Abstract
Background
No standard treatment exists for locally advanced prostate cancer (PC). This study evaluated the long-term treatment outcomes and toxicity in patients with clinically locally advanced and/or lymph node (LN)-positive PC who underwent high-dose-rate brachytherapy (HDR-BT) with external beam radiotherapy (EBRT).
Methods
The treatment outcomes and toxicities of 152 patients with PC who underwent HDR-BT with EBRT and had at least 2 years of observation were examined. The treatment dose was 19- and 13-Gy HDR-BT in two and single fractions, respectively, both combined with external irradiation of 46 Gy in 23 fractions. Long-term androgen deprivation therapy (ADT) for patients harboring very high-risk tumors was used in combination.
Results
The median observation period was 59.7 (24.4–182.1) months. The 5-year prostate cancer-specific and recurrence-free (RFS) survival rates were 99.0% and 91.8%, respectively, with only two PC mortalities. When 5-year RFS was examined for each parameter, RFS was significantly lower in pre-radiotherapy (pre-RT) prostate-specific antigen (PSA) > 0.5 ng/mL (77.1%; p = 0.008), and presence of LN metastasis (68.1%; p = 0.017). Multivariable analysis demonstrated that pre-RT PSA (HR, 4.68; 95% CI, 1.39–15.67; p = 0.012) and presence of LN metastasis (HR, 4.70; 95% CI, 1.24–17.74; p = 0.022) were independent recurrence predictors. The 5-year cumulative incidence rate of grade ≥ 2 toxicities in genitourinary and gastrointestinal tracts were 15.4% and 1.3%, respectively.
Conclusions
HDR-BT combined with EBRT and long-term ADT shows promising disease control and tolerant toxicities for clinically locally advanced and LN-positive PC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the introduction of prostate cancer (PC) screening, more men are continuously being diagnosed with clinically nonmetastatic PC. However, 17–31% of these men have high-risk localized or locally advanced disease requiring curative treatment [1]. Although most high-risk patients respond well to localized curative treatment with the goal of cure, especially for patients with locally advanced disease, clinical stage (c) T3–4 or confirmed lymph node (LN) metastasis, cancer progresses and leads to mortality. Locally advanced PC is usually treated with a combination of local and systemic treatment, mainly androgen deprivation therapy (ADT). However, currently, no standardized treatment exists. Furthermore, no direct comparative studies exist regarding the choice of local treatment, such as radical prostatectomy (RP) and radiotherapy (RT). Thus, the superiority or inferiority of these treatments is unknown.
High-dose-rate brachytherapy (HDR-BT) is an effective treatment modality that can be used either alone or in combination with external beam RT (EBRT) for patients with localized PC [2,3,4,5,6,7,8]. In contrast, few clinical studies have shown the efficacy of HDR-BT for locally advanced very high-risk PC. A previous study has barely reported the efficacy of HDR-BT with EBRT and long-term ADT in a small number of cases with a short observation period [9,10,11]. This study aimed to evaluate the long-term outcome and safety of patients with locally advanced and/or LN-positive PC who underwent HDR-BT with EBRT at Kanazawa University Hospital.
Materials and methods
Patients
Patients with PC who were treated by HDR-BT with EBRT between January 2005 and December 2018 at Kanazawa University Hospital and followed up for at least 2 years were included in this study. The treatment outcomes of 152 patients were retrospectively analyzed using their medical records. This study was approved by the Medical Ethics Committee of Kanazawa University.
Lesions were categorized according to the tumor–node–metastasis (TNM) classification [12]. All patients with cT3–4 or LN-positive PC were stratified into locally advanced high-risk PC according to the European Association of Urology (EAU) guidelines [13] based on clinical TNM stage, initial serum prostate-specific antigen (PSA) level, and grade groups according to the International Society of Urological Pathology (ISUP). Neoadjuvant ADT was generally administered to decrease prostate volume (< 50 mL) and prevent disease progression while awaiting treatment for 6 months. Adjuvant ADT for 2 years was recommended for patients with two or more high-risk factors according to localized high-risk categories of EAU, or cT3b–4 and/or LN-positive. Although in the case of regional LN metastasis, HDR-BT is indicated regardless of the size or number of LNs, HDR-BT is not indicated in the case of LN metastasis not in the external irradiation field to the pelvis. The clinical characteristics of all patients with LN-positive PC are shown in supplementary file 1.
High-dose-rate brachytherapy
Interstitial catheter implantation is usually conducted under spinal anesthesia in awake patients with transrectal ultrasound guidance in the lithotomy position using a perineal template in an operating room. Even if the invasion was present in the seminal vesicles or bladder, the current study consciously punctured the applicators into those areas and irradiated the invaded areas. Three gold markers were inserted to mark the bilateral base and apex of the prostate. The patient was transferred to a computed tomography table for treatment planning after catheter implantation. Treatment planning was conducted using a treatment planning system (Oncentra Brachy, Elekta AB, Stockholm, Sweden) according to the following dose constraints: prostate volume receiving 100% of the dose (> 90%), urethral volume receiving 125% of the dose (< 1 cm3), urethral volume receiving 150% of the dose (0 cm3), rectal volume receiving 75% of the dose (< 1 cm3), and rectal volume receiving 100% of the dose (0 cm3) based on the protocol of the radiotherapist [14]. The patient was then transferred to a treatment table in an HDR unit room, and irradiation was conducted using an 192Ir remote afterloading system (microSelectron, Nucletron, Veenendaal, the Netherlands) at 19 Gy in two fractions (2005 to March 2014) or 13 Gy in single fraction (from April 2014). The needles were removed from the patient in the bed after the irradiation session, and the 20-French triple-lumen urethral catheter with continuous irrigation with saline was left intact until the next day. The urethral catheter was removed, and the patient was released the following day.
External beam radiotherapy
EBRT delivering 46 Gy in 23 fractions was initiated using intensity-modulated RT usually 1 or 2 weeks after the HDR procedure and performed with a Monaco treatment planning system (Elekta AB). The linear accelerator was an Elekta Synergy (Elekta AB) [14]. Irradiation to the small pelvic cavity comprised internal/external iliac, obturator, and anterior sacral LN and surely included in the external beam fields for pelvic LN metastases. Although it has been reported that local control was enabled so that biologically effective dose (BED) was high and the biochemical freedom from failure was higher when the delivered BED was > 220 Gy [15], BED in the current protocol corresponded to 246 (19 Gy in two fractions) and 233 (13 Gy in a single fraction) Gy using an α/β ratio of 1.5 Gy.
Toxicity evaluation
Toxicities were recorded according to the Common Terminology Criteria for Adverse Events v5.0. Adverse events are events ≤ 3 or ≥ 3 months as acute or late toxicities, respectively.
Statistical analyses
Intervals for survival in this study were calculated from the first day of irradiation treatment to the event. Recurrence was defined as clinical and biochemical recurrence based on the Phoenix criteria [16]. Recurrence-free survival (RFS), cancer-specific survival (CSS), and cumulative incidence of toxicities were estimated using the Kaplan–Meier method. In addition, the differences were compared using the log-rank test. A Cox proportional hazards model was used for multivariate analysis. The cut-lines of clinicopathological factors were explored by binary or using receiver operating characteristic curve analysis. Statistical analyses were performed using GraphPad Prism version 6.07 (GraphPad Software Inc., San Diego, CA, USA) and IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as a p value of < 0.05.
Results
Patient’s background
Patient characteristics are shown in Table 1. The median follow-up duration was 59.7 (range, 24.4–182.1) months, and the median age of the patients was 69 (range, 50–82) years. Of the 152 patients included in this study, 75 (49.3%), 60 (39.5%) and 17 (11.2%) were in clinical stage T3a, T3b, and T4, respectively. Regional LN metastasis was included in 14 (9.2%). The median initial PSA level was 21.0 (range, 3.7–557.6) ng/mL. The ISUP grade groups were 1 (4; 2.6%), 2 (15; 9.9%), 3 (26; 17.1%), 4 (49; 32.2%), 5 (57; 37.5%), and unknown (1; 0.7%). All patients underwent neoadjuvant ADT for a median of 6 months, and 131 patients (86.2%) received adjuvant ADT for 2 years.
Treatment outcomes
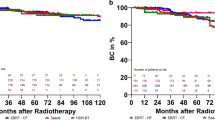
In the present study, 12 patients had disease recurrences (3 clinical recurrences and 9 biochemical recurrences) during the observation period. Clinical recurrences occurred in the ribs, sacrum, and perineum (the insertion route of the applicator). The 5-year RFS and CSS rates were 91.8% and 99.0%, respectively. (Fig. 1). The median time to biochemical recurrence was 41.9 (range, 21.4–114.4) months, and almost all patients had a recurrence during the off-phase of ADT. Moreover, mortalities occurred in five patients, including two PC mortalities. The other causes of deaths were due to hematopoietic tumors and biliary tract cancer.
Prognostic factors related to treatment outcomes
According to clinical and oncological parameters as shown in Fig. 2, pre-radiotherapy (pre-RT) PSA > 0.5 ng/mL (p = 0.008) and LN metastasis (p = 0.017) were significantly worse prognosis for RFS. As shown in Table 2, the results of the multivariate analysis of prognostic factors showed that pre-RT PSA > 0.5 ng/mL (hazard ratio [HR], 4.68; 95% confidence interval [CI], 1.39–15.67; p = 0.012) and presence of LN metastasis (HR, 4.70; 95% CI, 1.24–17.74; p = 0.022) were independent predictors of recurrence.
Treatment-related toxicities
Table 3 shows the treatment-related toxicities. The most frequent acute genitourinary (GU) complications were pollakisuria (24.3%), followed by urgency/incontinence (5.9%) and urethral retention (5.9%), both below grade 2 toxicities. Only one patient (0.7%) experienced grade 3 hematuria. Acute gastrointestinal (GI) complications were mainly observed as diarrhea (17.7%), followed by anal pain (2.6%), both below grade 2 toxicities. Although 11 patients (7.3%) experienced hematuria in the late phase, one patient (0.7%) had gross hematuria requiring transfusion. Urethral stricture and rectal hemorrhage, which are serious late complications, occurred in 6.0% and 2.0% of patients, respectively. In contrast, urethral stricture, which was often observed as an adverse effect of conventional fractionated HDR-BT, was not observed in the single-fraction irradiation era (data not shown). The 5-year cumulative incidence rate of grade ≥ 2 GU and GI toxicities were 15.4% and 1.3%, respectively (Fig. 3).
Discussion
Although the development of new imaging techniques has facilitated the diagnosis of locally advanced and LN-positive PC, the absence of standardized treatment is a current problem. Furthermore, no direct comparative randomized clinical trials exist on the choice of local treatment (RP or RT). Thus, the superiority or inferiority is unknown. However, many large-scale studies in recent years have demonstrated that combined ADT and RT are more effective than ADT alone for locally advanced PC [17,18,19,20]. In addition, the duration of ADT was reported to be superior in terms of local control and prognosis with long-term treatment (24–36 months) compared with short-term treatment (6 months or less) [19]. Thus, the significance of combined long-term ADT and RT for locally advanced PC has been established. Moreover, recently in Japan, open-label, randomized, phase 3 trials involving patients with locally advanced PC were conducted to investigate the management of treatment with adjuvant ADT concomitant with neoadjuvant ADT and EBRT [21]. Data showed that 5-year modified biochemical RFS rates were 84.8% and 82.8% for the patients assigned to long-term or intermittent adjuvant ADT, respectively (p = 0.5619), with the median follow-up time of 8.2 years.
However, the options for RT are diversifying, and which irradiation method is optimal is currently unclear. Radiobiologically, an α/β value of PC is as low as 1.5 Gy. Thus, RT for PC is considered to be more effective with higher doses in smaller fractions [22, 23]. Therefore, the efficacy of HDR-BT, which can deliver a high dose per irradiation, has been recognized.
Currently, few studies exist on multimodal therapy, HDR-BT with EBRT, and long-term ADT for patients with locally advanced high-risk PC. The present study showed a lower rate of disease recurrence and higher survival prognosis even after an overwhelmingly high number of very high-risk tumors and a long observation period compared to previous studies [2, 10, 24]. Interestingly, the current findings suggest that pre-RT PSA and the presence of LN metastasis could be used as predictive tools for patients who underwent HDR-BT. Several studies have shown that PSA nadir values after neoadjuvant ADT influences long-term biochemical RFS and could lead to more important survival outcomes (e.g., distant metastasis-free survival [DMFS], CSS, and overall survival [OS]) [25]. PSA nadir values cutoff points used in these studies ranged between 0.1 and 2.5 ng/mL, with the majority of the series reporting on PSA values of ≤ 0.5 ng/mL as discriminatory PSA level. McGuire et al. found that a PSA cutoff value of 0.5 ng/mL was the best discriminator for biochemical RFS, DMFS, CSS, and OS in high-risk PC treated in the era of dose-escalating RT (≥ 74 Gy) and long-term ADT [26]. The present study also showed that pre-RT PSA > 0.5 ng/mL had a significantly worse prognosis for disease control. Considering the new prognostic value of the biochemical response to neoadjuvant ADT among patients with PC treated with RT, the initial PSA response to ADT may be argued to indirectly reflect tumor sensitivity to hormone removal, intrinsic hormone-mediated radiosensitivity of tumor cells [25], or the existence of more aggressive hormone-insensitive cancer cells. Thus, predicting inherent radiosensitivity in the early stages of ADT may assist clinicians in their practice during follow-up after curative RT. In contrast, although imagining that the presence of LN metastasis is a poor prognostic factor is not difficult, only 3 of 14 (21%) patients with LN-positive had recurrence in the current study. Thus, even in the presence of LN metastasis, certain cases with such multidisciplinary treatment will result in a complete cure.
Although acute adverse events are mainly GU (e.g., low-grade pollakisuria and urgency/incontinence) and GI (e.g., low-grade diarrhea) toxicities, almost all of them are easily managed by temporary medication. In addition, grade 3 GU and GI toxicities were observed in only 4.0% of the patients during the acute and late phases. Therefore, this treatment is considered to be safe and well-tolerated. As the current study is a cohort of patients with locally advanced high-risk PC, receiving combined modality treatment of hormonal therapy and EBRT may contribute to acute GU and GI toxicities. In contrast, late adverse events that may be problematic were urethral stricture, hematuria, and rectal hemorrhage. Moreover, several studies have reported urethral stricture cases following conventional HDR-BT with EBRT, with a urethral stricture rate of 2–10% [5, 27, 28]. A previous study (19 Gy in two fractions of HDR-BT) showed a similar rate of urethral stricture (8.8%) wherein most urethral stricture occurred within 2 years of HDR-BT [4]. In contrast, urethral stricture has not been observed during a single fraction of the HDR-BT era [9]. Although the migration of applicators during two fractions of HDR-BT may cause excess irradiation to a distal portion of the urethral sphincter, a single fraction of HDR-BT may generally reduce the risk of migration of applicators. In addition, an α/β ratio of 1.5 Gy in PC, which is lower than an α/β ratio of 3–5 Gy in the normal adjacent organs (i.e., bladder, urethra, and rectum), may work in favor of the urethra in single irradiation.
The present study is limited by its retrospective, single-center, and nonrandomized design and the small number of events. Thus, caution should be taken in interpreting the results of statistical analyses. Furthermore, the current study should be considered to adapt an α/β ratio of 1.5 Gy unlike the BED by Stone et al., which was an important parameter of biochemical recurrence using an α/β ratio of 2 Gy [15]. This study is also limited by the fact that the differences potentially contributing to GU and GI toxicities between the radiation dose from interstitial irradiation and the external beam component cannot be evaluated. However, the current result demonstrates that multimodal therapy combining HDR-BT with EBRT and long-term ADT facilitates the improvement of disease control in locally advanced and LN-positive PC and can be delivered with tolerant toxicities.
In conclusion, the treatment outcomes of patients with locally advanced and LN-positive PC who undergo HDR-BT in combination with EBRT and long-term ADT have been reported. Although the present study shows excellent results in terms of disease control and safety, a longer follow-up period is required to assess long-term survival.
References
Cooperberg MR, Cowan J, Broering JM et al (2008) High-risk prostate cancer in the United States, 1990–2007. World J Urol 26(3):211–218. https://doi.org/10.1007/s00345-008-0250-7
Ishiyama H, Kamitani N, Kawamura H et al (2017) Nationwide multi-institutional retrospective analysis of high-dose-rate brachytherapy combined with external beam radiotherapy for localized prostate cancer: an Asian Prostate HDR-BT Consortium. Brachytherapy 16(3):503–510. https://doi.org/10.1016/j.brachy.2017.01.006
Yoshioka Y, Kotsuma T, Komiya A et al (2017) Nationwide, multicenter, retrospective study on high-dose-rate brachytherapy as monotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 97(5):952–961. https://doi.org/10.1016/j.ijrobp.2016.12.013
Makino T, Mizokami A, Namiki M (2015) Clinical outcomes of patients with localized and locally advanced prostate cancer undergoing high-dose-rate brachytherapy with external-beam radiotherapy at our institute. Anticancer Res 35(3):1723–1728
Hoskin PJ, Rojas AM, Bownes PJ et al (2012) Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol 103(2):217–222. https://doi.org/10.1016/j.radonc.2012.01.007
Hsu IC, Rodgers JP, Shinohara K et al (2021) Long-term results of nrg oncology/RTOG 0321: a phase II trial of combined high dose rate brachytherapy and external beam radiation therapy for adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 110(3):700–707. https://doi.org/10.1016/j.ijrobp.2020.11.008
Wedde TB, Smastuen MC, Brabrand S et al (2019) Ten-year survival after high-dose-rate brachytherapy combined with external beam radiation therapy in high-risk prostate cancer: a comparison with the Norwegian SPCG-7 cohort. Radiother Oncol 132:211–217. https://doi.org/10.1016/j.radonc.2018.10.013
Astrom L, Grusell E, Sandin F et al (2018) Two decades of high dose rate brachytherapy with external beam radiotherapy for prostate cancer. Radiother Oncol 127(1):81–87. https://doi.org/10.1016/j.radonc.2017.12.025
Makino T, Nakashima K, Iijima M et al (2019) Health-related quality of life and toxicity after single-fraction high-dose-rate brachytherapy with external beam radiotherapy for localized and locally advanced prostate cancer. Anticancer Res 39(1):477–486. https://doi.org/10.21873/anticanres.13137
Kasahara T, Ishizaki F, Kazama A et al (2020) High-dose-rate brachytherapy and hypofractionated external beam radiotherapy combined with long-term androgen deprivation therapy for very high-risk prostate cancer. Int J Urol 27(9):800–806. https://doi.org/10.1111/iju.14305
Yamazaki H, Suzuki G, Masui K et al (2021) Radiotherapy for clinically localized T3b or T4 very-high-risk prostate cancer-role of dose escalation using high-dose-rate brachytherapy boost or high dose intensity modulated radiotherapy. Cancers (Basel) 13(8):1856. https://doi.org/10.3390/cancers13081856
Brierley J, Gospodarowicz M, Wittekind C (2016) The TNM classification of malignant tumours, 8th edn. Wiley-Blackwell, United States
Mottet N, van den Bergh RCN, Briers E et al (2021) EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 79(2):243–262. https://doi.org/10.1016/j.eururo.2020.09.042
Sakurai T, Takamatsu S, Shibata S et al (2020) Toxicity and clinical outcomes of single-fraction high-dose-rate brachytherapy combined with external beam radiotherapy for high-/very high-risk prostate cancer: a dosimetric analysis of toxicity. Jpn J Radiol 38(12):1197–1208. https://doi.org/10.1007/s11604-020-01023-2
Stone NN, Potters L, Davis BJ et al (2009) Multicenter analysis of effect of high biologic effective dose on biochemical failure and survival outcomes in patients with Gleason score 7–10 prostate cancer treated with permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 73(2):341–346. https://doi.org/10.1016/j.ijrobp.2008.04.038
Roach M 3rd, Hanks G, Thames H Jr et al (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO phoenix consensus conference. Int J Radiat Oncol Biol Phys 65(4):965–974. https://doi.org/10.1016/j.ijrobp.2006.04.029
Bolla M, Van Tienhoven G, Warde P et al (2010) External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 11(11):1066–1073. https://doi.org/10.1016/s1470-2045(10)70223-0
Roach M 3rd, Bae K, Speight J et al (2008) Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol 26(4):585–591. https://doi.org/10.1200/JCO.2007.13.9881
Horwitz EM, Bae K, Hanks GE et al (2008) Ten-year follow-up of radiation therapy oncology group protocol 92–02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 26(15):2497–2504. https://doi.org/10.1200/JCO.2007.14.9021
Fossa SD, Wiklund F, Klepp O et al (2016) Ten- and 15-yr prostate cancer-specific mortality in patients with nonmetastatic locally advanced or aggressive intermediate prostate cancer, randomized to lifelong endocrine treatment alone or combined with radiotherapy: final results of the Scandinavian prostate cancer group-7. Eur Urol 70(4):684–691. https://doi.org/10.1016/j.eururo.2016.03.021
Ito K, Kobayashi M, Komiyama M et al (2020) Oncological outcomes for patients with locally advanced prostate cancer treated with neoadjuvant endocrine and external-beam radiation therapy followed by adjuvant continuous/intermittent endocrine therapy in an open-label, randomized, phase 3 trial. Cancer 126(17):3961–3971. https://doi.org/10.1002/cncr.33034
Brenner DJ, Martinez AA, Edmundson GK et al (2002) Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 52(1):6–13. https://doi.org/10.1016/s0360-3016(01)02664-5
Brenner DJ, Hall EJ (1999) Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 43(5):1095–1101. https://doi.org/10.1016/s0360-3016(98)00438-6
Boladeras A, Santorsa L, Gutierrez C et al (2014) External beam radiotherapy plus single-fraction high dose rate brachytherapy in the treatment of locally advanced prostate cancer. Radiother Oncol 112(2):227–232. https://doi.org/10.1016/j.radonc.2014.07.013
Zilli T, Dal Pra A, Kountouri M et al (2016) Prognostic value of biochemical response to neoadjuvant androgen deprivation before external beam radiotherapy for prostate cancer:a systematic review of the literature. Cancer Treat Rev 46:35–41. https://doi.org/10.1016/j.ctrv.2016.03.016
McGuire SE, Lee AK, Cerne JZ et al (2013) PSA response to neoadjuvant androgen deprivation therapy is a strong independent predictor of survival in high-risk prostate cancer in the dose-escalated radiation therapy era. Int J Radiat Oncol Biol Phys 85(1):e39-46. https://doi.org/10.1016/j.ijrobp.2012.08.036
Sullivan L, Williams SG, Tai KH et al (2009) Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol 91(2):232–236. https://doi.org/10.1016/j.radonc.2008.11.013
Ishiyama H, Satoh T, Kitano M et al (2014) High-dose-rate brachytherapy and hypofractionated external beam radiotherapy combined with long-term hormonal therapy for high-risk and very high-risk prostate cancer: outcomes after 5-year follow-up. J Radiat Res 55(3):509–517. https://doi.org/10.1093/jrr/rrt128
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Makino, T., Sakurai, T., Takamatsu, S. et al. The effectiveness of high-dose-rate brachytherapy with external beam radiotherapy for clinically locally advanced and node-positive prostate cancer: long-term results of a retrospective study. Int J Clin Oncol 26, 2310–2317 (2021). https://doi.org/10.1007/s10147-021-02023-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02023-6