Abstract
Purpose
To investigate salvage treatment approaches and treatment outcomes in high-risk prostate cancer after radical prostatectomy (RP).
Methods
In this retrospective, multicenter study, 272 patients who underwent salvage radiotherapy (RT) ± androgen deprivation therapy (ADT) for recurrent prostate cancer after RP between 2007 and 2021 were analysed. Univariate analyses of time to biochemical and clinical relapse after salvage therapies were conducted using Kaplan–Meier plots and log-rank tests. Multivariate analyses were performed using a Cox proportional hazards model to determine the risk factors for disease relapse.
Results
Median age was 65 (48–82) years. All patients underwent salvage prostate bed RT. Pelvic lymphatic RT was performed in 66 patients (24.3%) and ADT was included in 158 (58.1%) patients. The median PSA value before RT was 0.35 ng/mL. The median follow-up time was 64 (12–180) months. 5-years bRFS, cRFS, and OS were 75.1%, 84.8%, and 94.9% respectively. In multivariate cox regression analysis; seminal vesicle invasion (HR 8.64, 95% CI 3.47–21.48, p < 0.001), pre-RT PSA higher than 0.14 ng/mL (HR 3.79, 95% CI 1.47–9.78, p = 0.006), and ≥ 2 positive pelvic lymph nodes (HR 2.50, 95% CI 1.11–5.62, p = 0.027) were found to be unfavorable prognostic factors for bRFS.
Conclusion
Salvage RT ± ADT provided 5-years biochemical disease control in 75.1% of patients. Seminal vesicle invasion, ≥ 2 positive pelvic nodes and delayed administration of salvage RT (PSA levels higher than 0.14 ng/mL) were found to be adverse risk factors for relapse. Such factors should be taken into account during the decision process on salvage treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the most common malignancy in men throughout the world [1]. Active surveillance, radical prostatectomy (RP) and radiotherapy (RT) are the main definitive treatment methods of the disease for localized stages [2, 3]. RT can also be used postoperatively as an adjuvant or salvage treatment. More than 60% of men with adverse pathologic risk factors such as extraprostatic extension (EPE), seminal vesicle involvement (SVI) and positive surgical margins develop a biochemical recurrence (bR) within 10 years after RP. Former randomized trials concluded that adjuvant RT halves bR in patients with these adverse risk factors [4,5,6]. More recently, RADICALS-RT, RAVES and GETUG-17 trials have found that early salvage RT ± androgen deprivation therapy (ADT) has disease control rates comparable to adjuvant RT with low urinary morbidity [7,8,9]. The pre-planned ARTISTIC collaborative meta-analysis of these three randomized trials also confirmed these results [10]. In addition to prostate bed RT, the benefit of ADT in the salvage setting has been shown in RTOG 9601 and GETUG-AFU 16 [11, 12]. Moreover, NRG Oncology/RTOG 0534 SPPORT reported that the combination of ADT and pelvic RT adjunct to prostate bed RT provided better freedom from progression rates [13]. In the era of ultra-sensitive prostate-specific antigen (PSA) analysis, early salvage RT is widely accepted by clinicians in low-intermediate risk patients. However, there are still controversial points regarding the timing of postoperative management of PCa after RP in high-risk patients. Besides, there is no consensus on the use of ADT and pelvic RT in the postoperative setting. The definition of the patient at high risk and the definition of recurrence; timing, dose, and target volume of RT; the addition, duration and form of ADT are the main research topics of the latest randomized trials.
In daily practice, clinicians have different approaches to patients with adverse risk factors. Some clinicians consider certain risk factors, while others consider the combination of these when deciding on the treatment scheme. In this study, we aimed to analyse the salvage treatment approaches and treatment outcomes for relapsed PCa patients treated in four centers retrospectively.
Material and method
This study is a multicenter, retrospective evaluation of salvage RT outcomes of the PCa patients treated at four radiotherapy centers between 2007 and 2021. A total of 300 consecutive patient data were collected from 2 public, 1 academic and 1 private hospital. Eligibility criteria after radical prostatectomy were as follows; 1. Persistently detectable PSA level, 2. PSA ≥ 0.1 ng/ml at any time, 3. Three consecutive PSA rises after an initially undetectable level. Patients who underwent adjuvant RT at undetectable PSA levels, who were followed earlier than a year, and who had a pre-existing malignancy were excluded from the study. There were 272 patients included in the final analysis. TNM staging was performed according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. The Common Terminology Criteria for Adverse Events v4.0 was used to evaluate acute and late genitourinary and gastrointestinal side effects. The study protocol was approved by the Local Ethics Committee (91/04.04.2022).
Statistical analysis
Categorical variables were presented with frequency and proportion, and continuous variables with median, minimum and maximum values. A Receiver Operating Characteristics (ROC) analysis was used to find the predictive cut-off value of pre-RT PSA for disease progression. Biochemical relapse-free survival (bRFS) was calculated from the end of the salvage RT to the second PSA relapse. PSA relapse after salvage RT was defined as PSA level ≥ 0.4 ng/mL or second initiation of salvage therapy. Clinical relapse-free survival (cRFS) was calculated from the end of salvage RT to the time of lymph node, bone, or visceral metastasis. Overall survival (OS) was calculated from the end of salvage RT to any cause of death. Kaplan–Meier method was used to calculate the 5-years bRFS, cRFS, and OS rates. Univariate analyses of time to bR and clinical relapse were conducted using Kaplan–Meier plots and log-rank tests. Multivariate analyses of time to bR and clinical relapse were performed using a Cox proportional hazards model with 95% confidence intervals (CIs). Statistical analyses were performed with SPSS version 20 for Windows (IBM Corp., Armonk, NY). The p value was set at < 0.05 for significance.
Results
The median age was 65 (48–82) years. Staging before surgery was performed with Magnetic resonance imaging (MRI) in 128 (47%) patients, bone scintigraphy in 10 (3.7%) patients and 68Ga-PSMA-PET/CT in 55 (20.2%) patients. All patients had radical prostatectomy; laparoscopic surgery was used in 71 (26.1%), and pelvic lymph node dissection was performed in 155 (57%) patients. A total of 42 (15.4%) patients had pathologically involved nodes. Patients’ and disease characteristics are given in Table 1.
Treatment after the first PSA relapse
Persistent PSA (≥ 0.1 ng/ml) after RP was present in 132 (48.5%) of the patients. MRI was used for restaging at relapse in 118 (43.4%) patients and 68Ga-PSMA-PET/CT was performed additionally in 71 (26.2%) patients. Of these, 26 (9.6%) had local, 10 (3.7%) had pelvic lymphatic and 4 (1.5%) had local + pelvic lymphatic recurrence. Salvage RT was implemented a median of 8 (4–149) months after RP. RT was delivered mostly with intensity-modulated radiation therapy (IMRT) in 232 (85.3%) patients and with three-dimensional conformal radiotherapy (3D-CRT) in 40 (14.7%) patients. RT target volumes consisted of prostate bed alone in 206 (75.7%) and prostate bed + pelvic lymph nodes in 66 (24.3%) patients. The median dose to prostate bed and pelvic lymphatics were 66 (60–74) Gy and 50 (45–56) Gy, respectively. Thirteen patients received lymphatic boost up to a median of 66 (60–78) Gy in addition to pelvic lymphatic irradiation. ADT was added in 158 (58.1%) patients. Treatment characteristics are given in Table 1.
Side effects
Overall, side effects were generally mild and RT was well tolerated. Grade 3–4 acute genitourinary and gastrointestinal side effects were seen in only 1 patient. Grade 3–4 late genitourinary and gastrointestinal side effects were seen in 6 (2.2%) and 4 (1.5%) patients, respectively.
Follow-up
The median follow-up time was 64 (12–180) months. Each patient was followed-up according to the protocols of each institute. The patients were followed-up every 3 months for the first 2 years, every 6 months till 5th year, and annually thereafter. PSA was evaluated in addition to a physical examination at each visit. Imaging interventions were performed in case of a rising PSA.
Treatment outcomes
Biochemical relapse after salvage treatment was observed in 49 (18%) patients and clinical relapse was observed in 34 (12.5%) patients. Clinical relapse was seen in the pelvic lymphatics in 11, in the paraaortic lymphatics in 4, in the bone in 17, and in the visceral organs in 2 patients. At the time of evaluation, 27 (9.9%) patients died, and only 4 (1.5%) died due to prostate cancer. 5-years bRFS, cRFS, and OS were found 75.1%, 84.8%, and 94.9% respectively. The cut-off PSA value for lower bRFS was found to be 0.14 (AUC 0.607, p 0.019) in ROC analysis.
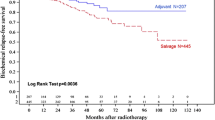
In univariate analysis, 5-years bRFS was found to be lower for patients ≤ 65 years old than for patients > 65 years old (68.4%, 83.8%, p 0.006), patients with pre-RT PSA level > 0.14 ng/mL than patients with ≤ 0.14 ng/mL (71.2%, 89.2%, p 0.035, Fig. 1a), patients with stages pT3b vs pT3a vs pT2 (49.1%, 82.8%, 90.3%, p < 0.001, Fig. 1b), and patients with pathologic pelvic lymph node involvement ≥ 2 vs 1 vs 0 (38.5%, 66.8%, 93.7%, p < 0.001). A trend for lower bRFS was also observed with increasing Gleason scores (p = 0.054). We did not find any difference between patients treated with and without pelvic lymph node RT, or between patients treated with and without ADT. This may be due to treatment selection bias. Not surprisingly, the proportion of patients with pN1, GS ≥ 8, and pT3b was higher in patients treated with pelvic lymph node RT and/or ADT. In multivariate analyses, stages pT3a-b, pre-RT PSA value above 0.14 ng/ml, and two or more pelvic lymphatic involvement were found independent adverse prognostic factors for bRFS. SVI was found to be the most significant adverse risk factor for disease progression (HR 8.6 95% CI, 3.4–21.5, p < 0.001). Results of univariate and multivariate analyses of bRFS are listed in Table 2. Similar results remained valid in the cRFS analysis.
Dıscussion
The optimal management of biochemically relapsed PCa after RP remains unclear. Several patient and disease characteristics are considered for the best-individualized salvage management strategy for PCa. In this study, 5-year bRFS for the whole cohort was 75.1% and the most important independent factors for worse disease control were found to be SVI, ≥ 2 pathologic nodal involvement and higher PSA levels (> 0.14 ng/ml) during salvage treatments. These findings are consistent with the latest randomized trials.
In NRG Oncology/RTOG 0534 SPPORT Trial; SVI, Gleason score of 8–9, and pre-RT PSA > 1 ng/mL were found to be significant adverse factors of freedom from progression. 5-years progression-free survival rates were improved from 70.9 to 81.3% with short-term ADT and to 87.4% both with short-term ADT and pelvic RT in addition to prostate bed RT. The 8-years estimated freedom from progression rate was worst in patients with SVI (37.2%), and improved to 50.8% when short-term ADT was added and to 59.6% when short-term ADT plus pelvic RT added [13]. In GETUG-AFU 16 study, better progression-free survival was achieved with 6 months of goserelin (5-years bRFS, 80% vs 62%, p < 0.0001). Similar to NRG Oncology/RTOG 0534 SPPORT, pre-RT PSA level and SVI were found to be unfovarable factors for relapse [12]. The advantage of long-term ADT in the salvage setting has also been demonstrated in two trials. In RTOG 9601 study, the 12-years incidence of prostate cancer mortality was 5.8% with 2 years of bicalutamide versus 13.4% with placebo [11]. In recently reported first results of the RADICALS-HD trial, 24 month ADT improved metastasis-free survival rates than 6 months ADT (HR 0.77; CI 0.61–0.97; 72% vs 78%) at 10 years [14]. In our study, 42% of the whole cohort and 30% of the patients with SVI did not use ADT. Adding at least 6 months of ADT could improve the bRFS rates of patients with SVI as well as the entire cohort. In the same way, pelvic RT was implemented in 24.3% of our patients. In our study staging with 68Ga-PSMA-PET/CT before salvage treatments was done in only 71 (26%) of patients. Pelvic lymph nodes were involved in 14 (20%) of them. These patients underwent pelvic RT with a boost dose to the involved lymph node(s) and no bR was observed until the time of evaluation. This underlines that 68Ga-PSMA-PET/CT must be performed prior to salvage RT for re-staging and modification of RT field and dose.
Early implementation of salvage RT is also important due to concerns about the progress to the metastatic stage. Tendulkar et al. reported that in a large, multicenter dataset of 2460 patients undergoing salvage RT for PCa, 5-year bRFS rates were 71% when salvage RT was performed at PSA levels of 0.01–0.2 ng/mL and decreased to 37% when PSA level was > 2.0 ng/mL. In this study, the overall 5-year bRFS was lower than our series (56% vs. 75%). This may be due to higher pre-RT PSA levels (median 0.50 ng/mL vs. 0.35 ng/mL) and a lower rate of ADT use (16% vs. 58%) in their patient cohort [15]. In the RADICALS-RT, RAVES and GETUG-AFU 17 trials, the median PSA before salvage RT was around 0.20–0.24 ng/mL and compared with historical data, the rates of disease control was higher than 85%. However, these randomized clinical trials have been criticized for the small number of high-risk patients (pN1, pT3b and GS ≥ 8), which may underpower subgroup analysis even at longer follow-ups. A huge study cohort of 26.118 patients recently reported by Tilki et al. found that adjuvant RT reduced all-cause mortality among men with pN1, GS ≥ 8 and pT3/T4 stages (HR 0.33, 0.13–0.85, p 0.02) [16]. They support adjuvant RT instead of early salvage RT in this high-risk patient group in their commantery [17]. Abdollah et al. also demonstrated the OS advantage of adjuvant RT in the presence of at least two of these risk factors [18].
Recent AUA/ASTRO guidelines state that patients with adverse risk factors, including R1, EPE, and SVI, “should be offered adjuvant RT”. EAU/ESTRO guidelines recommend adjuvant RT for patients with two of the risk factors, R1, pT3, and Gleason score ≥ 8. Both underlines the initiation of salvage RT at the earliest sign of PSA rise. ADT was strongly recommended in the AUA/ASTRO guidelines and weakly in the EAU/ESTRO guidelines for salvage setting, however, the duration was not pronounced [2, 19]. High-risk patients with SVI, GS ≥ 8, and positive pelvic nodes needs special attention. Early salvage prostate bed + pelvic RT and long-term ADT may be considered in patients with SVI and ≥ 2 positive pelvic lymph nodes. Given the high rates of comorbities in this age group, Adjuvant RT may also be an option in some of these patients who can not tolerate ADT and/or pelvic RT.
The main limitation of our study was its retrospective nature. Treatment biases based on risk factors limit the interpretation of results. Patients included in the study were treated at four different centers over a 15-years time period. The treatment protocols can be distinct and can have alterations during the years. 68Ga-PSMA-PET/CT was used for staging in a small number of patients both before the surgery and salvage RT. Some metastatic patients may have been undetected. PSA doubling time, which is an important factor, was not reached in all patients in this study. It can facilitate the postoperative management of PCa. Finally, the 5-years follow-up period may not be sufficient for PCa and longer follow-up may be required. However, the study addresses the real-life data of salvage treatment options for PCa.
Conclusion
Salvage RT ± ADT provided 5-year biochemical disease control in 75.1% patients. SVI, ≥ 2 positive pelvic nodes, and delayed administration of salvage RT (PSA > 0.14) were found to be independent adverse risk factors for the second relapse after salvage RT for PCa. Such factors should be taken into account during the decision process on salvage treatment.
Data availability
For data availability, please contact the corresponding author.
Change history
29 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00345-023-04498-6
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660. (Epub 2021 Feb 4)
https://uroweb.org/guideline/prostate-cancer/. Accessed 24 Feb 2023.
Maggi M, Cowan JE, Fasulo V, Washington SL 3rd, Lonergan PE, Sciarra A, Nguyen HG, Carroll PR (2020) The long-term risks of metastases in men on active surveillance for early stage prostate cancer. J Urol 204(6):1222–1228. https://doi.org/10.1097/JU.0000000000001313. (Epub 2020 Nov 6)
Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, Canby-Hagino E, Crawford ED (2009) Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 181(3):956–962. https://doi.org/10.1016/j.juro.2008.11.032. (Epub 2009 Jan 23)
Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven R, Colombel M, van de Beek C, Verhagen P, van den Bergh A, Sternberg C, Gasser T, van Tienhoven G, Scalliet P, Haustermans K, Collette L, European Organisation for Research and Treatment of Cancer, Radiation Oncology and Genito-Urinary Groups (2012) Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380(9858):2018–2027. https://doi.org/10.1016/S0140-6736(12)61253-7. (Epub 2012 Oct 19)
Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, Golz R, Störkel S, Willich N, Semjonow A, Stöckle M, Rübe C, Rebmann U, Kälble T, Feldmann HJ, Wirth M, Hofmann R, Engenhart-Cabillic R, Hinke A, Hinkelbein W, Miller K (2014) Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96–02/AUO AP 09/95 trial. Eur Urol 66(2):243–250. https://doi.org/10.1016/j.eururo.2014.03.011. (Epub 2014 Mar 21)
Parker CC, Clarke NW, Cook AD, Kynaston HG, Petersen PM, Catton C, Cross W, Logue J, Parulekar W, Payne H, Persad R, Pickering H, Saad F, Anderson J, Bahl A, Bottomley D, Brasso K, Chahal R, Cooke PW, Eddy B, Gibbs S, Goh C, Gujral S, Heath C, Henderson A, Jaganathan R, Jakobsen H, James ND, Kanaga Sundaram S, Lees K, Lester J, Lindberg H, Money-Kyrle J, Morris S, O’Sullivan J, Ostler P, Owen L, Patel P, Pope A, Popert R, Raman R, Røder MA, Sayers I, Simms M, Wilson J, Zarkar A, Parmar MKB, Sydes MR (2020) Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. Lancet 396(10260):1413–1421. https://doi.org/10.1016/S0140-6736(20)31553-1. (Epub 2020 Sep 28)
Sargos P, Chabaud S, Latorzeff I, Magné N, Benyoucef A, Supiot S, Pasquier D, Abdiche MS, Gilliot O, Graff-Cailleaud P, Silva M, Bergerot P, Baumann P, Belkacemi Y, Azria D, Brihoum M, Soulié M, Richaud P (2020) Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): a randomised, phase 3 trial. Lancet Oncol 21(10):1341–1352. https://doi.org/10.1016/S1470-2045(20)30454-X
Kneebone A, Fraser-Browne C, Duchesne GM, Fisher R, Frydenberg M, Herschtal A, Williams SG, Brown C, Delprado W, Haworth A, Joseph DJ, Martin JM, Matthews JHL, Millar JL, Sidhom M, Spry N, Tang CI, Turner S, Wiltshire KL, Woo HH, Davis ID, Lim TS, Pearse M (2020) Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol 21(10):1331–1340. https://doi.org/10.1016/S1470-2045(20)30456-3
Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, Sargos P, Sydes MR, Brawley C, Brihoum M, Brown C, Chabaud S, Cook A, Forcat S, Fraser-Browne C, Latorzeff I, Parmar MKB, Tierney JF, ARTISTIC Meta-analysis Group (2020) Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet 396(10260):1422–1431. https://doi.org/10.1016/S0140-6736(20)31952-8. (Epub 2020 Sep 28)
Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, Sartor O, Patel MP, Bahary JP, Zietman AL, Pisansky TM, Zeitzer KL, Lawton CA, Feng FY, Lovett RD, Balogh AG, Souhami L, Rosenthal SA, Kerlin KJ, Dignam JJ, Pugh SL, Sandler HM, NRG Oncology RTOG (2017) Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 376(5):417–428. https://doi.org/10.1056/NEJMoa1607529
Carrie C, Hasbini A, de Laroche G, Richaud P, Guerif S, Latorzeff I, Supiot S, Bosset M, Lagrange JL, Beckendorf V, Lesaunier F, Dubray B, Wagner JP, N’Guyen TD, Suchaud JP, Créhange G, Barbier N, Habibian M, Ferlay C, Fourneret P, Ruffion A, Dussart S (2016) Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol 17(6):747–756. https://doi.org/10.1016/S1470-2045(16)00111-X
Pollack A, Karrison TG, Balogh AG, Gomella LG, Low DA, Bruner DW, Wefel JS, Martin AG, Michalski JM, Angyalfi SJ, Lukka H, Faria SL, Rodrigues GB, Beauchemin MC, Lee RJ, Seaward SA, Allen AM, Monitto DC, Seiferheld W, Sartor O, Feng F, Sandler HM (2022) The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial. Lancet 399(10338):1886–1901. https://doi.org/10.1016/S0140-6736(21)01790-6
Parker CC, Clarke N, Cook A et al (2022) Ann Oncol 33(suppl_7):S808–S869. https://doi.org/10.1016/annonc/annonc1089; https://doi.org/10.1016/j.annonc.2022.08.064
Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM, Koontz BF, Hamstra DA, Feng FY, Liauw SL, Abramowitz MC, Pollack A, Anscher MS, Moghanaki D, Den RB, Stephans KL, Zietman AL, Lee WR, Kattan MW, Stephenson AJ (2016) Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol 34(30):3648–3654. https://doi.org/10.1200/JCO.2016.67.9647
Tilki D, Chen MH, Wu J, Huland H, Graefen M, Wiegel T, Böhmer D, Mohamad O, Cowan JE, Feng FY, Carroll PR, Trock BJ, Partin AW, D’Amico AV (2021) Adjuvant versus early salvage radiation therapy for men at high risk for recurrence following radical prostatectomy for prostate cancer and the risk of death. J Clin Oncol 39(20):2284–2293. https://doi.org/10.1200/JCO.20.03714. (Epub 2021 Jun 4)
Tilki D, D’Amico AV (2020) Timing of radiotherapy after radical prostatectomy. Lancet 396(10260):1374–1375. https://doi.org/10.1016/S0140-6736(20)31957-7. (Epub 2020 Sep 28)
Abdollah F, Suardi N, Cozzarini C, Gallina A, Capitanio U, Bianchi M, Sun M, Fossati N, Passoni NM, Fiorino C, Di Muzio N, Karakiewicz PI, Rigatti P, Montorsi F, Briganti A (2013) Selecting the optimal candidate for adjuvant radiotherapy after radical prostatectomy for prostate cancer: a long-term survival analysis. Eur Urol 63(6):998–1008. https://doi.org/10.1016/j.eururo.2012.10.036. (Epub 2012 Oct 26)
https://www.auanet.org/guidelines/guidelines/prostate-cancer-adjuvant-and-salvage-radiotherapy-guideline. Accessed 24 Feb 2023
Funding
None.
Author information
Authors and Affiliations
Contributions
Protocol/project development: FOD, HCY; Data collection or management: HCY, CB, NC, MP, NK; Data analysis: GC, HCY, CB; Manuscript writing/editing: HCY, STD, GY, FOD; Super-vision: FOD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study protocol was approved by the local ethics committee (91/04.04.2022).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yıldırım, H.C., Dinçer, S.T., Yaprak, G. et al. Adverse risk factors for salvage radiotherapy outcomes after radical prostatectomy in prostate cancer patients. World J Urol 41, 1503–1509 (2023). https://doi.org/10.1007/s00345-023-04419-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04419-7