Abstract
Background and purpose
To evaluate the effectiveness of high-dose-rate interstitial brachytherapy (HDR-ISBT) as the only form of radiotherapy for high-risk prostate cancer patients.
Patients and methods
Between July 2003 and June 2008, we retrospectively evaluated the outcomes of 48 high-risk patients who had undergone HDR-ISBT at the National Hospital Organization Osaka National Hospital. Risk group classification was according to the criteria described in the National Comprehensive Cancer Network (NCCN) guidelines. Median follow-up was 73 months (range 12–109 months). Neoadjuvant androgen deprivation therapy (ADT) was administered to all 48 patients; 12 patients also received adjuvant ADT. Maximal androgen blockade was performed in 37 patients. Median total treatment duration was 8 months (range 3–45 months). The planned prescribed dose was 54 Gy in 9 fractions over 5 days for the first 13 patients and 49 Gy in 7 fractions over 4 days for 34 patients. Only one patient who was over 80 years old received 38 Gy in 4 fractions over 3 days. The clinical target volume (CTV) was calculated for the prostate gland and the medial side of the seminal vesicles. A 10-mm cranial margin was added to the CTV to create the planning target volume (PTV).
Results
The 5-year overall survival and biochemical control rates were 98 and 87 %, respectively. Grade 3 late genitourinary and gastrointestinal complications occurred in 2 patients (4 %) and 1 patient (2 %), respectively; grade 2 late genitourinary and gastrointestinal complications occurred in 5 patients (10 %) and 1 patient (2 %), respectively.
Conclusion
Even for high-risk patients, HDR-ISBT as the only form of radiotherapy combined with ADT achieved promising biochemical control results, with acceptable late genitourinary and gastrointestinal complication rates.
Zusammenfassung
Hintergrund und Zweck
Beurteilung der Wirksamkeit von interstitieller Brachytherapie mit Hochdosisraten („high-dose-rate interstitial brachytherapy“, HDR-ISBT) als einzige Form der Radiotherapie für Hochrisiko-Prostatakarzinompatienten.
Patienten und Methodik
Zwischen Juli 2003 und Juni 2008 werteten wir retrospektiv die Ergebnisse von 48 Patienten mit hohem Risiko aus, die im National Hospital Organization Osaka National Hospital mittels HDR-ISBT behandelt worden waren. Die Klassifikation der Risikogruppen wurde gemäß der Richtlinien des „National Comprehensive Cancer Network“ (NCCN) durchgeführt. Die mittlere Nachbeobachtungszeit betrug 73 Monate (Bereich 12–109 Monate). Eine neoadjuvante Androgendeprivationstherapie (ADT) erhielten alle 48 Patienten, darunter 12 Patienten zusätzlich als adjuvante Behandlung. Einer maximalen Androgenblockade unterzogen sich 37 Patienten. Die Gesamtbehandlungszeit umfasste im Mittel 8 Monate (Bereich 3–45 Monate). Die geplante vorgeschriebene Dosis betrug bei den ersten 13 Patienten 54 Gy in 9 Fraktionen über 5 Tage bzw. bei den anderen 34 Patienten 49 Gy in 7 Fraktionen über 4 Tage. Nur ein einziger Patient im Alter von über 80 Jahren erhielt eine Dosis von 38 Gy in 4 Fraktionen über 3 Tage. Das klinische Zielvolumen (CTV) wurde für die Prostatadrüse und die mediale Seite der Samenblase (SV) berechnet. Ein 10 mm breiter kranialer Rand wurde dem CTV als Planzielvolumen (PTV) hinzugefügt.
Ergebnisse
Die Gesamtüberlebensrate und die biochemische Kontrollrate über 5 Jahre betrug 98 bzw. 87 %. Späte Grad-3-Urogenital- und -Gastrointestinalkomplikationen traten bei 2 bzw. 1 Patienten (4 bzw. 2 %) auf, während späte Grad-2-Urogenital- und -Gastrointestinalkomplikationen bei 5 bzw. 1 Patienten (10 bzw. 2 %) festgestellt wurden.
Schlussfolgerung
Auch bei Hochrisiko-Patienten erzielte die HDR-ISBT als alleinige Form der Radiotherapie in Kombination mit ADT vielversprechende biochemische Kontrollergebnisse mit akzeptablen späten urogenitalen und gastrointestinalen Komplikationsraten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
External beam radiotherapy (EBRT), interstitial brachytherapy (ISBT) and the combination of both are effective radiotherapeutic treatment modalities for clinically localized prostate cancer. ISBT is particularly useful for delivering high-dose radiation to the prostate gland without increasing doses to the surrounding normal tissues. ISBT can be administered in the following two ways: low-dose-rate (LDR) permanent seed implantation and high-dose-rate (HDR) temporal implantation. HDR-ISBT has some merits, such as a stepping source and dose optimization to improve target coverage after implantation. Furthermore, HDR-ISBT has a radiobiological advantage because the α/b value is lower in the malignant tissue than in the late-responding normal tissue, thereby resulting in superiority of the hypofractionation schedule. In contrast, HDR-ISBT has the disadvantage of prolonged discomfort due to applicator implantation, which decreases the patient’s quality of life. Therefore, HDR-ISBT is commonly used as a boost therapy after EBRT [4, 10, 15, 22]. Although several recent studies have indicated that HDR-ISBT monotherapy achieved good biochemical control—even in high-risk patients [7, 27, 32]—this finding remains controversial [11]. We introduced HDR-ISBT as monotherapy more than a decade ago [23–25, 28–30]. In this study, we present our results of treatment with HDR-ISBT and androgen deprivation therapy (ADT) in a retrospective analysis, with a special focus on high-risk patients.
Patients and methods
Patient characteristics
Between July 2003 and June 2008, a total of 113 patients received HDR-ISBT at the National Hospital Organization Osaka National Hospital. The 3 patients who were lost to follow-up before 12 months after HDR-ISBT were excluded from this study. The median age of the 110 included patients was 73 years (range 52–86 years) and median follow-up duration was 71 months (range 12–109 months).
Pretreatment staging included digital rectal examination, computed tomography (CT), magnetic resonance imaging (MRI) and bone scintigraphy. Using the National Comprehensive Cancer Network (NCCN) risk group classification, 17, 45 and 48 patients were classified as low-risk, intermediate-risk and high-risk, respectively. In this study, 48 high-risk patients, including 9 very high-risk patients, were investigated (◉ Table 1).
Of the 48 high-risk patients, 6 were classified as having stage T1 disease, 23 as T2, 18 as T3 and 1 patient as T4 according to the 2002 NCCN classification criteria. Histological examination of all patients confirmed adenocarcinoma. Gleason scores (GS) were < 7 in 7 patients, 7 in 19 patients, > 7 in 20 patients and unknown in 2 patients. The median percentage of positive biopsies was 50 % (range 10 –100 %). Perineural invasion was observed in 3 patients. The median pretreatment prostate-specific antigen (PSA) level was 25.1 ng/mL (range 3.8–98.6 ng/mL).
ADT was administered to all 48 patients as a neoadjuvant treatment for a median duration of 8 months (range 3–22 months). Maximal androgen blockade was performed in 37 patients. In addition, 12 patients received adjuvant ADT for a median period of 24 months (range 10–36 months). Patients who were T3 (7 patients) or GS ≥ 8 (6 patients) or iPSA > 20 (6 patients) are included. Total treatment duration for the 48 patients was a median of 8 months (range 3–45 months). Seventeen patients received ADT for > 12 months.
Applicator implantation
The applicator implantation procedure has been previously described elsewhere [23, 24]. In brief, implantation was performed under lumbar anesthesia. And, epidural anesthesia was continued until applicator extraction. We adopted metal needle applicators for the first 5 patients and flexible needle applicators (ProGuide Sharp Needle®, Nucletron an Elekta Company, Veenendaal, Netherlands) for the remaining 43 patients. We implanted 9–15 (median 13) applicators. In the first 15 patients, this was performed via an nonambulatory implant technique using a nonremovable template (Taisei Medical, Osaka, Japan) for guidance. In the remaining 33 patients, the applicators were implanted using a removable template (Taisei Medical), or by hand, with an ambulatory implant technique [23]. A 6F flexible applicator was used in the ambulatory implant technique. A colored bead was fixed to the applicator using an adhesive; the length between the needle tip and the bead is color-coded (for example, purple bead: 12 cm; green bead: 13 cm; orange bead: 14 cm). After bead fixation, we applied a color button with thread to the side of the bead with a tip. Subsequently, we tightly sutured the button to the patient’s perineal skin using thread. Guided by transrectal ultrasonography (SSD-1000® and Prosound α7®, Hitachi Aloka Medical, Ltd., Tokyo, Japan), we implanted the treatment applicator in and around the prostate gland and proximal seminal vesicles (SVs). To prevent dorsal SV displacement near the rectum, we implanted a dummy needle at the template hole that was one template hole dorsal to the hole we initially judged as adequate. This dummy needle ventrally displaced the SV. After the dummy needle was implanted, we implanted the true applicator into the template hole immediately ventral to the one initially judged to be adequate. If the applicator position was considered to give good SV coverage, the dummy needle was extracted [24]. The top 2–3 cm of the applicators were placed within the urinary bladder to allow the planning target volume (PTV) to include a 10-mm margin added to the clinical target volume (CTV) in the cranial direction. This margin was established for the prevention of caudal displacement of the applicators [25]. This technique is similar to those described in other Japanese reports [9, 27].
Treatment planning
After applicator implantation, we performed the treatment planning. CT scans were obtained for all patients and MRI was performed for the 43 patients treated with flexible needle applicators and nonmetallic stoppers. CT-based planning with or without MRI-assistance using the PLATO® and Oncentra® Brachy treatment planning systems (Nucletron) was performed with manual modification [26].
The planned prescribed dose was 54 Gy in 9 fractions over 5 days for the first 13 patients and 49 Gy in 7 fractions over 4 days for the remaining 34 patients. Only 1 patient who was > 80 years old was administered 38 Gy in 4 fractions over 3 days. CTV was calculated for the prostate gland and the medial side of the SVs. A 10-mm cranial margin was added to the CTV to generate the PTV. The median dose nonuniformity ratio was 0.32 (range 0.23–0.40).
The treatment machine used was the microSelectron-HDR® (Nucletron). One hour before each irradiation fraction, a urinary balloon catheter was clamped in place to keep the urine within the urinary bladder so that the cranial side of the bladder wall and the rectosigmoid colon were kept away from the irradiation field [9, 27]. Beginning in May 2007, corrective action for applicator displacement was initiated in 10 patients after performing daily CT [20].
Statistical analysis
Statistical analyses were performed using the StatView v. 5.0 (SAS Institute, Cary, NC, USA) software program. We analyzed biochemical control and survival rates using the Kaplan–Meier method. A probability (p) value of < 0.05 was considered statistically significant for the log-rank test and dose-volume histogram (DVH) analysis using the Mann–Whitney method.
Results
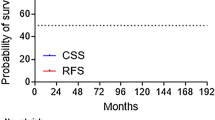
All 48 patients received the planned treatment dose. The 5-year overall survival rate was 98 %. The 5-year biochemical control rate was 87 % (◉ Fig. 1). A total of 4 patients died: 1 patient succumbed to bone metastasis of prostate cancer and 3 died from intercurrent diseases. Of the 7 patients exhibiting biochemical failure, this was observed within 60 months in 6 cases (86 %). One instance of biochemical failure was judged as a case of transient PSA bounce and the patient’s prostate-specific antigen (PSA) level decreased without treatment. No clinically apparent incidence of local recurrence was observed in the other 6 patients with biochemical failure. Two patients showed bone metastasis, but no clinical tumors were detected in the other 4 patients who restarted ADT.
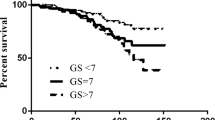
The 5-year biochemical control rates for each tumor-defining factor were as follows: 100, 87 and 84 % for stages T1–2a, T2b–c and T3–4, respectively; 100, 94 and 75 % for GS < 7, 7 and > 7, respectively (p = 0.002); 100, 70 and 90 % for PSA < 10, 10–20 and 20 ng/mL, respectively.
Subsequently, we investigated the relationship between biochemical control rate and ADT duration. The 5-year biochemical control rates were 84 and 94 % for ≤ 12 months and > 12 months of ADT, respectively. No significant difference was observed between the two groups.
The incidence of late complications was evaluated using the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0). No grade 4 late genitourinary complications were observed. Grade 3 late genitourinary complications occurred in 2 patients (4 %; urinary retention and incontinence). Grade 2 late genitourinary complications occurred in 5 patients (10 %). Grade 3 late gastrointestinal complications (rectal bleeding healed by hyperbaric oxygen therapy) were observed in 1 patient (2 %). Grade 2 late gastrointestinal complications (rectal bleeding) occurred in 1 patient (2 %).
Discussion
The number of reports of HDR-ISBT monotherapy eliciting good treatment results has increased recently [1, 3, 5–7, 9, 13, 14, 17, 19, 27, 32]. HDR-ISBT as monotherapy has been used to treat low- and intermediate-risk patients [1, 3, 5, 6, 9, 13, 14, 17, 19]; however, several groups have also reported its effectiveness in high-risk patients [7, 27, 32] (◉ Table 2). For example, Hoskin et al. [7] analyzed 197 patients using MRI and the NCCN classification for staging workup; these authors reported that the 3-year biochemical control rate was 91 % for high-risk patients. In particular, Zamboglou et al. [32] evaluated > 700 patients treated with HDR-ISBT as monotherapy using dose fractionation schedules of 34.5–38 Gy in 3–4 fractions; these authors reported a 5-year biochemical control rate of 93 % for high-risk patients.
In contrast, Krauss [11] commented that there were two points of caution in the report by Zamboglou et al. [32]: Krauss pointed out that the staging workup (via MRI) and risk group definition led to the superior outcomes observed in the subset of high-risk patients of the latter publication. The prognostic value of MRI-staged disease is not clearly defined. Furthermore, they used risk classification criteria identifying high-risk as ≥ T2c. According to the NCCN criteria, T2c is considered intermediate risk. If these criteria are used, several patients classified as high-risk would have been reclassified as intermediate-risk.
Krauss [11] suggested the over-evaluation of HDR-ISBT as monotherapy for high-risk patients by patient risk-group migration. However, this factor seems to have a minor influence on the significance of the study. For example, we previously reported our results of a multi-institutional retrospective analysis of conventional 60–70 Gy EBRT for prostate cancer [31], which included 436 patients treated between 1999 and 2006. In this study, MRI was included in the staging workup for almost all patients. In the same study, the 5-year progression-free survival rates were 69 and 67 % for high-risk and very-high-risk patients, respectively, using the NCCN classification criteria. In contrast, the European Groupe d’Etude des Tumeurs Uro-Génitales (GETUG)-01 trial performed during the same period in Europe [16] evaluated 444 patients who were treated with an irradiation dose of 66–70 Gy between 1998 and 2004. However, in the GETUG-01 trial, MRI was not included in the staging workup. In the high-risk group (T3 and/or Gleason score≥ 7 and/or PSA ≥ 3 × the upper normal limit), the 5-year progression-free survival rates were 63 and 60 % for EBRT to the prostate alone and to the pelvis plus prostate, respectively, which were similar to the results reported in this study. On the basis of these results, we reasoned that the influence of MRI for staging workup does not have a significant impact.
In this study, we evaluated biochemical control results and showed a rate of 87 % among 48 high-risk patients, including 9 very-high-risk patients. Because this result was lower than that reported by Zamboglou et al. [11], patient risk-group migration may explain such differences. However, we considered the difference to be a relatively minor problem; although it may not be negligible in all cases. From these arguments, we believe that the treatment results reported by Zamboglou et al. remain valuable.
Pelvic nodal irradiation should be considered in cases where HDR-ISBT as monotherapy is an option in high-risk patients. Roach et al. [18] reported that pelvic nodal irradiation was effective for patients with a 15 % risk of lymph node involvement. However, opposing opinions have also been reported [8, 21]. Although EBRT is administered to the pelvis, it often results in unsatisfactory outcomes if the total prostate doses are insufficient (total dose 66–70 Gy). Compared with the results of HDR-ISBT as monotherapy [16], the results of conventional EBRT to the pelvis plus prostate in the GETUG-01 trial appear poor, as the 5-year progression free survival rate was only 60 % (◉ Table 2). The Radiation Therapy Oncology Group(RTOG) 94-13 trial demonstrated similar results [12]; here, the 5-year biochemical control rate was approximately 70 % (estimated from figure) for conventional EBRT to the pelvis plus prostate with both adjuvant and neoadjuvant ADT arms. Challapalli et al. [2] reviewed the treatment results of a series that combined EBRT and HDR-ISBT and reported that the 4–10 year biochemical control rate was 62–97 % for high-risk patients. These reports showed that HDR-ISBT as monotherapy elicited better treatment results than conventional EBRT to the pelvis plus prostate; they also showed that its efficacy was almost equivalent to that of combined HDR-ISBT and EBRT—even among high-risk patients.
With regard to toxicity profiles, HDR-ISBT as monotherapy showed outcomes equivocal to other treatments. For example, Zamboglou et al. [32] reported that the rate of late grade ≥ 3 genitourinary complications was 2–7 % for three types of dose fractionation schedules (◉ Table 3). The present study showed comparable results (4 %). Furthermore, Hoskin et al. [7] reported that the rate of late grade ≥ 3 gastrointestinal complications was 0–1 %; whereas Zamboglou et al. reported that this was 0.4–4.2 %. Similarly, one (2 %) grade 3 gastrointestinal complication was observed in the present study (◉ Table 3). Challapalli et al. [2] reviewed the late complication rates of combined EBRT and HDR-ISBT, and showed that the median grade ≥ 3 genitourinary toxicity rate was 4.5 % (range 0–14.4 %) and the median grade ≥ 3 gastrointestinal toxicity rate was 0.5 % (range 0–4.1 %). These reports showed that HDR-ISBT as monotherapy showed results similar to those of combined HDR-ISBT and EBRT.
Conclusion
Our results showed that HDR-ISBT combined with ADT demonstrates promising biochemical control, even in high-risk patients. In addition, the results showed that the late genitourinary and gastrointestinal complication rates were acceptable.
References
Barkati M, Williams SG, Foroudi F et al (2012) High-dose-rate brachytherapy as a monotherapy for favorable-risk prostate cancer: a Phase II trial. Int J Radiat Oncol Biol Phys 82:1889–1896
Challapalli A, Jones E, Harvey C et al (2012) High dose rate prostate brachytherapy: an overview of the rationale, experience and emerging applications in the treatment of prostate cancer. Br J Radiol 85:18–27
Demanes DJ, Martinez AA, Ghilezan M et al (2011) High-dose-rate monotherapy: safe and effective brachytherapy for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 81:1286–1292
Galalae RM, Kovacs G, Schultze J et al (2002) Long-term outcome after elective irradiation of the pelvic lymphatics and local dose escalation using high-dose-rate brachytherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys 52:81–90
Ghadjar P, Keller T, Rentsch CA et al (2009) Toxicity and early treatment outcomes in low- and intermediate-risk prostate cancer managed by high-dose-rate brachytherapy as a monotherapy. Brachytherapy 8:45–51
Grills IS, Martinez AA, Hollander M et al (2004) High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol 171:1098–1104
Hoskin P, Rojas A, Lowe G et al (2012) High-dose-rate brachytherapy alone for localized prostate cancer in patients at moderate or high risk of biochemical recurrence. Int J Radiat Oncol Biol Phys 82:1376–1384
Jacob R, Hanlon AL, Horwitz EM et al (2005) Role of prostate dose escalation in patients with greater than 15 % risk of pelvic lymph node involvement. Int J Radiat Oncol Biol Phys 61:695–701
Komiya A, Fujiuchi Y, Ito T et al (2013) Early quality of life outcomes in patients with prostate cancer managed by high-dose-rate brachytherapy as monotherapy. Int J Urol 20:185–192
Kovács G, Pötter R, Loch T et al (2005) GEC/ESTRO-EAU recommendations on temporary brachytherapy using stepping sources for localised prostate cancer. Radiother Oncol 74:137–148
Krauss DJ (2013) HDR brachytherapy alone for high-risk prostate cancer: a safe approach for patients with localized disease? Int J Radiat Oncol Biol Phys 85:1161–1162
Lawton CA, DeSilvio M, Roach M 3rd et al (2007) An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94–13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys 69:646–655
Martin T, Baltas D, Kurek R et al (2004) 3-D conformal HDR brachytherapy as monotherapy for localized prostate cancer. A pilot study. Strahlenther Onkol 180:225–232
Martinez A, Pataki I, Edmundson G et al (2001) Phase II prospective study of the use of conformal high-dose-rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer: a feasibility report. Int J Radiat Oncol Biol Phys 49:61–69
Mate TP, Gottesman JE, Hatton J et al (1998) High dose-rate afterloading Ir-192 prostate brachytherapy: feasibility report. Int J Radiat Oncol Biol Phys 41:525–533
Pommier P, Chabaud S, Lagrange JL et al (2007) Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol 25:5366–5373
Prada PJ, Jimenez I, González-Suárez H et al (2012) High-dose-rate interstitial brachytherapy as monotherapy in one fraction and transperineal hyaluronic acid injection into the perirectal fat for the treatment of favorable stage prostate cancer: treatment description and preliminary results. Brachytherapy 11:105–110
Roach M 3rd, DeSilvio M, Valicenti R et al (2006) Whole-pelvis, “mini-pelvis,” or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J Radiat Oncol Biol Phys 66:647–653
Rogers CL, Alder SC, Rogers RL et al (2012) High dose brachytherapy as monotherapy for intermediate risk prostate cancer. J Urol 187:109–116
Takenaka T, Yoshida K, Ueda M et al (2012) Assessment of daily needle applicator displacement during high-dose-rate interstitial brachytherapy for prostate cancer using daily CT examinations. J Radiat Res 53:469–474. http://www.ncbi.nlm.nih.gov/pubmed/22485020
Vargas CE, Demanes J, Boike TP et al (2006) Matched-pair analysis of prostate cancer patients with a high risk of positive pelvic lymph nodes treated with and without pelvic RT and high-dose radiation using high dose rate brachytherapy. Am J Clin Oncol 29:451–457
Yamada Y, Rogers L, Demanes DJ et al (2012) American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy 11:20–32
Yoshida K, Nose T, Shiomi H et al (2006) New ambulatory implant technique of high-dose-rate interstitial brachytherapy for prostate cancer. Radiat Med 24:595–599
Yoshida K, Kuroda S, Yoshida M et al (2007) New implant technique for separation of seminal vesicle and rectal mucosa for high-dose-rate prostate brachytherapy. Brachytherapy 6:180–186
Yoshida K, Yamazaki H, Nose T et al (2010) Needle applicator displacement during high-dose-rate interstitial brachytherapy for prostate cancer. Brachytherapy 9:36–41
Yoshida K, Nose T, Koizumi M et al (2002) The usefulness of metal markers for CTV-based dose prescription in high-dose-rate interstitial brachytherapy. J Jpn Soc Ther Radiol Oncol 13:253–260
Yoshioka Y, Konishi K, Sumida I et al (2011) Monotherapeutic high-dose-rate brachytherapy for prostate cancer: five-year results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int J Radiat Oncol Biol Phys 80:469–475
Yoshioka Y, Nose T, Yoshida K et al (2000) High-dose-rate interstitial brachytherapy as a monotherapy for localized prostate cancer: Treatment description and preliminary results of a phase I/II clinical trial. Int J Radiat Oncol Biol Phys 48:675–681
Yoshioka Y, Nose T, Yoshida K et al (2003) High-dose-rate brachytherapy as monotherapy for localized prostate cancer: a retrospective analysis with special focus on tolerance and chronic toxicity. Int J Radiat Oncol Biol Phys 56:213–220
Yoshioka Y, Konishi K, Oh RJ et al (2006) High-dose-rate brachytherapy without external beam irradiation for locally advanced prostate cancer. Radiother Oncol 80:62–68
Yoshioka Y, Suzuki O, Kobayashi K et al (2009) External-beam radiotherapy for clinically localized prostate cancer in Osaka, Japan, 1995–2006: time trends, outcome, and risk stratification. Strahlenther Onkol 185:446–452. http://www.ncbi.nlm.nih.gov/pubmed/19714306
Zamboglou N, Tselis N, Baltas D et al (2013) High-dose-rate interstitial brachytherapy as monotherapy for clinically localized prostate cancer: treatment evolution and mature results. Int J Radiat Oncol Biol Phys 85:672–678
Compliance with ethical guidelines
Conflict of interest
K. Yoshida, H. Yamazaki, T. Takenaka, T. Kotsuma, M. Yoshida, K. Masui, Y. Yoshioka, Y. Narumi, T. Oka and E. Tanaka state that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, K., Yamazaki, H., Takenaka, T. et al. High-dose-rate interstitial brachytherapy in combination with androgen deprivation therapy for prostate cancer. Strahlenther Onkol 190, 1015–1020 (2014). https://doi.org/10.1007/s00066-014-0675-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0675-4