Abstract
Background
Survival upon diagnosis of brain metastases (BM) in patients with non-small cell lung cancer (NSCLC) is highly variable and established prognostic scores do not include tissue-based parameters.
Methods
Patients who underwent neurosurgical resection as first-line therapy for newly diagnosed NSCLC BM were included. Microvascular density (MVD), Ki67 tumor cell proliferation index and hypoxia-inducible factor 1 alpha (HIF-1 alpha) index were determined by immunohistochemistry.
Results
NSCLC BM specimens from 230 patients (151 male, 79 female; median age 56 years; 199 nonsquamous histology) and 53/230 (23.0 %) matched primary tumor samples were available. Adjuvant whole-brain radiation therapy (WBRT) was given to 153/230 (66.5 %) patients after neurosurgical resection. MVD and HIF-1 alpha indices were significantly higher in BM than in matched primary tumors. In patients treated with adjuvant WBRT, low BM HIF-1 alpha expression was associated with favorable overall survival (OS), while among patients not treated with adjuvant WBRT, BM HIF-1 alpha expression did not correlate with OS. Low diagnosis-specific graded prognostic assessment score (DS-GPA), low Ki67 index, high MVD, low HIF-1 alpha index and administration of adjuvant WBRT were independently associated with favorable OS. Incorporation of tissue-based parameters into the commonly used DS-GPA allowed refined discrimination of prognostic subgroups.
Conclusion
Ki67 index, MVD and HIF-1 alpha index have promising prognostic value in BM and should be validated in further studies.
Zusammenfassung
Hintergrund
Die Überlebensprognose von Patienten mit zerebralen Metastasen eines nicht-kleinzelligen Lungenkarzinoms (NSCLC) ist sehr variabel. Bisher werden gewebsbasierte Parameter nicht in die prognostische Beurteilung inkludiert.
Methoden
Neurochirurgische Resektate zerebraler NSCLC-Metastasen wurden in dieser Studie untersucht. Die Gefäßdichte („microvascular density“, MVD), der Ki67-Proliferationsindex sowie der HIF-1α-Index wurden mittels immunhistochemischer Methoden analysiert.
Ergebnisse
Insgesamt wurden Proben von 230 Patienten (151 Männer, 79 Frauen; mittleres Alter 56 Jahre; 199 Adenokarzinome) und korrespondierenden Primärtumoren (53/230; 23,0 %) eingeschlossen. Mit einer Ganzhirnbestrahlung nach neurochirurgischer Resektion wurden 153/230 Patienten (66,5 %) behandelt. Eine höhere Gefäßdichte sowie ein niedrigerer HIF-1α-Index wurden in den korrespondierenden Primärtumoren im Vergleich zu den zerebralen Metastasen beobachtet. Ein niedriger HIF-1α-Index zeigte eine signifikante Korrelation mit dem Gesamtüberleben in Patienten mit postoperativer Ganghirnbestrahlung. In der Gruppe der Patienten ohne postoperative Ganzhirnbestrahlung hingegen konnte kein prognostischer Einfluss des HIF-1α-Index beobachtet werden. Ein niedriger diagnosespezifischer Prognosescore (DS-GPA), ein niedriger Ki67-Proliferationsindex, eine hohe Gefäßdichte, ein niedriger HIF-1α-Index sowie die Durchführung einer postoperativen Ganzhirnbestrahlung zeigten eine unabhängige und signifikante Korrelation mit dem Gesamtüberleben. Der Einschluss von gewebebasierten Parametern in der üblich verwendeten DS-GPA ermöglicht die Unterscheidung prognostischer Subgruppen.
Schlussfolgerung
Die Analyse des Ki67-Proliferationsindex, der Gefäßdichte sowie des HIF-1α-Index sollte in die prognostische Beurteilung von Patienten mit zerebralen NSCLC-Metastasen inkludiert werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The development of brain metastases (BM) is a devastating complication in cancer patients, for which there are only limited treatment options. Unfortunately, the incidence of BM has increased over the last decade. Non-small cell lung cancer (NSCLC) is the primary tumor most frequently responsible for BM and approximately 40 % of patients suffering from metastatic NSCLC eventually develop BM [1].
The prognosis upon diagnosis of BM is poor, with a median overall survival (OS) of only a few months [2]. Several prognostic scores have been established in order to provide survival estimations for patients with newly diagnosed BM. These prognostic scores rely mainly on established clinical prognostic factors such as age, Karnofsky Performance Status, status of extracranial disease and number of BM [2–5]. The diagnosis-specific graded prognostic assessment score (DS-GPA) is an established prognostic score for survival estimation in patients with newly diagnosed NSCLC BM [2]. Herein, the calculated median OS from diagnosis of BM ranges from 3 months in the least favorable group, to 14.8 months in the most favorable group [2]. Tissue-based prognostic factors are not currently included in any of the established prognostic scores for BM patients.
Neoangiogenesis, hypoxia and proliferation are hallmarks of cancer. These factors have been shown to influence patients’ prognosis and response to therapy in many tumor types, including primary and metastatic NSCLC [6–10]. However, the prognostic value of these parameters in NSCLC BM has not been systematically studied.
In the present study we investigated the association of Ki67 tumor cell proliferation index, the expression of hypoxia-inducible factor 1 alpha (HIF-1 alpha) and the expression of CD34 (as an endothelial marker) with outcome parameters in order to explore their prognostic value. The study cohort comprised a large and well-defined series of NSCLC patients treated with first-line neurosurgical resection upon diagnosis of BM.
Methods
Patients
All patients diagnosed with NSCLC BM having undergone first-line neurosurgical resection between January 1990 and February 2011 were identified from the Neuro-Biobank, Medical University of Vienna. Histological confirmation of BM originating from NSCLC was mandatory for inclusion. Clinical data, including clinical prognostic factors, were identified by chart review. DS-GPA was calculated based on clinical factors [2, 3]. Survival data was obtained from the National Cancer Registry of Austria database and the Austrian Brain Tumor Registry [11]. The ethics committee of the Medical University of Vienna approved the study (vote 078/2004).
Tissue-based analysis
Formalin-fixed and paraffin-embedded tissue blocks were assembled according to standard laboratory practice. Tissue blocks were cut into 3-μm slices with a microtome. Immunohistochemistry was performed using an automated horizontal slide processing system (Autostainer Plus Link; Ki67; Dako Denmark, Glostrup, Denmark) and a fully automated multimodal slide staining system (BenchMark ULTRA; HIF-1 alpha, CD34; Ventana Medical Systems, Tucson, AZ, USA) according to standard protocol [12–14]. In brief, slides underwent heat-induced epitope retrieval in pH6.0 citrate buffer (HIF-1 alpha: 92 min; Ki67: 20 min) or in pH8.0 buffer (CD34: 36 min). Afterwards, sections were incubated with antibody: HIF-1 alpha: polyclonal rabbit purified anti-human HIF-1 alpha/610959 BD Transduction Laboratories™ (BD Biosciences, East Rutherford, NJ, USA) 1:10; Ki67: monoclonal mouse Ki67 clone MIB-1/M7240 (Dako), 1:200; CD34: Novocastra™ lyophilized mouse monoclonal antibody endothelial cell marker (CD34) (Novocastra™, Leica Biosystems, Wetzlar, Germany) 1:50.
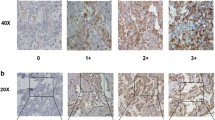
For the Ki67 proliferation index, 500 cells were counted within the area of strongest staining to give the percentage of positive cells (0–100 %) [12]. HIF-1 alpha score was calculated according to the modified H-score [15–17]. HIF-1 alpha intensity groups were defined as follows: 0 = no appreciable staining in the tumor cell nucleus; 1 = barely detectable staining intensity in the nucleus; 2 = moderate staining intensity distinctly in the tumor cell nucleus; 3 = strong staining intensity of the tumor cell nucleus. For each intensity group the fraction of cells (0–100 %) was recorded. The HIF-1 alpha index was calculated by multiplying the intensity by the fraction of cells producing this intensity, producing a total range of 0–300. The mean microvascular density (MVD) was defined by the number of CD34-positive vessels within the area of the highest density at a 200x magnification (“hot spot”) [18]. Furthermore, the vascular pattern was analyzed in the CD34 staining to differentiate between the “angiogenic type” defined by the predominance of sprouting vessels (characterized by multilayer endothelium) and the “silent type” defined by predominance of vessels with thin monolayer endothelium. Specimens with an equal distribution of angiogenic and silent types were defined as the “balanced type” [17].
Statistical analysis
The Spearman’s rank correlation coefficient was used to assess monotone associations between two continuous variables. For assessing group differences, χ-square, paired and unpaired t-, Mann–Whitney U and Kruskal–Wallis tests were used as appropriate. A significance level of 0.05 was applied. OS of patients was estimated with the Kaplan–Meier product limit method and group differences were assessed with the log-rank test. The median was used as the cutoff value for continuous variables entered in univariate analysis.
Variables with significant results in univariate analysis were entered into a multivariate Cox proportional hazards model. Overall and partial measures of dependence (R squared, R2 values) were computed according to Kent and O’Quigley [19, 20]. Due to the exploratory and hypothesis-generating design of the present study, no adjustment for multiple testing was applied [21].
For calculation of the tissue GPA (tGPA) prognostic score a multivariate Cox regression model was used. For graphical representation, the patient cohort was divided into three equal sized classes according to the terciles of the tGPA.
All statistical analyses were performed with the Statistical Package for the Social Sciences version 20.0 software (IBM, SPSS, Armonk, NY, USA) and SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
A total of 230 patients (151 male, 79 female) with a median age of 56 years (range 33–78 years) at first diagnosis of NSCLC BM were included. All patients underwent neurosurgical resection as first line therapy for newly diagnosed BM. Histology was as follows: 165/230 (71.7 %) patients had adenocarcinoma, 29/230 (12.6 %) squamous cell carcinoma, 15/230 (6.5 %) adenosquamous carcinoma, 17/230 (7.4 %) large cell carcinoma and 4/230 (1.7 %) patients had unknown histology. A history of cigarette smoking was reported by 169/230 (73.5 %) patients. Further patient characteristics are listed in Table 1.
Tissue-based findings in brain metastasis specimens
Median Ki67 proliferation index was 39.8 % (range 5–97 %), median HIF-1 alpha index was 60 (range 0–270) and median MVD was 71/0.7 mm2 (range 7–298/0.7 mm2). Of all specimens, 102/230 (44.3 %) showed a predominance of microvascular sprouting (angiogenic type); 62/230 (27.0 %) specimens showed a silent angiogenesis with predominance of mature vessels and without signs of angiogenesis (silent type); 61/230 (26.5 %) specimens showed an equal distribution of microvascular sprouting and silent angiogenesis (balanced type) and 5/230 (2.2 %) specimens were not definable due to too few classifiable vessels.
In BM specimens, MVD showed a significant association with vascular pattern: MVD values increased when going from specimens with silent, to balanced, to angiogenic vascular patterns (Kruskal–Wallis test, p< 0.001; Fig. 1a).
Ki67 proliferation index was significantly higher in BM specimens with squamous histology as compared to cases with nonsquamous histology (Mann–Whitney U test, p = 0.002; Fig. 1b).
Among BM specimens, no correlation was observed between Ki67 proliferation index and MVD (Spearman’s correlation coefficient − 0.039, p = 0.556) or vascular pattern (Kruskal–Wallis test, p = 0.587). Furthermore, no correlation between HIF-1 alpha index and MVD (Spearman’s correlation coefficient − 0.049, p = 0.459) or vascular pattern (Kruskal–Wallis test, p = 0.572) was present. No correlation between histology and MVD (Kruskal–Wallis test, p = 0.311), HIF-1 alpha index (Kruskal–Wallis test, p = 0.321) or vascular pattern (χ-square test, p = 0.799) was evident. Weak correlation was observed between Ki67 proliferation index and HIF-1 alpha index (Spearman’s correlation coefficient 0.298, p < 0.001).
Comparative analyses of brain metastases and corresponding primary tumors
Tumor tissue from the corresponding primary tumor was available in 53/230 (23.0 %) cases. Median Ki67 proliferation index of the primary tumor was 39 % (range 4–79 %) and did not significantly differ from the BM Ki67 proliferation index (paired t-test, p = 0.897). Median primary tumor MVD was 65/0.7 mm2 (range 26–179/0.7 mm2), which was significantly lower than in BM (71/0.7 mm2; paired t-test, p = 0.032). Median primary tumor HIF-1 alpha index was 30 (0–210), which was significantly lower than in BM (60; paired t-test, p = 0.013).
Survival analyses
Impact of parameters on time to diagnosis of brain metastases
Time to diagnosis of BM was only evaluated in patients with subsequent diagnosis of BM and no synchronous diagnosis of primary tumor and BM (n = 103). No impact of Ki67 proliferation index, HIF-1 alpha index, MVD or vascular pattern of the primary tumor on time to development of BM (TTBM) was observed. Patients with large cell carcinoma histology (median TTBM: 4 months) developed BM earlier than patients with adenocarcinoma (median TTBM: 12 months), squamous cell (median TTBM: 11 months) or adenosquamous cell carcinoma histology (median TTBM: 9 months; log-rank test, p = 0.052; Fig. 2). Furthermore, patients with large cell carcinoma (11/17, 64.7 %) or adenocarcinoma (99/165, 60.0 %) had more synchronous diagnosis of NSCLC and BM than patients with adenosquamous (7/15, 46.7 %) or squamous cell carcinoma (8/29, 27.6 %; χ-square test, p = 0.009).
Impact of clinical characteristics on overall survival from diagnosis of brain metastasis
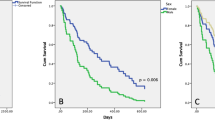
DS-GPA showed a statistically significant correlation with OS measured from first diagnosis of BM. Patients with DS-GPS class 1 had a median OS of 17 months, compared to 7 months in class 2, 5 months in class 3 and only 1 month in patients with class 4 (log-rank test, p < 0.001; Fig. 3a). However, no significant difference in OS was observed between DS-GPA class 2 and DS-GPA class 3 (7 vs. 5 months; log-rank test, p = 0.205).
a Overall survival (OS) from diagnosis of brain metastases (BM) according to diagnosis-specific graded prognostic assessment (DS-GPA) class. b OS from diagnosis of BM according to primary tumor histology. c OS from diagnosis of BM according to adjuvant treatment after surgery for BM. d OS from diagnosis of BM in patients with synchronous diagnosis of BM and primary non-small cell lung cancer (NSCLC) according to surgical strategy. WBRT whole-brain radiation therapy
Histology significantly influenced survival prognosis. Patients with adenocarcinoma had a median OS of 9 months from diagnosis of BM, compared to 8 months in patients with large cell histology, 6 months in patients with adenosquamous histology and 4 months in patients with squamous histology (log-rank test, p = 0.008; Fig. 3b).
Patients scheduled for adjuvant WBRT after neurosurgical resection of BM had a significantly longer median OS than patients without adjuvant WBRT after neurosurgical resection of BM (9 vs. 5 months; log rank test, p < 0.001). Patients with a single BM received WBRT significantly less frequently after neurosurgical resection (100/164, 61.0 %) than did patients with 2–3 BM (38/47, 80.9 %) or > 3 BM (15/18, 83.3 %; χ-square test, p = 0.012). Furthermore, patients receiving chemotherapy after diagnosis of BM survived significantly longer than patients not receiving chemotherapy (15 vs. 6 months; log-rank test, p.0.005). Patients receiving combination therapy comprising WBRT and chemotherapy after surgery had improved survival (15 months) compared to patients receiving WBRT (8 months) or chemotherapy alone (14 months; log-rank test, p < 0.001; Fig. 3c).
A synchronous diagnosis of BM and primary NSCLC had been made for 127/230 (55.2 %) patients. In this cohort, patients treated with surgical treatment of both primary tumor and BM had a more favorable OS compared to patients in whom only the BM was resected (15 vs. 7 months; log-rank test, p = 0.004; Fig. 3d).
Impact of tissue-based characteristics on overall survival from diagnosis of brain metastasis
Patients with a low Ki67 proliferation index in the BM tissue had an improved survival compared to patients with high Ki67 proliferation index (10 vs. 6 months; log-rank test, p = 0.011; Fig. 4a). Patients with a low HIF-1 alpha index in the BM tissue had more favorable OS than patients with a high HIF-1 alpha index (11 vs. 7 months; log-rank test, p = 0.013; Fig. 4b). Patients with high MVD in the BM tissue survived significantly longer than patients with low MVD (10 vs. 6 months; log-rank test, p = 0.049; Fig. 4c). The vascular pattern did not show a significant impact on prognosis (log-rank test, p = 0.850).
In the cohort of patients treated with WBRT after neurosurgery, patients with a low HIF-1 alpha index had a more favorable median OS than patients with a high HIF-1 alpha index (15 vs. 7 months; p = 0.004; Fig. 5a). However, HIF-1 alpha expression had no impact on OS in the cohort of patients without WBRT (log-rank test, p = 0.904; Fig. 5b). A trend was observed in multivariate interaction analysis of WBRT and HIF-1 alpha index (Cox regression model, p = 0.074).
a Overall survival (OS) from diagnosis of brain metastases (BM) in patients receiving adjuvant whole-brain radiation therapy (WBRT) after surgery of BM related to HIF-1 alpha index. b OS from diagnosis of BM in patients not receiving adjuvant WBRT after surgery of BM related to HIF-1 alpha index. c OS from diagnosis of BM related to tissue graded prognostic assessment (tGPA) class, d OS from diagnosis of BM in patients with GPA class 2 related to tGPA
Multivariable analysis of overall survival
According to the results of univariate analysis, we entered the following parameters into multivariate survival analyses using the Cox regression model: DS-GPA, primary tumor histology, chemotherapy (yes/no), adjuvant WBRT (yes/no), HIF-1 alpha index, Ki67 proliferation index and MVD. DS-GPA, WBRT, HIF-1 alpha index, Ki67 proliferation index and MVD remained independent prognostic parameters for survival from diagnosis of BM in multivariable analysis (Table 2). Furthermore, the tissue-based characteristics were shown to add statistically significant information to the model (Cox regression model,three degrees of freedom, p < 0.001). Overall, the R2 measure showed that the seven prognostic factors explained 34.04 % of the variability in OS time. Moreover, the partial R2 measure showed that the tissue-based prognostic characteristics in addition of the clinical prognostic characteristics explained 10.62 % variability in OS time. After accounting for the effects of the clinical prognostic factors and the other respective tissue-based characteristics, the partial R2 values for MVD, HIF-1 alpha index and ki67 proliferation index were 2.72, 2.18 and 4.38 %, respectively (Table 2).
Calculation of a prognostic score including tissue-based characteristics
To illustrate the potential of improved prognostication by incorporation of tissue-based prognostic parameters into the DS-GPA, we calculated a tGPA. Using the terciles as cutoffs, the entire cohort was divided into three tGPA classes (class 1: 76 patients; class 2: 77 patients; class 3: 77 patients). A statistically significant association was observed between tGPA and median OS (class 1: 15 months, class 2: 9 months, class 3: 4 months; log-rank test, p < 0.001; Fig. 5c). As no significant discrimination was observed between DS-GPA class 2 and DS-GPA class 3, we applied the tGPA to this group of patients (n = 161). Herein, tGPA showed a statically significant discrimination. Patients with tGPA class 1 had median OS of 11 months; for patients with class 2 this was 8 months and patients with tGPA class 3 had a median OS of 5 months (log-rank test, p < 0.001; Fig. 5d).
Discussion
In this project we investigated for the first time the prognostic value of Ki67 proliferation index, HIF-I alpha index and MVD in a large and well-defined cohort of NSCLC BM. Our findings clearly show that these parameters are biologically and clinically relevant and may help to guide the management of patients with NSCLC BM.
In our large cohort of patients with NSCLC BM, we observed an independent, significant prognostic impact of Ki67 proliferation index, HIF-1 alpha index and MVD on OS from diagnosis of BM. Our results are well in line with previous findings in primary NSCLC, which postulated that high Ki67 proliferation and high HIF-1 alpha indices be unfavorable prognosis parameters [22, 23]. However, our study is, to our best knowledge, the first to investigate these tissue-based prognostic factors in BM tissue. The association of high MVD and favorable OS prognosis in our cohort might be perceived as somewhat surprising, as a high MVD was shown to be associated with an impaired survival prognosis in primary NSCLC [24, 25]. However, a previous study of MVD in NSCLC lymph node metastases postulated a switch of the prognostic value of MVD in the metastatic setting, as a high MVD in the lymph node metastases correlated with an improved OS prognosis [26]. Taken together, these results suggest a differential influence of MVD on prognosis in primary NSCLC tumors and NSCLC metastatic lesions. The surrounding microenvironment might influence the pathobiological behavior and the angiogenic potential of a given tumor, as postulated in the “seed and soil” theory [27]. While some primary NSCLCs were shown to grow in an alveolar pattern with only low neoangiogensis, preclinical data demonstrated a highly angiogenic behavior of NSCLC BM cells after extravasation into the perivascular niche [28, 29]. Previously, chemotherapy drug delivery through the increased capillary surface was postulated to account for the improved survival of patients with highly angiogenic tumors [26]. However, we observed an independent impact of high MVD in the multivariate analysis, irrespective of chemotherapy administration. Nevertheless, the reasons for the unexpectedly observed beneficial effect of increased BM angiogenesis on OS are not clear.
Our findings underscore the heterogeneity of tumor behavior among NSCLC subtypes and the relevance of these differences for the biology and clinical course of BM. Firstly, we found a higher proliferation rate in squamous, compared to nonsquamous BM tumors—a finding that mirrors the situation in primary NSCLC [30]. In line with these results, we found that patients with squamous cell BM had a poor outcome, with median OS reaching only 4 months and thereby significantly shorter than that observed for other tumor types (6–9 months).
Interestingly, we found a profound effect of tumor histology on the time to BM development from first diagnosis of NSCLC. Patients with large cell carcinomas developed BM within a median 4 months from diagnosis of the primary tumor, while in other tumor types BM became evident only after median follow-up times of more than 11 months. To the best of our knowledge, this result has not been described previously and warrants further investigation. It is of note that a relatively high incidence of BM has been documented in adenocarcinoma and large cell carcinoma previously [31]. Additionally, a higher incidence of synchronous diagnosis of primary tumor and BM was evident for large cell and adenocarcinoma in our series, thus further highlighting the higher propensity of BM in these histological entities.
Concerning cases with synchronous diagnosis of BM and primary NSCLC tumor, we observed a strong effect of surgical strategy on patient outcome. Patients treated with resection of both the CNS and the primary tumors fared significantly better than patients treated with neurosurgery only. Our study is limited by its retrospective nature; in particular, the prescription bias with respect to the administered therapies may be a potential issue of concern resulting from poorly standardized therapy approaches in patients with BM. Furthermore, statistical power may be an additional issue and prospective, randomized trials are urgently warranted to reappraise our findings. However, compared to previous studies, we were able to include and investigate an appreciable sample size. Some small phase II prospective trials already exist and underscore our findings: these have shown that a subgroup of patients with synchronous diagnosis of NSCLC and oligometastatic disease benefits from multimodality treatment including BM and lung resection [32–34].
We observed significant longer survival in patients receiving a multimodal therapy approach including surgery, chemotherapy and WBRT as compared to patients receiving only adjuvant WBRT after surgery. Although a selection bias cannot be ruled out, our findings once more underscore the shaky and ambiguous value of adjuvant WBRT after neurosurgical resection of NSCLC BM [35, 36]. A prospective phase III trial failed to demonstrate an impact of adjuvant WBRT on OS in patients with one to three BM treated with neurosurgical resection or radiosurgery [37]. However, time to intracranial disease progression was significantly prolonged in patients receiving WBRT. Considering the relative radioresistance of NSCLC and the long-term side effects of WBRT (e.g. neurocognitive decline), a predictive marker for patients profiting from adjuvant WBRT would be of clinical relevance [38–40]. Our data suggest that the HIF-1 alpha index, which is frequently used a as surrogate marker for hypoxia, might serve as a predictive marker for WBRT. This is in good agreement with the results of previous studies indicating the value of HIF-1 alpha expression as a predictor for the response to radiotherapy in various primary tumors [41]. Our results clearly suggest the need for prospective studies to further investigate whether stratification of BM patients for adjuvant WBRT based on HIF-1 alpha expression is a feasible strategy.
Conclusion
In order to make appropriate personalized and prognostic-based treatment decisions, prognostic scoring systems have to be as precise as possible and include all relevant prognostic aspects. To date, established prognostic scores only include clinical prognostic factors and do not include radiological or pathological findings [2–4, 42]. In the present study, we could demonstrate the impact of proliferation, MVD and hypoxia on survival in our cohort of patients with NSCLC BM. We illustrate by example that the addition of tissue-based parameters to traditional prognostic scores based on clinical parameters alone may improve discrimination between prognostic subgroups. Therefore, the inclusion of tissue-based prognostic factors should be considered, but needs to be validated in independent patient cohorts and prospective studies.
References
Yamanaka R (2009) Medical management of brain metastases from lung cancer (Review). Oncol Rep 22:1269–1276
Sperduto PW et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425
Gaspar L et al (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751
Sperduto PW et al (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70:510–514
Rades D et al (2013) A new survival score for patients with brain metastases from non-small cell lung cancer. Strahlenther Onkol 189:777–781
Huang C et al (2005) Clinical application of biological markers for treatments of resectable non-small-cell lung cancers. Br J Cancer 92:1231–1239
Trivella M et al (2007) Microvessel density as a prognostic factor in non-small-cell lung carcinoma: a meta-analysis of individual patient data. Lancet Oncol 8:488–499
Yohena T et al (2009) Upregulation of hypoxia-inducible factor-1 alpha mRNA and its clinical significance in non-small cell lung cancer. J Thorac Oncol 4:284–290
Park S et al (2011) Prognostic implications of hypoxia-inducible factor-1 alpha in epidermal growth factor receptor-negative non-small cell lung cancer. Lung Cancer 72:100–107
Kim SJ et al (2005) Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer 49:325–335
Wohrer A et al (2009) The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol 95:401–411
Preusser M et al (2008) Ki67 index in intracranial ependymoma: a promising histopathological candidate biomarker. Histopathology 53:39–47
Birner P et al (2003) Vascular patterns in glioblastoma influence clinical outcome and associate with variable expression of angiogenic proteins: evidence for distinct angiogenic subtypes. Brain Pathol 13:133–143
Zhong H et al (1999) Overexpression of hypoxia-inducible factor 1 alpha in common human cancers and their metastases. Cancer Res 59:5830–5835
Cappuzzo F et al (2005) Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst 97:643–655
Hirsch FR et al (2003) Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 21:3798–3807
Spanberger T et al (2013) Extent of peritumoral brain edema correlates with prognosis, tumoral growth pattern, HIF1a expression and angiogenic activity in patients with single brain metastases. Clin Exp Metastasis 30:357–368
Weidner N (1995) Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 36:169–180
Heinzl H (2000) Using SAS to calculate the Kent and O’Quigley measure of dependence for Cox proportional hazards regression model. Comput Methods Programs Biomed 63:71–76
Kent J, Quigley J (1988) Measures of dependence for censored survival data. Biometrika 75:525–534
Bender R, Lange S (2001) Adjusting for multiple testing—when and how? J Clin Epidemiol 54:343–349
Radovic S et al (2007) Non-small cell lung carcinoma: cyclin D1, bcl-2, p53, Ki-67 and HER-2 proteins expression in resected tumors. Bosn J Basic Med Sci 7:205–211
Saad AG et al (2008) Immunohistochemical markers associated with brain metastases in patients with nonsmall cell lung carcinoma. Cancer 113:2129–2138
Giatromanolaki A et al (1996) Prognostic value of angiogenesis in operable non-small cell lung cancer. J Pathol 179:80–88
Medetoglu B et al (2010) Tumor angiogenesis in predicting the survival of patients with stage I lung cancer. J Thorac Cardiovasc Surg 140:996–1000
Kreuter M et al (2009) Prognostic relevance of angiogenesis in stage III NSCLC receiving multimodality treatment. Eur Respir J 33:1383–1388
Fidler IJ (2011) The role of the organ microenvironment in brain metastasis. Semin Cancer Biol 21:107–112
Pezzella F et al (1997) Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol 151:1417–1423
Kienast Y et al (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16:116–122
Mangili F et al (1998) Cell loss and proliferation in non-small cell lung carcinoma: correlation with histological subtype. Eur J Histochem 42:287–295
Guo S et al (2012) Impact of non-small cell lung cancer histology on survival predicted from the graded prognostic assessment for patients with brain metastases. Lung Cancer 77:389–393
Collaud S et al (2012) Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung Cancer 78:234–238
De Ruysscher D et al (2012) Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 7:1547–1555
Hu C et al (2006) Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 106:1998–2004
Nieder C et al (2007) The role of postoperative radiotherapy after resection of a single brain metastasis. Combined analysis of 643 patients. Strahlenther Onkol 183:576–580
Nieder C et al (1998) Patterns of relapse and late toxicity after resection and whole-brain radiotherapy for solitary brain metastases. Strahlenther Onkol 174:275–278
Kocher M et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141
Monaco EA 3rd et al (2013) Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer 119:226–232
Soffietti R et al (2013) A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 31:65–72
Rades D, Schild SE (2012) Do patients with a limited number of brain metastases need whole-brain radiotherapy in addition to radiosurgery? Strahlenther Onkol 188:702–706
Bachtiary B et al (2003) Overexpression of hypoxia-inducible factor 1 alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res 9:2234–2240
Sperduto CM et al (2008) A validation study of a new prognostic index for patients with brain metastases: the Graded Prognostic Assessment. J Neurosurg 109(Suppl):87–89
Acknowledgements
We thank Carina Dinhof, Gerda Riecken, Irene Leisser, Ursula Rajky and Bettina Jesch for excellence technical assistance. The costs for this project were covered by the research budget of the Medical University of Vienna. This study was performed within the PhD thesis project of Anna Sophie Berghoff in the PhD program “Clinical Neuroscience (CLINS)” at the Medical University Vienna.
Compliance with ethical guidelines
Conflict of interest
A. S. Berghoff, A. Ilhan-Mutlu, A. Wöhrer, M. Hackl, G. Widhalm, J. A. Hainfellner, K. Dieckmann, T. Melchardt, B. Dome, H. Heinzl, P. Birner and M. Preusser state that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berghoff, A.S., Ilhan-Mutlu, A., Wöhrer, A. et al. Prognostic significance of Ki67 proliferation index, HIF1 alpha index and microvascular density in patients with non-small cell lung cancer brain metastases. Strahlenther Onkol 190, 676–685 (2014). https://doi.org/10.1007/s00066-014-0639-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0639-8