SCC of esophagus from the present results; further investigations with larger patient populations are needed to compare CRT versus RT alone in esophageal SCC patients.
Early assessment for a radiotherapeutic effect may reduce delays in clinical management and might be of complementary value in the optimization of early treatment modifications. In our study, serial PET/CT of both 18F-FLT and 18F-FDG effectively revealed the gradual reduction of tracer uptake in SCC of the esophagus during RT or CRT. However, compared with 18F-FDG PET/CT, 18F-FLT PET/CT demonstrated faster and more substantial SUVmax reduction early during the course of therapy. In our study, we adopted quantitative methods using ΔSUVmax and ΔGTV to predict the 2-year PFS, LRC, and OS. In 18F-FLT PET/CT, we ultimately found that the parameters extracted from the interim scan showed the best results in terms of predicting clinical outcomes, with a particularly strong association in the subgroup of patients who received CRT. The 2-year PFS and LRC rates according to interim FLT PET/CT results were 75.0 % and 91.7 % for PET-negative patients and 28.6 % and 42.9 % for PET-positive patients when the ΔSUVmax12 cutoff of 60 % was used (Fig. 3). It is also observed that SUVmax for 18F-FLT was not much different between the interim and final scan in tumors (P = 0.525), indicating that evaluation might be best done in week 4 of treatment, later measurements did not add much information, and the 18F-FLT PET signal became more difficult to quantify due to decreasing signal-to-noise ratios.
As for 18F-FDG PET/CT, a statistically significant difference was not demonstrated until the final scan. The parameter extracted from the final 18F-FDG PET/CT (ΔSUV13 ≥ 71 %) was found to be the best discriminative values for predicting clinical outcome, also with strongest association in the CRT-treated group. Thus, our studies demonstrated the utility of both interim 18F-FLT PET/CT and final 18F-FDG PET/CT in providing prognostic information and as a tool for treatment evaluation in patients with SCC of the esophagus. However, posttherapy evaluation may be less helpful when one is aiming for early treatment modification to improve outcome or reduce overtreatment. Similar results were reported in patients with head and neck cancer [18, 19]. The poor correlation of early 18F-FDG PET and RT response may be attributed to the interference of radiation-related mucosal inflammation. Such inflammation-induced FDG uptake will lead to difficulty in evaluation of residual tumor. Given that the biopsies during active (chemo)radiotherapy are often difficult to perform, our study showed that the interim 18F-FLT PET/CT may have the potential to noninvasively discriminate high-risk patients more precisely than 18F-FDG PET/CT early during (chemo)radiotherapy. In addition, it might help oncologists provide individualized management to patients with esophageal cancer, although future prospective clinical trials are needed.
However, it is worth noting the literature about MUNICON II study, which was an initial trial to prospectively evaluate the feasibility and potential effect on prognosis of administering PET-response-guided chemotherapy to patients with locally adenocarcinoma of the esophagus and the esophagogastric junction [20]. In the MUNICON II study, 18F-FDG PET/CT was performed 14 days after initiation of the first chemotherapy cycle in patients with esophageal cancer, and the chemotherapy-induced changes in 18F-FDG uptake identified patients who benefitted from preoperative chemotherapy and those who do not. The prognosis for chemotherapy metabolic nonresponders was poorer than for metabolic responders. Therefore, many scholars hold the view that response of esophageal cancers can be evaluated by 18F-FDG PET/CT as early as 14 days after therapy [21–22]. However, in our study, we performed the interim FDG PET/CT 4 weeks after the start of therapy. This was due to our previous research on nasopharyngeal cancer [23]: we had found the 5-week assessment of treatment response allows for the accurate replanning during the course of RT, reducing treatment volume, and/or dose dependency according to the reduction of disease activity. Therefore, we chose this time-point for imaging based on our previous experience. However, from the results of present study, we observed the serve inflammatory response on the 4-week 18F-FDG PET/CT, which result in false-positive 18F-FDG PET/CT readings and can hamper accurate tumor volume measurements for this tracer. Therefore, in order to avoid the interference of radiation-related inflammation, the timing of the interim 18F-FDG PET/CT scan may better be performed earlier (such as 2 weeks) after initiation of therapy.
Early evaluation of the response to therapy has been evaluated using 18F-FLT PET/CT in several malignancies. In a study of 31 patients with non-small cell lung cancer, the decrease of 18F-FLT uptake after 7 days of therapy with gefitinib was highly predictive of tumor response on CT at 6 weeks and PFS [24]. Hoeben et al. [25] proposed a 45 % decline in 18F-FLT uptake 2 weeks after (chemo)radiotherapy as a strong indicator for long-term outcome in head and neck cancer. In another study on head and neck cancer [26], a decrease in GTVFLT-PET above the median 4 weeks after (chemo)radiotherapy was associated with better 4-year DFS (90 % vs 53 %). In a study of 30 patients with recurrent glioma, changes in 18F-FLT uptake after 6 weeks of bevacizumab treatment were predictive for patient outcome, with a median OS 3.3 time longer in PET responders than in nonresponders [27].
As a matter of fact, no standardized guidelines for the timing of 18F-FLT PET/CT scans, particularly in terms of the imaging time-point of interim scan, have been established. We performed the interim 18F-FLT PET/CT 4 weeks after the start of therapy, which is based on research by Yue et al. [28] about SCC of the esophagus. In their study, tumor proliferation was almost absent after delivery of about 40 Gy, that is, after 4 weeks of uninterrupted radiotherapy. Therefore, we hypothesized the 4-week 18F-FLT PET/CT might demonstrate the early biologic response of esophageal SCC to (chemo)radiotherapy. Given that some studies showed a very early decline of 18F-FLT uptake after the start of treatment, earlier FLT PET acquisition in comparison with our present schedule will be considered in our future work.
Our study also presents the advantage of 18F-FLT PET/CT over 18F-FDG PET/CT in distinguishing inflammation from residual tumor, as currently used imaging modalities are nonspecific in this regard. Endoscopic ultrasound after radiotherapy is poor at distinguishing tumor from posttherapeutic fibrosis or inflammation [29]. 18F-FDG PET/CT, relying on differences in glucose metabolism, represents either residual tumor or esophagitis. Our research suggests that 18F-FLT PET/CT may have the potential to distinguish the tumor residual from radiation esophagitis effectively, which could have important clinical applications. However, in our study, 18F-FDG uptake in the inflammatory lesion was not verified by pathological examination in every patient due to severe inflammatory response in some patients’ esophagus after RT. Typical imaging (the “long line uptake”) of radiation inflammation may not necessarily indicate the presence of inflammation in tumor tissue; additional research is needed to confirm the validity of these findings in our next investigation.
Finally, some limitations of our study should be noted. Because of the small patient population and relatively short follow-up time, well-designed prospective trials with larger patient populations and longer observations are ongoing. Meanwhile, further studies to establish a standard protocol for 18F-FLT PET/CT and interpretation criteria are needed.
Conclusion
The present investigation results suggest that interim 18F-FLT PET/CT might be a promising imaging modality for early evaluation of radiotherapeutic or chemoradiotherapy effects in esophageal SCC. A SUVmax decrease in 18F-FLT uptake in the fourth week of treatment may provide superior prediction of 2-year PFS and LRC. 18F-FLT PET/CT may, thus, aid in personalized patient management by steering treatment modifications during the course of therapy.
References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Semrau R, Herzog SL, Vallbohmer D et al (2012) Radiotherapy in elderly patients with inoperable esophageal cancer. Is there a benefit? Strahlenther Onkol 188:226–232
Westerterp M, van Westreenen HL, Reitsma JB et al (2005) Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy–systematic review. Radiology 236:841–851
Tixier F, Le Rest CC, Hatt M et al (2011) Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 52:369–378
Essler M, Wantke J, Mayer B et al (2013) Positron-emission tomography CT to identify local recurrence in stage I lung cancer patients 1 year after stereotactic body radiation therapy. Strahlenther Onkol 189:495–501
Troost EG, Schinagl DA, Bussink J et al (2010) Innovations in radiotherapy planning of head and neck cancers: role of PET. J Nucl Med 51:66–76
Arslan N, Miller TR, Dehdashti F et al (2002) Evaluation of response to neoadjuvant therapy by quantitative 2-deoxy-2-[18F]fluoro-D-glucose with positron emission tomography in patients with esophageal cancer. Mol Imaging Biol 4:301–310
Jadvar H (2013) Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging 40:5–10
Yang YJ, Ryu JS, Kim SY et al (2006) Use of 3’-deoxy-3’-[18F]fluorothymidine PET to monitor early responses to radiation therapy in murine SCCVII tumors. Eur J Nucl Med Mol Imaging 33:412–419
Herrmann K, Buck AK, Schuster T et al (2011) Predictive value of initial 18F-FLT uptake in patients with aggressive non-Hodgkin lymphoma receiving R-CHOP treatment. J Nucl Med 52:690–696
Lee H, Kim SK, Kim YI et al (2014) Early determination of prognosis by interim 3’-deoxy-3’-18F-fluorothymidine PET in patients with non-Hodgkin lymphoma. J Nucl Med 55:216–222
Machulla H-J, Blocher A, Kuntzsch M et al (2000) Simplified labeling approach for synthesizing 3’-deoxy-3’-[18F] fluorothymidine ([18F] FLT). J Radioanal Nucl Chem 243:843–846
Kim JJ, Tannock IF (2005) Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer 5:516–525
Withers HR, Taylor JM (1993) Critical volume model. Int J Radiat Oncol Biol Phys 25:151–152
Cvek J, Kubes J, Skacelikova E et al (2012) Hyperfractionated accelerated radiotherapy with concomitant integrated boost of 70–75 Gy in 5 weeks for advanced head and neck cancer. A phase I dose escalation study. Strahlenther Onkol 188:666–670
Balermpas P, Bauer C, Fraunholz I et al (2014) Concomitant chemoradiotherapy versus induction chemotherapy followed by chemoradiotherapy as definitive, first line treatment of squamous cell carcinoma of the head and neck. A retrospective single center analysis. Strahlenther Onkol 190:256–262
Bonner JA, Harari PM, Giralt J et al (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11:21–28
Murphy JD, La TH, Chu K et al (2011) Postradiation metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys 80:514–521
Castaldi P, Rufini V, Bussu F et al (2012) Can “early” and “late”18F-FDG PET-CT be used as prognostic factors for the clinical outcome of patients with locally advanced head and neck cancer treated with radio-chemotherapy? Radiother Oncol 103:63–68
Lordick F, Ott K, Krause BJ et al (2007) PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 8:797–805
zum Buschenfelde CM, Herrmann, Schuster T et al (2011) (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med 52:1189–1196
Ott K, Weber WA, Lordick F et al (2006) Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 24:4692–4698
Lin Q, Yang R, Sun L et al (2012) Biological response of nasopharyngeal carcinoma to radiation therapy: a pilot study using serial 18F-FDG PET/CT scans. Cancer Invest 30:528–536
Sohn HJ, Yang YJ, Ryu JS et al (2008) [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin Cancer Res 14:7423–7429
Hoeben BA, Troost EG, Span PN et al (2013) 18F-FLT PET during radiotherapy or chemoradiotherapy in head and neck squamous cell carcinoma is an early predictor of outcome. J Nucl Med 54:532–540
Arens AI, Troost EG, Hoeben BA et al (2014) Semiautomatic methods for segmentation of the proliferative tumour volume on sequential FLT PET/CT images in head and neck carcinomas and their relation to clinical outcome. Eur J Nucl Med Mol Imaging 41:915–924
Schwarzenberg J, Czernin J, Cloughesy TF et al (2012) 3’-deoxy-3’-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med 53:29–36
Yue J, Chen L, Cabrera AR et al (2010) Measuring tumor cell proliferation with 18F-FLT PET during radiotherapy of esophageal squamous cell carcinoma: a pilot clinical study. J Nucl Med 51:528–534
Pastor C, Subtil JC, Sola J et al (2011) Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis Colon Rectum 54:1141–1146
Compliance with ethical guidelines
Acknowledgements
This study was supported by grant No. 81101067 from National Nature Science Foundation of China.
Conflict of interest
H. Chen, Y. Li, H. Wu, L. Sun, Q. Lin, L. Zhao and H. An state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Consent was obtained from all patients identifiable from images or other information within the manuscript. In the case of underage patients, consent was obtained from a parent or legal guardian.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Haojun Chen and Yimin Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, H., Li, Y., Wu, H. et al. 3’-Deoxy-3’-[18F]-fluorothymidine PET/CT in early determination of prognosis in patients with esophageal squamous cell cancer. Strahlenther Onkol 191, 141–152 (2015). https://doi.org/10.1007/s00066-014-0744-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0744-8