Abstract
A 59-year-old patient with dilated cardiomyopathy and incessant ventricular tachycardia leading to progressive cardiogenic shock is presented. Due to hemodynamic instability, high dose catecholamines were required in addition to the implantation of an intraaortic balloon pump (IABP), which, however, appeared to further augment the frequency and duration of ventricular tachycardias. The implantation of a microaxial blood pump allowed catecholamine administration to be terminated, thereby, ending this vicious circle of catecholamine-driven electrical storm. Within 5 days, the patient was hemodynamically stabilized and kidney and liver function recovered with the support of intensive antiarrhythmic therapy (amiodarone, mexiletine, sotalol). During a 24-month follow-up, the patient had no further ICD shocks and no rehospitalization was required for treatment of congestive heart failure.
Zusammenfassung
Wir berichten über den Fall eines 59-jährigen Patienten, der auf dem Boden einer dilatativen Kardiomyopathie im elektrischen Sturm zunehmend in einen kardiogenen Schock geriet. Aufgrund der hämodynamischen Instabilität war eine hochdosierte Katecholamingabe notwendig sowie die Implantation einer intraaortalen Ballonpumpe (IABP). Unter dieser Therapie kam es jedoch zu einer Zunahme der Häufigkeit sowie der Dauer der ventrikulären Tachykardien. Erst die Implantation einer mikroaxialen Blutpumpe ermöglichte es uns, die Katecholamine auszuschleichen und damit den durch diese aufrechterhaltenen Teufelskreis zu durchbrechen. Innerhalb von 5 Tagen war der Patient unter zusätzlichem Einsatz einer intensiven antiarrhythmischen Therapie (Amiodarone, Mexiletin, Sotalol) hämodynamisch stabil; Leber- und Nierenfunktion erholten sich. Über ein Follow-up von 24 Monaten traten keine weiteren ICD-Schockabgaben auf, und es war keine weitere Hospitalisierung auf dem Boden der Herzinsuffizienz notwendig.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A 59-year-old patient with dilatative cardiomyopathy and incessant ventricular tachycardia (VT, 200 beats/minute) was admitted to our hospital (Fig. 1). In 2000, an automatic implantable cardioverter–defibrillator (AICD) had been implanted for primary prevention of sudden cardiac death due to a reduced left ventricular function and the inducibility of a persistent ventricular tachycardia (VT) upon programmed stimulation during an electrophysiological study. On the day of admission, the AICD had delivered multiple painful adequate shocks without persistent success.
On admission, the administration and loading with amiodarone was able to decrease the incidence of incessant VTs (150/minute). However, multiple episodes of incessant VTs did not respond to antitachycardia pacing (ATP). There was no hint for external inflammatory or endocrinological triggers (normal thyroid function). Coronary angiography ruled out coronary artery disease. An electrophysiological study showed four different types of VTs with similar cycle lengths ranging from 390–450 ms (133–153/min) originating from the anterolateral mitral valve annulus, inferior left ventricle, and two forms deriving from the inferolateral left ventricle near the origin of the papillary muscle. An ablation of the dominant form originating from the inferolateral left ventricle was performed. However, the VT was not terminable using adequate energy levels with respect to the close proximity to the insertion of the papillary muscle.
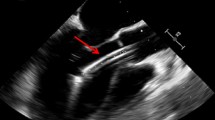
Recurrent incessant VTs continuously destabilized our patient, who suffered from progressive cardiogenic shock. Various therapeutic strategies including deep sedation/intubation did not reduce the number of ventricular arrhythmias. The implantation of an intraaortic balloon pump (IABP) only temporarily allowed the doses of catecholamines to be reduced (Fig. 2). Finally, a transfemoral microaxial blood pump (Impella® 2.5) was placed into the left ventricle (Fig. 3), which developed a blood flow of 2.5 l/minute This treatment allowed this vicious circle to be stopped and to avert hepatorenal failure. Catecholamines were continuously reduced (Fig. 2), which was accompanied by a reduced incidence of arrhythmias. The pump was removed after 5 days and the patient was extubated on the following day. Due to the hemolysis, which mostly occurs under the treatment with the Impella® 2.5, the patient required one homologous red cell concentrate to maintain a hemoglobin level above 8 g/dl.
Thereafter, the VTs continued to be refractory to antitachycardic pacing after loading with 1 g amiodarone over 10 days. The addition of mexiletine i.v. to amiodarone led to a significant reduction of the rate of the VTs. Using esmolol i.v. followed by carvedilol p.o. further stabilized sinus rhythm temporarily. However, recurrent VTs, now with a cycle length of about 480 ms (HR 125–130/minute) still remained resistant to ATPs. Finally the antiarrhythmic “cocktail” of amiodarone, mexiletine, and sotalol allowed the VTs to be terminated by ATP treatment and yielded a stable sinus rhythm.
Due to the severely reduced left ventricular systolic function and the severe and life-threatening course, we decided to list the patient for heart transplantation. In the course of mobilization, an ergometry up to 75 W was performed without inducing incessant VTs. Further VTs occurred at a cycle length of 500 ms (120/minute) and remained terminable via ATP (RAMP+). Four weeks after admission, the patient was completely recompensated. The patient was discharged with amiodarone 200 mg/day, mexiletine 200 mg 4x/day, and sotalol 80 mg 2x/day.
A follow-up investigation after 3 months revealed 147 VTs in the ICD memory, all of them successfully treated by ATP. After another 6 months, our patient reported regularly exercising up to 75 W on his home trainer. The ICD memory had stored 794 VT episodes (cycle lengths between 530 and 580 ms) all being successfully treated by ATP except two. These two episodes had continued up to 1 hour and had finally stopped spontaneously. The patient pointed out to feel “better than ever.” At 24 months after the acute event, there was no hospitalization due to congestive heart failure. The combination of amiodarone, mexitile, and sotalol was well tolerated. Due to his good quality of life, the patient refused to be further listed for heart transplantation.
Discussion
This patient presented with a continuously worsening cardiogenic shock due to repeated and incessant ventricular tachycardias. The tachycardias were resistant to ATP treatment by his AICD. Amiodarone therapy reduced the heart rate, but did not achieve the conversion to sinus rhythm or its stabilization after repeated defibrillations. The combination of two measures helped us to change the course of treatment: first, the implantation of the left ventricular microaxial blood pump (Impella® 2.5) allowed us to continuously reduce the use of catecholamines, which were essentially required as life-saving medications, on the one hand, but supported the incidence and rate of ventricular tachycardias, on the other hand. The Impella® pump assisted in ending this vicious circle. Second, the rather uncommon combination of the three antiarrhythmic agents (i.e., amiodarone, mexiletine, and sotalol) led to a continuous stabilization of the heart rhythm.
Using the Impella® 2.5 in patients with cardiogenic shock is an established option and has been validated in multiple reports showing good usability [1, 2, 5, 7]. The device has previously been successfully used as a bridge to recovery in patients with septic or cardiogenic shock secondary to acute fulminant myocarditis [3] or acute myocardial infarction [5]. The Impella® 2.5 is also frequently used periinterventionally in patients undergoing high-risk percutaneous coronary interventions [4, 6]. In addition, this device is routinely used in many centers perioperatively for heart surgery in patients with low output failure [1]. The device has also previously been used in patients with tachyarrhythmias: it has been used during catheter ablation of an intraatrial reentrant tachycardia [8] and during ablation therapy of a hemodynamically unstable ventricular tachycardia allowing successful completion of the procedure [9].
The Impella® 2.5 is relatively easy to implant by an experienced interventionalist. The most commonly reported complications of Impella 2.5 placement and support include bleeding, vascular injury, and limb ischemia [10, 11]. Hemolysis has been reported but seems to be a transient side effect [5, 12]. There seems to be no significant long-term adverse effect on the aortic valve [13]. Device failure, although not often reported, can occur. Our patient required one concentrate of red blood cells.
This report shows the successful treatment of cardiogenic shock due to incessant VT by combining temporary circulatory support (Impella® 2.5) with a “cocktail” of antiarrhythmic drugs.
References
Siegenthaler MP, Brehm K, Strecker T et al (2004) The Impella recover microaxial left ventricular assist device reduces mortality for postcardiotomy failure: a three-center experience. J Thorac Cardiovasc Surg 127:812
Jurmann MJ, Siniawski H, Erb M et al (2004) Initial experience with miniature axial flow ventricular assist devices for postcardiotomy heart failure. Ann Thorac Surg 77:1642
Colombo T, Garatti A, Vitali E et al (2003) First successful bridge to recovery with the Impella recover 100 left ventricular assist device for fulminant acute myocarditis. Ital Heart J 4(9):642–645
Thomopoulou S, Manginas A, Cokkinos DV (2008) Initial experience with the Impella recover LP 2.5 micro-axial pump in patients undergoing high-risk coronary angioplasty. Hellenic J Cardiol 49(6):382–387
Seyfarth M, Sibbing D, Schömig A et al (2008) A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 52(19):1584–1588
Dixon SR, Henriques JP, O’Neill WW et al (2009) A prospective feasibility trial investigating the use of the impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial U.S. experience. JACC Cardiovasc Interv 2(2):91–96
Mueller XM, Boone Y, Segesser LK von et al (2001) Biventricular axial micropump: impact on blood cell integrity. Swiss Surg 7(5):213–217
Fishberger SB, Asnes JD, Cleman MW et al (2010) Percutaneous right ventricular support during catheter ablation of intra-atrial reentrant tachycardia in an adult with a mustard baffle. A novel use of the Impella device. J Interv Card Electrophysiol 29(1):69–72
Abuissa H, Roshan J, Asirvatham SJ et al (2010) Use of the Impella microaxial blood pump for ablation of hemodynamically unstable ventricular tachycardia. J Cardiovasc Electrophysiol 21(4):458–461
Remmelink M, Sjauw KD, Henriques JP et al (2007) Effects of left ventricular unloading by impella recover LP 2.5 on coronary hemodynamics. Catheter Cardiovasc Interv 70(4):532–537
Henriques JPS, Remmelink M, Baan J et al (2006) Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella recover LP 2.5. Am J Cardiol 97(7):990–992
Dixon SR, Henriques JPS, Mauri L et al (2009) A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (the PROTECT I trial): initial U.S. experience. J Am Coll Cardiol 2:91–96
Engström A, Sjauw K, Henriques J et al (2011) Long-term safety and sustained left ventricular recovery: long-term results of percutaneous left ventricular support with Impella LP2.5 in ST-elevation myocardial infarction. EuroIntervention 6(7):860–865
Conflict of interest
The corresponding author states that there are no conflicts of interest for any author of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henning, A., Schreieck, J., Riessen, R. et al. Successful bridge to recovery using a microaxial blood pump in a patient with electrical storm and cardiogenic shock. Med Klin 106, 132–136 (2011). https://doi.org/10.1007/s00063-011-0047-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00063-011-0047-0