Abstract
Purpose
3′-Deoxy-3′-[18F]fluorothymidine (FLT) is a promising new radiopharmaceutical for imaging cell proliferation. We evaluated whether FLT PET can be used to monitor early responses to radiation treatment.
Methods
C3H/HeN mice bearing murine squamous cell carcinomas were randomized to irradiation with 0, 10, or 20 Gy. Twenty-four hours later, the mice were sacrificed for histopathological and biological assessment such as cell cycle analysis, Hoechst staining, and clonogenic cell survival assay. PET scans were performed on other mice after injection of [18F]FLT or [18F]fluorodeoxyglucose (FDG) before and after radiation treatment, and tumor growth was assessed over 9 days.
Results
Histopathological examination detected no morphological changes 24 h after radiation treatment, but cell cycle analysis showed that irradiated tumors had a decreased fraction of cells in S phase and an increased fraction in G2–M phase, compared with nonirradiated tumors. Irradiated tumors also had a higher incidence of apoptotic features and reduced clonogenic cell survival. Tumor growth was significantly delayed in irradiated mice (p<0.001) compared with control mice. PET images showed increased tumoral uptake of both FLT and FDG before radiation treatment. Following irradiation, FLT uptake differed significantly (p=0.020) from that in control mice. In contrast, FDG uptake after irradiation did not differ significantly from that in control mice.
Conclusion
Our finding that tumor uptake of FLT was reduced at 24 h after radiation treatment suggests that FLT PET may be a promising imaging modality for monitoring the early effects of radiation therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation therapy has long been used for curative or palliative management in many cancers. Considering the known heterogeneity of radiobiological properties that govern tissue response to radiation therapy among individual patients, there is a need to monitor early responses to radiation therapy as a predictor of prognosis [1]. Radiation therapy also damages the surrounding normal tissues, making rapid identification of lack of tumor response crucial to avoid ineffective treatment, undesirable side-effects, and unnecessary cost [2].
Over the past decades, several in vitro/ex vivo assays have been proposed for predicting response to radiation therapy. These include measurements of variables such as the survival fraction at 2 Gy, potential tumor doubling time, thymidine labeling index, and tumor hypoxia [3]. Most of these techniques require invasive biopsies, making repeated determinations during or after treatment problematic in clinical practice. The current method for assessing response of a solid tumor to radiation therapy is measurement of the change in tumor size on anatomical imaging modalities [4]. However, it takes weeks to months to detect a change in tumor size, making it difficult to evaluate early responses to radiation therapy.
As functional or biochemical change precedes anatomical change, positron emission tomography (PET) has been recently applied to the evaluation of responses to radiation therapy [5–8]. [18F]fluorodeoxyglucose (FDG) is the most widely used radiopharmaceutical. Since alterations in glycolysis are not the direct biological effects of radiation, increased glycolysis is not specific to tumors [9, 10]. Radiotherapy may induce early acute inflammatory hypermetabolism on FDG PET, which is likely related to the healing of tissues damaged by radiation [11]. Although FDG PET may be a more effective tool than computed tomography in the evaluation of responses to radiation, most FDG PET scans are performed about 3–4 weeks after the completion of radiation therapy and FDG PET is rarely applied during radiation treatment for the monitoring of early responses. Furthermore, most responders show a variable degree of reduction or even an increase in tumoral FDG uptake [12], providing further evidence of drawbacks to the use of FDG PET for monitoring early responses to radiation therapy.
Increased cellular proliferation is a hallmark of the cancer phenotype. Consequently, a quantitative estimation of tumor proliferation activity obtained through noninvasive medical imaging could predict or allow the rapid assessment of response to radiotherapy [9]. There have been many efforts to synthesize agents to image cell proliferation. For example, 11C-labeled thymidine has enabled the noninvasive imaging of thymidine uptake and cell growth. Because thymidine is the only nucleoside incorporated exclusively into DNA and not RNA, uptake of [11C]thymidine has been assumed to measure the rate of DNA synthesis [13–15]. However, the short half-life of 11C and the rapid catabolism of thymidine have made use of [11C]thymidine impractical for routine clinical use.
3′-Deoxy-3′-[18F]fluorothymidine (FLT) is a newly developed thymidine analog that has enabled delayed imaging owing to the longer half-life of 18F [16]. FLT is also stable in vivo because it is a substrate for thymidine kinase (TK) but not for thymidine phosphorylase. FLT retention has been shown to correlate with thymidine uptake, TK1 activity, and the percentage of cells in S phase [17, 18]. TK activity is extremely sensitive to ionizing radiation, and the changes in FLT uptake are thought to reflect the direct biological effect of radiation therapy [19, 20]. Therefore, FLT has been proposed as a promising imaging agent for monitoring early responses to radiation therapy [9, 21]. Although the potential of monitoring early responses to chemotherapy with FLT PET has been supported by findings obtained both in vitro [22] and in animal studies [23, 24], which have shown early changes in FLT uptake after cytotoxic or cytostatic chemotherapy [25], FLT PET has not been fully evaluated for monitoring tumor response after radiation therapy. Previous studies demonstrated that changes in FLT uptake after radiotherapy were correlated with those in [3H]thymidine uptake and the PCNA labeling index and that decreased FLT uptake preceded the decreased uptake of FDG [21]. These data, however, were derived from biodistribution studies in different groups of animals, not during serial imaging studies in the same individual animals. We have therefore used a murine tumor model to evaluate whether FLT PET can monitor early responses to radiation therapy. To accomplish this, we performed serial FLT PET scanning before and soon after radiation treatment in the same individual animals, and compared the results of FLT PET scanning with the biological effects of radiation.
Materials and methods
Animals, tumor model, and X-ray irradiation
Tumors were induced by subcutaneous inoculation of 6-week-old male C3H/HeN mice with 1×106 squamous cell carcinoma (SCC)VII cells on the left forelimb. Tumor dimensions including the largest diameter (a), the perpendicular diameter (b), and the vertical diameter (c) were measured daily using calipers, and daily tumor volume was calculated according to the formula (π/6)×a×b×c. Experiments were performed when the tumors had reached a volume of ∼300 mm3 (10–14 days after inoculation). All animal manipulations were performed using sterile techniques.
Tumor-bearing mice were randomized to three groups of 11 mice each and anesthetized with intraperitoneal injections of ketamine (50 μg/g) and xylazine (12 μg/g). The tumors were irradiated with a single dose of 0, 10, or 20 Gy X-rays at a dose rate of 300 MU/min using a linear accelerator (CLINAC CL1800, Varian, Milpitas, CA, USA). Twenty-four hours later, six mice in each group were sacrificed for histopathological and biological assessment of radiation response. The other tumor-bearing mice were used for serial PET images and kept alive to assess tumor growth over 9 days.
Histopathological evaluation
Tumor-bearing mice were sacrificed by cervical dislocation, and the tumors were dissected, immersed in 10% neutral buffered formalin for 3 days at 4°C, and embedded in paraffin. Each paraffin block was cut into 4.0-μm-thick slices, which were stained with hematoxylin and eosin.
Primary cell culture
Dissected tumors were minced using surgical scissors and added to 30 ml of RPMI 1640 medium containing 0.25% trypsin and 5 μg/ml of collagenase (Wako Pure Chemical Industries, Osaka, Japan) for 30–45 min in a 37°C water bath. Cells were passed through a 70-μm cell strainer (FALCON, Becton Dickinson Labware, Franklin Lakes, NJ, USA) and washed twice with warmed plain medium. Red blood cells were removed by Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. The cells were counted and plated in RPMI 1640 medium containing 15% heat-inactivated fetal bovine serum.
Cell cycle analysis
For cell cycle analysis, about 1–5×105 cells were recovered by trypsin digestion and resuspended in 200 μl of PBS. Ice-cold 70% EtOH was added dropwise, and the cells were allowed to stand for 30 min on ice. The cells were centrifuged, resuspended in 400 μl PBS containing 50 μl 1 mg/ml RNase and 50 μl propidium iodide solution (400 μg/ml), and incubated for another 30 min at 37°C. The DNA content was analyzed in duplicate using FACS Calibur (Becton Dickinson, Immunocytometry System, San Jose, CA, USA).
Hoechst 33342 staining
For assessment of apoptosis, primary cultured cells were stained with 5 μg/ml Hoechst 33342 (Molecular Probe, Eugene, OR, USA) in dH2O at 37°C for 30 min. The fixed cells were assessed for morphological signs of apoptosis under a fluorescence microscope.
Clonogenic cell survival assay
Primary cultured cells (100–30,000 per well) were plated into each well of a six-well plate and cultured for 9 days. The cells were stained with 0.5% crystal violet in 5% methanol (Sigma Aldrich), and colonies containing more than 50 cells were counted. For each sample, the resulting number of colonies was normalized relative to the untreated control group. All assays were performed in triplicate.
Radiopharmaceuticals
[18F]Fluoride was produced in house with a 18-MeV cyclotron (Cyclone 18/9, IBA, Louvain-La-Neuve, Belgium) using the 18O (p,n) 18F nuclear reaction by irradiation of a water target containing 18O-enriched water. A TracerLab MX (GEMS, Liege, Benelux SA, Belgium) FDG module with a disposable cassette was used for automatic preparation of [18F]FLT [26]. [18F]FLT was prepared by [18F]fluorination of (5′-O-DMTr-2′-deoxy-3′-O-nosyl-β-D-threopentafuranosyl)-3-N-BOC-thymidine, followed by hydrolysis with 1 N HCl. After high-performance liquid chromatography purification, the decay-corrected radiochemical yield was 45±5.7%, and the radiochemical purity of the [18F]FLT injectable solution was 98±1.2%. The specific activity of the final injectable [18F]FLT solution was 1.2×108–2.8×108 MBq/mmol. [18F]FDG was synthesized conventionally using the same automated module.
PET studies and image analysis
PET images of tumor-bearing mice were obtained using the small animal PET (R4, Concord Microsystems, Knoxville, TN, USA) before and 24 and 48 h after radiation treatment. The mice were anesthetized, and [18F]FLT (n=3 in each group) or [18F]FDG (n=2 in each group) was injected intraperitoneally at a dose of 3.7 MBq in 0.1 ml saline. Image data were acquired for 10 min at 1 h post injection by list mode. For image reconstruction, list-mode data were sorted into three-dimensional sinograms, followed by Fourier rebinning and two-dimensional ordered subset expectation maximization reconstruction with 4 iterations and 16 subsets. Image pixel size was 0.85 mm transaxially with a 1.21-mm slice thickness.
For quantitation of tumor uptake of [18F]FLT or [18F]FDG, region of interest (ROI) analysis was performed using vendor software (ASI Pro 1.1, Concord Microsystems) on reconstructed images. Three consecutive coronal slice images containing tumors were selected visually and ROIs were drawn on the tumor and the contralateral lung as background, and the tumor to background (T/B) uptake ratio was calculated.
Statistical analysis
Statistical analyses were performed using the software SPSS for Windows, version 12.0.1 (SPSS, Inc., Chicago, IL, USA). All data were expressed as mean±1 SD. Tumor growth in the different groups of animals was compared using repeated measures analysis of variance, followed by Tukey’s procedure. Differences in radiopharmaceutical uptake before and after radiation treatment in each individual mouse were compared using the Wilcoxon signed rank test. Differences in radiopharmaceutical tumor uptake between the control and the irradiated group and clonogenic cell survival fraction were analyzed by the Wilcoxon rank sum or the Kruskal-Wallis test. Unless otherwise stated, p≤0.05 was considered statistically significant.
Results
Histopathological and biological response of SCCVII tumors 24 h after radiation treatment
Histopathological evaluation of SCCVII tumors 24 h after irradiation with 10 or 20 Gy X-rays showed no morphological difference between irradiated and nonirradiated tumors. When we analyzed DNA content 1 day after the tumor cells had been placed in primary culture, we found that irradiated tumors had fewer cells in S phase and more cells in G2–M phase than did the control cells (Fig. 1). In addition, cell death analysis performed 5 days after the cells had been placed in primary culture showed a dose-dependent increase in apoptotic features. Hoechst staining of dissected SCCVII tumors 24 h after irradiation also showed a dose-dependent increase in apoptotic features, such as nuclear fragmentation and chromatin condensation, and necrotic features, such as enlarged atypical cells (Fig. 2). Clonogenic cell survival analysis showed that radiation had a dose-dependent inverse correlation with cell survival, with cells irradiated with 10 and 20 Gy having cell survival of 71±6% and 13±3%, respectively (p<0.05) (Fig. 3).
DNA content analysis of murine SCCVII tumors after radiation treatment. Tumors were irradiated with 0, 10, or 20 Gy. Twenty-four hours later, they were dissected out and cultured. a In cell cycle analysis on day 1 by flow cytometry, compared with nonirradiated tumors, irradiated tumors had a decreased fraction of cells in S phase (20%, 5%, and 3% in the 0-Gy, 10-Gy, and 20-Gy groups, respectively) and an increased fraction of cells in G2–M phase (7%, 12%, and 23%, respectively). b In cell death analysis on day 5 by flow cytometry, compared with nonirradiated tumors, irradiated tumors had an increased number of dead cells
Radiation-associated changes in tumor uptake of FLT and FDG
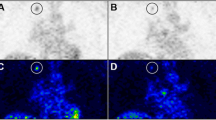
Prior to radiation treatment, PET images showed increased tumor uptake of both FLT and FDG (Fig. 4). ROI analysis showed that, prior to radiation, the baseline T/B ratios of FLT and FDG did not differ significantly among the three groups (Fig. 5), although the baseline T/B ratios of FDG were higher than those of FLT. The T/B ratios of FLT decreased 24 and 48 h after irradiation with 10 Gy (2.8±0.5 before irradiation vs 1.3±0.1 at 24 h vs 1.4±0.2 at 48 h) and 20 Gy (2.6±0.2 vs 1.3±0.1 vs 1.2±0.1), but not in control mice (2.4±0.3 vs 2.5±0.3 vs 2.1±0.7). The change in T/B ratio after radiation treatment was statistically significant (p=0.027), as were the differences in the T/B ratio between control and irradiated mice at 24 h and 48 h (p=0.020). In contrast, the tumor uptake of FDG after irradiation was equivalent in the three groups of mice.
Serial PET images of murine SCCVII tumors after radiation treatment. a FLT PET images showing that the tumor uptake of FLT (arrows) was markedly reduced 24 h and 48 h after irradiation. b FDG PET images showing that tumor uptake was not affected by radiation treatment (one FDG image at 48 h is missing)
Changes in T/B ratios after radiation treatment of murine SCCVII tumors. a The T/B ratios of FLT decreased significantly 24 h after irradiation with 10 Gy or 20 Gy (p=0.027), while there was no significant change in the control group. b The T/B ratios of FDG of irradiated and nonirradiated tumors did not differ significantly
Tumor growth curve after PET imaging
We found that irradiation had a significant, dose-dependent effect on tumor growth (p<0.001, Fig. 6). In untreated mice, there was a continuous increase in tumor volume. The slope of the growth curve in mice treated with 20 Gy was flatter than that in mice treated with 10 Gy, but there was no statistically significant difference between these two groups.
Discussion
We have shown here that a single dose of radiation treatment induced cell cycle arrest and apoptotic features and reduced clonogenic cell survival of murine SCCVII tumors within 24 h at a time when there was no visible shrinkage in tumor size or morphological changes. At the same time, the tumor uptake of FLT in irradiated mice was significantly reduced compared with that in control mice. As significant growth delay occurred well after the serial PET images were obtained, our findings suggest that the reduction in FLT uptake at 24 h is an indication of the early response of tumors to irradiation. Our results are consistent with previous studies, which demonstrated correlations between FLT uptake and thymidine uptake, TK1 activity, and S-phase fraction [17, 18]. These results demonstrate the potential of FLT PET as a noninvasive imaging modality for monitoring early biological responses of tumors to radiation therapy.
Early responses to radiation therapy are thought to derive from the signal transduction cascade, which consists of sensors, transducers, and effectors [27]. Among the effector responses to DNA damage are cell growth arrest, DNA damage repair, and apoptosis [28]. Following irradiation, mammalian cells were halted in the G1 and/or G2 phases and delayed in the S phase [29]. Similarly, our cell cycle analysis showed that irradiated tumors were delayed in the S phase and arrested in the G2 phase. We also observed apoptotic features in irradiated tumors. DNA damage repair requires a supply of deoxynucleoside precursors [30]. TK1 is a key enzyme in the salvage pathway of DNA precursor biosynthesis [30]. TK activity is associated with two isoforms, TK1 and TK2, of which TK1 activity is known to fluctuate with cellular DNA synthesis [31]. TK1 activity is high in proliferating and malignant cells, but low or absent in quiescent cells [32, 33]. TK1 activity in malignant cells has been reported to decrease 24 h after X-ray irradiation [19]. As [18F]FLT is a substrate of TK and FLT uptake is positively correlated with cell growth and TK1 activity, the rapid reduction in FLT uptake observed in these experiments may be due to a rapid reduction in TK1 activity 24 h after radiation treatment [17, 19]. A better understanding of radiation-associated changes in FLT uptake would require measurement of TK activity in correlation with FLT uptake.
The eukaryotic strategy to deal with damaged DNA can be split into three components: recognition of the injured DNA; a period of damage assessment; and the implementation of the appropriate response, DNA repair or cell death [34]. The tumor suppressor p53 is a key protein in checkpoint pathways of signal transduction cascades after irradiation, and this protein provides the decision between cell cycle progression and apoptosis [35, 36]. In the absence of functional p53, TK1 activity is directly stimulated by ionizing radiation, whereas in the presence of functional p53, radiation induction of TK1 activity is inhibited [20, 37, 38]. SCCVII tumors have been suggesting that the reduction of FLT uptake in our animal model may be due to the rapid activation of p53 after radiation treatment [39]. Thus FLT uptake should be low in tumors with a normal p53 response after irradiation, but both TK1 activity and FLT uptake may remain high in p53-deficient tumors even when the percentage of cells in S phase is low [34]. It is therefore of interest to determine whether FLT PET has a role in early monitoring of radiation therapy in p53-deficient tumors.
Despite a marked reduction in FLT uptake to background level at 24 h after single irradiation, the cell surviving fraction on clonogenic assay was 80% with 10 Gy. These results suggest that the reduction of FLT does not reflect killed tumor cells but rather biologically impaired cells in proliferation.
Although clonogenic cell survival after 24 h was dependent on the dose of radiation, we found that neither FLT PET nor the tumor growth curve could detect significant differences between radiation doses. The doses of radiation used here may have been too high to show a dose-dependent effect on FLT PET, since FLT uptake was reduced almost to background level by 10 Gy. To determine whether an early reduction in FLT uptake can predict ultimate dose-dependent response to radiation treatment, it is necessary to perform experiments using lower doses of radiation, as well as using tumor cell lines with different radiosensitivity.
We found that the tumor uptake of FDG did not decrease 24 h after radiation treatment, at a time when there was a significant reduction in FLT tumor uptake. These results are consistent with previous biodistribution data [21], which showed that the tumor uptake of [18F]FDG and [14C]deoxyglucose (DG) was not reduced significantly 24 h after irradiation but showed a gradual decrease only 3 days after radiation. In radiosensitive rat AH109A tumors, FDG uptake gradually and constantly decreased with time after irradiation, significantly decreasing only after 2 days [40]. In contrast, FDG uptake by radiosensitive tumor cells has been reported to increase shortly after irradiation [41]. Radiation-induced acute inflammatory reactions have been suggested as a cause of the unchanged or early increase in FDG uptake by tumor cells after irradiation [12, 41], but we observed no inflammatory reactions in our model. These results suggest that any change in FDG uptake after irradiation may be the result of radiation-induced alterations in glucose metabolism. Tumor uptake of FDG is partially due to hexokinase activity [42] or the number of glucose transporters [43], suggesting either may be altered after radiation treatment. We also found that reduced FLT uptake preceded reduced FDG uptake, suggesting that decreased cell proliferation precedes changes in glucose metabolism and that FLT uptake may be more suitable for early monitoring of the effects of radiation. Our findings are in good agreement with results showing that FLT uptake provided a more accurate assessment of the early response to anticancer therapy than FDG uptake [9, 24].
Among the limitations of this study was our use of different mice for serial FLT PET and FDG PET. Comparison of FDG and FLT uptake in the same tumor at the same time is impossible. As evaluation of cell-based biological responses requires dissection of tumor, it was performed in different animals from those used for serial PET imaging. In addition, we performed these experiments in only one tumor model with a relatively small number of animals. To make firm conclusions, further evaluation with various tumors and a larger number of animals will be needed. The serum concentration of thymidine has been reported to be tenfold higher in rodents than in humans. Endogenous thymidine may compete with FLT for uptake transporter and TK1, suggesting that tumor uptake and biodistribution of FLT in rodents and humans may be different [24]. However, the rapid reduction of FLT uptake after irradiation corresponded to results from cell-based responses. Another study limitation derives from the use of single 10- or 20-Gy treatments, rather than the more usual fractionation dose such as 2 Gy, which limits direct clinical application of our results. Experiments with fractionation of radiation dose should also be performed in radiosensitive and resistant tumors.
Further preclinical and clinical research is required to clarify the role of FLT in early monitoring and prediction of the effects of radiation therapy and to understand the mechanisms underlying changes in FLT uptake.
Conclusion
The tumor uptake of FLT was reduced 24 h after radiation treatment. This reduced uptake may be associated with early responses of tumor cells to radiation. Our findings suggest that FLT PET may be a promising imaging modality for monitoring the early effects of radiation therapy.
References
Van de Wiele C, Lahorte C, Oyen W, Boerman O, Goethals I, Slegers G, et al. Nuclear medicine imaging to predict response to radiotherapy: a review. Int J Radiat Oncol Biol Phys 2003;55:5–15
Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am 2005;43:189–204
Fornace A FZ, Weichselbaum RR, Milas L. Radiation therapy. In. Mendelsohn J, Howley PM, Israel MA, Liotta LA, editors. The molecular basis of cancer. Philadelphia: Saunders; 2001; p. 423–54
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16
Hicks RJ, MacManus MP, Matthews JP, Hogg A, Binns D, Rischin D, et al. Early FDG-PET imaging after radical radiotherapy for non-small-cell lung cancer: inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys 2004;60:412–8
Erdi YE, Macapinlac H, Rosenzweig KE, Humm JL, Larson SM, Erdi AK, et al. Use of PET to monitor the response of lung cancer to radiation treatment. Eur J Nucl Med 2000;27:861–6
Nam SY, Lee SW, Im KC, Kim JS, Kim SY, Choi SH, et al. Early evaluation of the response to radiotherapy of patients with squamous cell carcinoma of the head and neck using18FDG-PET. Oral Oncol 2005;41:390–5
Castellucci P, Zinzani P, Nanni C, Farsad M, Moretti A, Alinari L, et al.18F-FDG PET early after radiotherapy in lymphoma patients. Cancer Biother Radiopharm 2004;19:606–12
Mankoff DA, Shields AF, Krohn KA. PET imaging of cellular proliferation. Radiol Clin North Am 2005;43:153–67
Lonneux M, Sibomana M, Pauwels S, Gregoire V. Current data and perspectives on positron emission tomography oncology-radiotherapy [in French]. Cancer Radiother 1999;3:275–88
Lowe V. PET and PET-CT in oncology. Berlin Heidelberg New York: Springer; 2003; p. 303–8
Hautzel H, Muller-Gartner HW. Early changes in fluorine-18-FDG uptake during radiotherapy. J Nucl Med 1997;38:1384–6
Sundoro-Wu BM, Schmall B, Conti PS, Dahl JR, Drumm P, Jacobsen JK. Selective alkylation of pyrimidyldianions: synthesis and purification of11C labeled thymidine for tumor visualization using positron emission tomography. Int J Appl Radiat Isot 1984;35:705–8
Christman D, Crawford EJ, Friedkin M, Wolf AP. Detection of DNA synthesis in intact organisms with positron-emitting (methyl-11C)thymidine. Proc Natl Acad Sci U S A 1972;69:988–92
Vander Borght T. Laber D, Pauwels S, Lambotte L. Production of [2–11C]thymidine for quantification of cellular proliferation with PET. Int J Rad Appl Instrum [A] 1991;42:103–4
Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 1998;4:1334–6
Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 2002;43:1210–7
Toyohara J, Waki A, Takamatsu S, Yonekura Y, Magata Y, Fujibayashi Y. Basis of FLT as a cell proliferation marker: comparative uptake studies with [3H]thymidine and [3H]arabinothymidine, and cell-analysis in 22 asynchronously growing tumor cell lines. Nucl Med Biol 2002;29:281–7
He Q, Skog S, Welander I, Tribukait B. X-irradiation effects on thymidine kinase (TK): I. TK1 and 2 in normal and malignant cells. Cell Prolif 2002;35:69–81
Schwartz JL, Tamura Y, Jordan R, Grierson JR, Krohn KA. Effect of p53 activation on cell growth, thymidine kinase-1 activity, and 3′-deoxy-3′fluorothymidine uptake. Nucl Med Biol 2004;31:419–23
Sugiyama M, Sakahara H, Sato K, Harada N, Fukumoto D, Kakiuchi T, et al. Evaluation of 3′-deoxy-3′-18F-fluorothymidine for monitoring tumor response to radiotherapy and photodynamic therapy in mice. J Nucl Med 2004;45:1754–8
Dittmann H, Dohmen BM, Kehlbach R, Bartusek G, Pritzkow M, Sarbia M, et al. Early changes in [18F]FLT uptake after chemotherapy: an experimental study. Eur J Nucl Med Mol Imaging 2002;29:1462–9
Barthel H, Cleij MC, Collingridge DR, Hutchinson OC, Osman S, He Q, et al. 3′-deoxy-3′-[18F]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res 2003;63:3791–8
Leyton J, Latigo JR, Perumal M, Dhaliwal H, He Q, Aboagye EO. Early detection of tumor response to chemotherapy by 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography: the effect of cisplatin on a fibrosarcoma tumor model in vivo. Cancer Res 2005;65:4202–10
Waldherr C, Mellinghoff IK, Tran C, Halpern BS, Rozengurt N, Safaei A, et al. Monitoring antiproliferative responses to kinase inhibitor therapy in mice with 3′-deoxy-3′-18F-fluorothymidine PET. J Nucl Med 2005;46:114–20
Oh SJ, Mosdzianowski C, Chi DY, Kim JY, Kang SH, Ryu JS, et al. Fully automated synthesis system of 3′-deoxy-3′-[18F]fluorothymidine. Nucl Med Biol 2004;31:803–9
Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature 2000;408:433–9
Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 2001;27:247–54
Little JB. Delayed initiation of DNA synthesis in irradiated human diploid cells. Nature 1968;218:1064–5
Munch-Petersen B, Cloos L, Jensen HK, Tyrsted G. Human thymidine kinase 1. Regulation in normal and malignant cells. Adv Enzyme Regul 1995;35:69–89
Coloma J, Castell JV. Isozyme pattern of thymidine kinase during liver regeneration. Hoppe Seylers Z Physiol Chem 1984;365:457–62
Ellims PH, Van der Weyden MB, Medley G. Thymidine kinase isoenzymes in human malignant lymphoma. Cancer Res 1981;41:691–5
Sakamoto S, Iwama T, Tsukada K, Utsunomiya J, Kawasaki T, Okamoto R. Increased activity of thymidine kinase isozyme in human colon tumor. Carcinogenesis 1984;5:183–5
Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science 1996;274:1664–72
Lane DP. Cancer. p53, guardian of the genome. Nature 1992;358:15–6
Fei P, El-Deiry WS. P53 and radiation responses. Oncogene 2003;22:5774–83
Boothman DA, Davis TW, Sahijdak WM. Enhanced expression of thymidine kinase in human cells following ionizing radiation. Int J Radiat Oncol Biol Phys 1994;30:391–8
Kreder NC, van Bree C, Peters GJ, Loves WJ, Haveman J. Enhanced levels of deoxycytidine kinase and thymidine kinase 1 and 2 after pulsed low dose rate irradiation as an adaptive response to radiation. Oncol Rep 2002;9:141–4
Saito Y, Milross CG, Hittelman WN, Li D, Jibu T, Peters LJ, et al. Effect of radiation and paclitaxel on p53 expression in murine tumors sensitive or resistant to apoptosis induction. Int J Radiat Oncol Biol Phys 1997;38:623–31
Kubota K, Ishiwata K, Kubota R, Yamada S, Tada M, Sato T, et al. Tracer feasibility for monitoring tumor radiotherapy: a quadruple tracer study with fluorine-18-fluorodeoxyglucose or fluorine-18-fluorodeoxyuridine,L-[methyl-14C]methionine, [6-3H]thymidine, and gallium-67. J Nucl Med 1991;32:2118–23
Furuta M, Hasegawa M, Hayakawa K, Yamakawa M, Ishikawa H, Nonaka T, et al. Rapid rise in FDG uptake in an irradiated human tumour xenograft. Eur J Nucl Med 1997;24:435–8
Weber G. Enzymology of cancer cells (second of two parts). N Engl J Med 1977;296:541–51
Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science 1987;235:1492–5
Acknowledgements
Supported by a grant of the National Cancer Control R&D Program 2003, Ministry of Health & Welfare, Republic of Korea (No. 0320280–2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, YJ., Ryu, JS., Kim, SY. et al. Use of 3′-deoxy-3′-[18F]fluorothymidine PET to monitor early responses to radiation therapy in murine SCCVII tumors. Eur J Nucl Med Mol Imaging 33, 412–419 (2006). https://doi.org/10.1007/s00259-005-0011-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0011-4