Abstract
Background or purpose
A joint analysis of data from three contributing centres within the intraoperative electron-beam radiation therapy (IOERT) Spanish program was performed to investigate the main contributions of IORT to the multidisciplinary treatment of high-risk extremity soft tissue sarcoma (STS).
Methods and materials
Patients with an histologic diagnosis of primary extremity STS, with absence of distant metastases, undergoing limb-sparing surgery with radical intent, external beam radiotherapy (median dose 45 Gy) and IOERT (median dose 12.5 Gy) were considered eligible for participation in this study.
Results
From 1986–2012, a total of 159 patients were analysed in the study from three Spanish institutions. With a median follow-up time of 53 months (range 4–316 years), 5-year local control (LC) was 82 %. The 5-year IOERT in-field control, disease-free survival (DFS) and overall survival (OS) were 86, 62 and 72 %, respectively. On multivariate analysis, only microscopically involved margin (R1) resection status retained significance in relation to LC (HR 5.20, p < 0.001). With regard to IOERT in-field control, incomplete resection (HR 4.88, p = 0.001) and higher IOERT dose (≥ 12.5 Gy; HR 0.32, p = 0.02) retained a significant association in multivariate analysis.
Conclusion
From this joint analysis emerges the fact that an IOERT dose ≥ 12.5 Gy increases the rate of IOERT in-field control, but DFS remains modest, given the high risk of distant metastases. Intensified local treatment needs to be tested in the context of more efficient concurrent, neo- and adjuvant systemic therapy.
Zusammenfassung
Ziel
Um den therapeutischen Beitrag einer intraoperativen Bestrahlung mit Elektronen (IOERT) als Teil eines multidisziplinären Behandlungskonzepts von Weichteilsarkomen (STS) im Extremitätenbereich mit hohem Risikoprofil evaluieren zu können, wurde anhand des spanischen IOERT-Programms eine gepoolte Datenanalyse von drei teilnehmenden Zentren vorgenommen.
Patienten und Methoden
Eingeschlossen in diese Studie wurden Patienten mit histologisch bestätigtem primären STS der Extremitäten ohne Fernmetastasierung, welche nach radikaler extremitätenerhaltenden Operation eine externe Radiotherapie (mediane Dosis 45 Gy) in Kombination mit einer IOERT (mediane Dosis 12,5 Gy) erhielten.
Ergebnisse
In einem Zeitraum von 1986–2012 wurden insgesamt 159 Patienten ausgewertet. Bei einer medianen Nachbeobachtungszeit von 53 Monaten (Spanne 4–316 Monate) wurde eine Lokalkontrolle (LC) nach 5 Jahren von 82 % errechnet. Die 5-Jahres-Raten der LC innerhalb des IOERT-Felds, das krankheitsfreie Überleben (DFS) und das Gesamtüberleben (OS) lagen entsprechend bei 86, 62 und 72 %. In multivariaten Analysen erwiesen sich lediglich mikroskopisch positive Resektionsränder (R1) als signifikant prädiktiv hinsichtlich der LC (HR 5,20; p < 0,001). Innerhalb des ehemaligen IOERT-Felds zeigte in der multivariaten Analyse neben der inkompletten Resektion (HR 4,88; p = 0,001) auch die höhere IOERT-Dosis ≥ 12,5 Gy (HR 0,32; p = 0,02) einen statistisch signifikanten Einfluss.
Schlussfolgerung
Die Ergebnisse aus dieser multiinstitutionellen Analyse lassen den Schluss zu, dass IOERT-Dosen ≥ 12,5 Gy die lokale Kontrollrate im ehemaligen IOERT-Bestrahlungsfeld erhöhen, bei jedoch insgesamt moderatem DFS aufgrund des hohen Metastasierungsrisikos bei dieser Art der Sarkomerkrankung. Diese Behandlungsoption zur intensivierten Erhöhung der LC sollte mit effizienterer konkomitanter, neo- und adjuvanter Systemtherapie weiter untersucht werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Felipe A. Calvo and Claudio V. Sole contributed equally to this work.
Successful treatment of high-risk soft tissue sarcomas (STS) of the extremities remains challenging in the 21st century [1]. External-beam radiation therapy (EBRT) combined with limb-sparing surgery local control (LC) rates are comparable to those achieved with amputation [2]. The benefit of adding EBRT to limb-sparing surgery has been addressed in two randomized trials; both showed that combined treatment reduced the risk of local recurrence by 20–25 % when compared to limb-sparing surgery alone [3, 4]. Thus, the use of adjuvant EBRT maximizes functional and cancer outcomes without the significant morbidity and cosmetic deformity of radical surgery [4]. Margin status has been reported to be the most important prognostic factor for LC even in patients treated with combined surgery and radiotherapy [5]. Complete negative margin resection can sometimes not be achieved due to close proximity or proven invasion into adjacent unresectable structures [6, 7]. Therefore, higher EBRT doses are sometimes used to compensate for close or positive margins.
Total escalated doses of radiotherapy that can be delivered even with the most sophisticated and updated EBRT precision techniques is limited by the presence of dose-limiting surrounding organs or structures in the planning treatment volume (PTV) [8]. Accordingly, high radiation therapy doses delivered with EBRT have often been associated with significant late toxicity [9]. Because a cumulative EBRT dose of 64 Gy or greater is needed in the positive and/or close margin setting [10], this scenario is an ideal situation to consider intraoperative electron-beam radiation therapy (IOERT) as a component of treatment, as this modality has the advantage of delivering a high boost dose to deep-seated sarcoma residues or risk surgical bed areas adjacent to radiosensitive critical organs by mobilizing these structures temporarily out of the radiation field [11]. Since 1986, three Spanish institutions have approached the treatment of patients with extremity STS using an IOERT-boost component in high-risk areas (post-resection and pre-reconstruction), EBRT and limb-sparing surgery. In this study, a joint analysis of data from three institutions was performed in order to evaluate, on a large and mature cohort of patients, evidence of the contribution of an IOERT-containing multimodality approach in promoting LC with acceptable tolerance.
Materials and methods
Patient characteristics and staging evaluation
From June 1986 to April 2012, patients aged ≥ 16 years (Karnofsky performance status ≥ 70) with pathologically confirmed [macroscopically resected (non-R2)] nonmetastatic extremity STS were eligible for multimodal treatment. Patients (n = 159) with primary (nonrecurrent) tumours, with either close (< 1 cm) and/or positive surgical margins (limb-preserving surgery) underwent EBRT and IOERT. Additionally, during the study period, 95 patients (with margins ≥ 1 cm) were treated exclusively with surgical resection and postoperative EBRT. Patients with the diagnosis of desmoid tumour, dermatofibrosarcoma protuberans, rhabdomyosarcoma and peripheral neuroectodermal tumour were not included in the study, the former two because of the often indolent clinical course, and the latter two from the well-known radioresponsiveness. Pretreatment evaluation consisted of a complete history and physical examination, complete blood count, renal and liver function tests, chest X-ray, and computerized tomography (CT) or magnetic resonance imaging (MRI) of the tumour site, chest and abdomen. Data were prospectively collected and retrospectively analysed at the time of scheduled follow-up. Patients were reclassified according to the 7th AJCC/UICC staging system for the analysis. Patient and treatment characteristics are listed in ◉ Table 1; there were no significant differences in baseline variables between the patients treated for lower and upper extremity STS. The protocol followed the recommendations of the Declaration of Helsinki. The Institutional Ethics Committee approved the protocol, and signed informed consent was obtained from all patients.
Treatment characteristics
Details of EBRT technique, IOERT and adjuvant chemotherapy (CT) followed previously described standards [11]. A total median EBRT dose of 45 Gy (range 40–54 Gy; 1.8–2.0 Gy/5 days/week) was applied postoperatively [79 %, 45 Gy (range 40–54 Gy)] or preoperatively [21 %, 45 Gy (range 40–50 Gy)] and delivered with megavoltage equipment (6 to 15 MV) using a three-dimensional (3D) conformal field technique. The technique for the EBRT component consisted of conventional (2D-RT) EBRT for patients treated between 1986 and 1992 (n = 28, 18 %) and conformal (3D-CRT) EBRT for patients treated after 1992 (n = 131, 82 %). PTV for 2D-RT was defined as tumour bed plus 3 cm in the radial directions in all cases, and for the longitudinal directions a 5 cm margin was applied. Clinical target volume (CTV) for the 3D-CRT technique included the surgical tumour bed plus a 2 cm margin in the radial directions and a 3 cm margin in the longitudinal (proximal and distal) directions, while the PTV was defined as CTV plus a 1 cm margin in the longitudinal and radial directions. At least one third of the circumference of the extremity was spared from irradiation to prevent development of chronic lymphedema. Surgical procedures (4–6 weeks before postoperative or after preoperative treatment) were categorized as marginal resection [(n = 19, 12 %) defined as resection through the tumour pseudocapsule or surrounding reactive tissue] or wide [(n = 140, 88 %) resection including normal tissue]. In all, 40 patients (25 %) underwent a tumour bed re-excision after prior excision, excisional biopsy, or intralesional surgical procedure. The remaining 119 patients (75 %) underwent a single attempt at definitive resection after incisional or core needle biopsy. For patients who had more than one procedure, the most radical procedure is listed. The IOERT program was performed in a non-dedicated linear accelerator with outpatient radiotherapy activity by the three institutions. After surgery and before reconstruction, 10–20 Gy (median 12.5 Gy) were delivered in a single fraction to one- (n = 128, 81 %) or two-field (n = 31, 19 %) PTVs, using a median energy of 6 MeV (range 4–20 MeV) (◉ Table 2). Dose was prescribed to the 90 % isodose line, covering the entire surgical bed. The intraoperative margin status was assessed using frozen pathologic sections. The IOERT dose was chosen according to the margin status and surgical bed volumes. Beveled (15–45º) Lucite circular applicators (size range 5–15 cm) were adjusted to collimate the target surface air gap, allowing dosimetric adaptation and uniform dose distribution. CT-guided treatment has been available since 2008 for IOERT planning [12]. Patients with higher histologic grade (grade 3) and tumour size (≥ 5 cm) were offered adjuvant CT (most commonly CT consisted of 4 or 5 cycles of doxorubicin 75 mg/m2 and ifosfamide 5 g/m2, every 3 weeks).
Follow-up and toxicity evaluation
All patients were required to be followed according to a common protocol every 3 months after treatment completion for the initial 3 years and every 6 months for 3 additional years thereafter. Patients were restaged 4 weeks after EBRT and routinely every 6 months with chest X-ray, and CT or MRI of the initial tumour site. Acute and late toxicities were evaluated according to Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer score [13].
Statistical analysis
The collected data were analyzed using SPSS (version 19.0) statistical software. The primary endpoint was LC. Secondary endpoints included IOERT in-field control, disease-free survival (DFS) and overall survival (OS).
The Kaplan–Meier method was used to estimate LC, IOERT in-field control, DFS and OS probabilities (all time-to-event end points were defined as the time from treatment initiation to event or the day of last follow-up). For survival outcomes potential associations were assessed in univariate and multivariate analysis using the Cox proportional hazards model. Based on, first, p values ≤ 0.10 in univariate analyses, and second, on clinical relevance, multivariate analysis was performed using a stepwise regression model to identify variables that have an effect (two-sided p test ≤ 0.05) on survival outcomes.
Results
Median follow-up time for all patients was 53 months (range 4–316 months). A total of 118 patients were alive at the time of analysis. Median follow-up for surviving patients was 67 months (range 4–316 months). Of the 41 deceased patients, 37 (90 %) died from cancer progression, and 4 (10 %) died from causes unrelated to their tumours or treatment. Crude local relapse (LR) rate was 16 % (n = 25), IOERT in-field rate was 12 % (n = 19) and 30 % (n = 48) developed distant metastases [most commonly pulmonary (n = 29, 60 %)]. Of the 25 patients who had local progression, 8 (5 %) were rescued with extremity amputation. Actuarial 5-year amputation-free survival was 94 %. The other 17 patients (15 had synchronic distant metastases) with local relapse underwent wide excision (n = 1), received chemotherapy alone (n = 14), or received no therapy (n = 2).
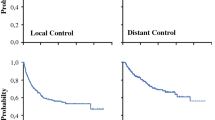
Actuarial local control for the study population at 5 and 10 years was 82 and 81 % (Supplemental Fig. 1a). Univariate Cox proportional hazard analyses showed that an R1 resection (Fig. 1e (p = 0.001)) and marginal excision (p = 0.05) were associated with a higher probability of LR (◉ Table 3). After adjustment for other covariates only R1 resection (p < 0.001) remained significantly associated with LR (◉ Table 4). We then evaluated patients with upper and lower extremity STS separately. For the subset with lower extremity STS (86 %), patients with R1 resections experienced a significantly higher risk of LR in univariate analysis (HR 3.67; 95 %CI 1.54–8.76; p = 0.003). Alternatively, for the subset with upper extremity STS (14 %), univariate analysis did not show that patients with R1 resection status had an increased risk of LR (HR 2.02; 95 %CI 0.54–7.02; p = 0.35). Actuarial IOERT in field-control at 5 and 10 years was 86 and 85 % (Supplemental Fig. 1b). Univariate analyses showed that R1 resection (p = 0.004) was associated with a higher probability of IOERT in-field relapse (◉ Table 3). An IOERT boost dose ≥ 12.5 Gy (Fig. 1f; p = 0.01) was associated with a lower probability of IOERT in-field relapse. In multivariate analysis an R1 resection (p = 0.001) and higher IOERT dose (≥ 12.5 Gy) retained a significant association with regard to IOERT in-field relapse (◉ Table 4). When IOERT dose was evaluated separately in patients with R0 and R1 resection margin status, we found that only for the subset of patients with R0 resections (84 %), receiving an IOERT dose ≥ 12.5 Gy was associated with a lower probability of IOERT in field relapse (HR 0.14; 95 %CI 0.02–0.98; p = 0.05). Actuarial DFS at 5 and 10 years was 62 and 57 % (Supplemental Fig. 1c). Univariate Cox proportional hazard analyses showed that stage III (p = 0.02) and R1 margin status (p = 0.004) were associated with a higher probability of overall metastases (◉ Table 3). After adjustment for other covariates stage III (p = 0.008) and R1 resection (p = 0.001) retained a significant association with DFS (◉ Table 4). Actuarial OS at 5 and 10 years was 72 and 64 % (Supplemental Fig. 1d). On univariate analysis, only stage III patients (p = 0.04) were at a significantly higher risk of overall death (◉ Table 3). We found on multivariate analysis that stage III (p = 0.04) and age ≥ 50 (p = 0.05) were significantly associated with OS (◉ Table 4).
Overall 23 patients (14 %) had grade ≥ 3 acute toxicity [severe skin reactions (n = 14, grade 3) and wound-healing disturbances (n = 8, grade 3; n = 1, grade 4)]. Sixteen patients (10 %) developed grade ≥ 3 chronic toxicity [neuropathy (n = 4, grade 3; n = 2, grade 4), necrosis/fistula/ulcer (n = 1, grade 3; n = 1, grade 4), joint function impairment due to fibrosis (n = 4, grade 3) and severe chronic lymphedema (n = 2, grade 3)]. No perioperative or long-term death from treatment occurred. In relation to acute [14 % (n = 19) vs. 17 % (n = 4); p = 0.41)] and chronic toxicity [10 % (n = 13) vs. 13 % (n = 3); p = 0.65)] no differences between patients with upper and lower extremity STS were observed.
Discussion
To our knowledge, this is the largest reported study that focuses on the outcomes of patients with primary STS treated with IOERT and EBRT. Discrimination between patients receiving treatment for an initial diagnosis and recurrence is important because it has been consistently reported that patients treated for LR have worse overall outcomes (◉ Table 5).
Our relevant findings can be summarized as follows. First, in a group of patients with high-risk features for local relapse (all incomplete or close margin resections), the 5-year LC and OS rates of 82 and 72 % compare well with more favourable cohorts of patients treated with limb-preserving surgery and EBRT without IOERT [5-year OS (71–87 %) and LC (72–96 %)] [2–6]. Second, we found that an IOERT dose ≥ 12.5 Gy reduces the risk of IOERT in-field relapse. Interestingly, this maintained significance when patients with complete resection (R0) were analysed separately. Finally, we found that patients with an R1 resection had an increased probability of local and distant relapse that could not be compensated by a moderate IOERT boost to the high-risk region. In a subgroup analysis margin status retained significance with regard to LC only for patients with lower extremity tumours.
Several groups have successfully implemented and reported combined management (IORT and EBRT) for patients with extremity sarcomas [5-year LC (73–95 %) and OS (70–80 %)] [14–18]. Although margin status is a common listed risk factor for local recurrence, what constitutes adequate surgical margins is not well defined. Positive surgical margins have been consistently reported as an adverse prognostic factor for LC (◉ Table 5). Oertel et al. [15] reported the largest single institution experience with IOERT plus EBRT for the management of extremity STS (n = 153). Although detailed data on the site of recurrence and rescue are not provided, LC was more favourable for patients receiving an IOERT dose ≥ 15 Gy (5-year LRC 85 vs. 50 %, p = 0.003) and complete margin resection (5-year LRC 85 vs. 60 %, p = 0.03). Azinovic et al. [16] analysed 45 patients with extremity sarcomas (58 % primary tumours) treated with postoperative EBRT (45 to 50 Gy) and IOERT. The 5-year local control was 87 % and margin status [negative or close margins vs. positive margins (p= 0.04)] significantly affected LC. Consistently, we also found that patients with an IOERT dose ≥ 12.5 Gy achieved better IOERT in-field control. In the current analysis positive microscopic resection margins (16 %) was the only factor that remained significantly associated with LC in the multivariate analysis. Additionally, the lack of the significant association of margin status with LC in the upper extremity and IOERT dose with IOERT in-field control in patients with R1 resection margin are likely due to small patient numbers in those subgroups. However, margin resection status may have a different prognostic impact in different settings [17]. Call et al. [17] analyzed 61 patients (treated with EBRT plus IORT) with upper extremity STS by margin status. The patients with positive margins had similar prognoses to patients with negative margins (5- and 10-year LC rates 100 % and 86 % vs. 89 % at both; p = 0.98). Likewise in the current analysis, margin status had no impact on LC for patients with upper extremity STS. In contrast for the subset of patients with lower extremity STS we found that R1 margins status was associated with an increased chance of LR.
Dickie et al. [19] examined the geometric relationship between LR and EBRT volumes of 768 STS patients treated with function-preserving surgery. Sixty (7.8 %) STS patients developed LR, 49 tumors relapsed in-field (6.4 % overall), 9 out-field (1.1 % overall) and 2 were marginal (0.3 % overall). Because the majority of STS tumours reoccur in-field, these data support that an accurate delivery of a higher radiation dose could potentially improve LC in select patients with limb STS. Al Yami et al. [7] reported no benefit for adding a postoperative EBRT boost for extremity STS patients treated with preoperative radiotherapy. This treatment strategy has several disadvantages such as a significant delay before boost delivery (potentially allowing tumour repopulation and systemic dissemination), diminished effectiveness due to tumour bed hypoxia after surgery and increased treated volume. An IOERT boost has several advantages over an EBRT escalated strategy such as a more precise delivery of radiation to a surgically identified high-risk area, mobilization of dose-sensitive critical organs temporarily out of the radiation boost field and to shorten overall treatment time (dose–dense radiotherapy). The observation that patients receiving a higher IOERT boost dose (≥ 12.5 Gy) had a decreased chance of IOERT in-field LR in the current analysis is an argument in favour of an IOERT dose escalation strategy, by implementing field within a field technique (Supplemental Fig. 2).
Distant metastases remain as the dominant pattern of progression for high-risk extremity STS [3, 20]. Although the effect of adjuvant CT on survival for resected soft-tissue sarcoma remains to be recognized [21], intensified local treatment needs to be tested in the context of more efficient concurrent, neo-, and adjuvant systemic therapy.
Concerning treatment-related toxicity, a treatment regimen that included IOERT for extremity sarcomas was tolerable for our 159 patients. The low rate of severe toxic events and the high limb preservation rates (95 % limb preservation) suggest that a multimodality approach with EBRT and an IOERT-boost component is feasible with acceptable risks and without prohibitive long-term side effects [22].
We acknowledge several limitations of our study. First, on average only two patients were treated per institution per year [study period is very long (almost 25 years)]. Second, the population was heterogeneous, receiving different treatment combinations, sequences and doses. Radiation therapy technology, treatment guidelines and surgical consensus have changed over time, and cannot be completely assessed in all three hospitals. Third, although we did observe a significant association between IOERT dose and IOERT in-field control after adjustment for several potential confounding factors, we certainly acknowledge the presence of a selection bias for patients referred for radiation therapy to a higher dose. Fourth, a systematic method of follow-up, including imaging, would be optimal to evaluate patterns of failure after radiation therapy. Given the retrospective nature of this analysis, consistent homogeneous imaging did not occur in a proportion of patients. Finally, we acknowledge the limitation that this series does not compare boost to no boost, and it is therefore difficult to assess the benefit of the IOERT boost.
Conclusion
We found that patients with extremity STS receiving EBRT and IOERT could be treated safely and had high LC rates. In addition, patients with radical resections experienced the largest benefit of a higher IOERT dose. A level of adverse prognostic features (R1 resections) might be compensated in upper extremity STS. Our results suggest that patients with close or positive margins could benefit from further intensified local treatment strategies.
Compliance with ethical guidelines
Conflict of interest
F. A. Calvo, C. V. Sole, A. Polo, M. Cambeiro, A. Montero, A. Alvarez, M. Cuervo, M. S. Julian, R. Martinez-Monge state that there are no conflicts of interest.
This work was supported in part by a grant from the Health Institute of Research Carlos III, Spanish Ministry of Science and Innovation (project code PI11-02908).
References
Haas RL, Delaney TF, O’Sullivan B (2012) Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? Int J Radiat Oncol Biol Phys 84:572–580
Rosenberg SA, Tepper J, Glatstein E et al (1982) The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196:305
Pisters PW, Harrison LB, Leung DH et al (1996) Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 14:859
Yang JC, Chang AE, Baker AR et al (1998) Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16:197
Pisters PW, Leung DH, Woodruff J et al (1996) Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 14:1679
Zagars GK, Ballo MT, Pisters PW et al (2003) Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 97:2530
Al Yami AG, Ferguson PC et al (2010) Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys 77:1191
Delaney TF (2012) Radiation therapy: neoadjuvant, adjuvant, or not at all. Surg Oncol Clin N Am 21:215–241
Zagars GK, Ballo MT, Benjamin RS et al (2003) Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: Analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys 57:739–747
Davis AM, O’Sullivan B, Pater J et al (2005) Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75:48–53
Gunderson LL, Willet CG, Calvo FA, Harrison LB (2011) Intraoperative irradiation techniques and results. Current Clinical Oncology. Humana Press. Second edition
Pascau J, Santos Miranda JA et al (2012) An innovative tool for intraoperative electron beam radiotherapy simulation and planning: description and initial evaluation by radiation oncologists. Int J Radiat Oncol Biol Phys 3:287–295
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341–1346
Haddock MG, Petersen IA, Pritchard D et al (1997) IORT in the management of extremity and limb girdle soft tissue sarcomas. Front Radiat Ther Oncol 31:151–152
Oertel S, Treiber M, Zahlten-Hinguranage A et al (2006) Intraoperative electron boost radiation followed by moderate doses of external beam radiotherapy in limb-sparing treatment of patients with extremity soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 64:1416–1423
Azinovic I, Martinez Monge R, Javier Aristu J et al (2003) Intraoperative radiotherapy electron boost followed by moderate doses of external beam radiotherapy in resected soft-tissue sarcoma of the extremities. Radiother Oncol 67:331–337
Call JA, Stafford SL, Petersen IA et al (2012) Use of Intraoperative radiotherapy for upper-extremity soft-tissue sarcomas: analysis of disease outcomes and toxicity. Am J Clin Oncol Oct 29 (Epub ahead of print)
Dubois JB, Debrigode C, Hay M et al (1995) Intra-operative radiotherapy in soft tissue sarcomas. Radiother Oncol 34:160–163
Dickie CI, Griffin AM, Parent AL et al (2012) The relationship between local recurrence and radiotherapy treatment volume for soft tissue sarcomas treated with external beam radiotherapy and function preservation surgery. Int J Radiat Oncol Biol Phys 82:1528–1534
Eckert F, Gani C, Kluba T et al (2013) Effect of concurrent chemotherapy and hyperthermia on outcome of preoperative radiotherapy of high-risk soft tissue sarcoma. Strahlenther Onkol 189:482–485
Pervaiz N, Colterjohn N, Farrokhyar F et al (2008) A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113:573–581
Kretzler A, Molls M, Gradinger R et al (2004) Intraoperative radiotherapy of soft tissue sarcoma of the extremity. Strahlenther Onkol 180:365–370
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Calvo, F., Sole, C., Polo, A. et al. Limb-sparing management with surgical resection, external-beam and intraoperative electron-beam radiation therapy boost for patients with primary soft tissue sarcoma of the extremity. Strahlenther Onkol 190, 891–898 (2014). https://doi.org/10.1007/s00066-014-0640-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0640-2