Abstract
Short-term mechanical circulatory support (MCS) devices are designed to provide hemodynamic support for a wide range of clinical conditions such as high-risk cardiac surgery or interventional procedures, post-cardiotomy cardiogenic shock, acute decompensated heart failure. Electrical storm (defined as three or more sustained episodes of ventricular fibrillation-VF- in a 24-h period) is a rare but critical complication following revascularization in patients with ischemic heart disease and it is associated with a very high mortality (80–90%) both during the incident alone and during further observation. Here we report the case of a 38-year-old patient affected by coronary artery disease with moderate to severe left ventricular systolic dysfunction (EF 30–35%) who underwent emergency coronary artery bypass grafting (CABG) complicated by electrical storm and severe haemodynamic instability, successfully managed with a novel approach of biventricular mechanical circulatory support with extracorporeal life support (ECLS) in first instance, subsequently switched to Impella CP and ProtekDuo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Short-term mechanical circulatory support (MCS) devices are designed to provide hemodynamic support for a wide range of clinical conditions such as high-risk cardiac surgery or interventional procedures, post-cardiotomy cardiogenic shock, acute decompensated heart failure, or cardiopulmonary arrest [1, 2]. Impella CP heart pump (Abiomed, Danvers, MA) is an intravascular microaxial blood pump that delivers up to 4.3 L/min of antegrade blood flow from the left ventricle (LV) into the ascending aorta, thus achieving a significant unloading of the failing LV. The transluminal placement of the pump crossing the aortic valve into the LV is performed via femoral or axillary artery access and under transesophageal echocardiography and fluoroscopy guidance [3]. The ProtekDuo cannula (CardiacAssist, Inc, Pittsburgh, PA) is a flexible, dual lumen and partially wire-reinforced cannula providing drainage of venous blood through the outer 29 Fr lumen from the right atrium and output through the tip of the inner 16 Fr cannula into the main pulmonary artery. When required, it is possible to add an oxygenator into the circuit to support the lung. The cannula can be combined with any centrifugal pump as a temporary right ventricle (RV) assist device [4].
Electrical storm (defined as three or more sustained episodes of ventricular fibrillation-VF- in a 24-h period) is a rare but critical complication following revascularization in patients with ischemic heart disease and it is associated with a very high mortality (80–90%) both during the incident alone and during further observation [5]. The pathogenesis of this condition can include myocaridal ischemia (e.g., occluded grafts), electrolyte derangements, pacing-induced R-on-T (temporary epicardial pacing wire), reperfusion syndrome.
Here we report the case of a 38-year-old patient affected by coronary artery disease with moderate to severe left ventricular systolic dysfunction (EF 30–35%) who underwent emergency coronary artery bypass grafting (CABG) complicated by electrical storm and severe haemodynamic instability, successfully managed with a novel approach of biventricular mechanical circulatory support with extracorporeal life support (ECLS) in first instance, subsequently switched to Impella CP and ProtekDuo.
Case report
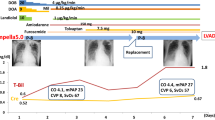
A 38-year-old gentleman with type 1 diabetes mellitus and previous myocardial infarction treated with percutaneous coronary intervention (PCI) plus stents on left anterior descending (LAD) artery, circumflex artery (Cx) and right coronary artery (RCA) presented to our emergency department with a non-ST elevation myocardial infarction (NSTEMI). A coronary angiogram showed significant LAD and Cx intra-stent restenosis and new progressive disease affecting left main with a clear indication to surgical revascularization. A transthoracic echocardiogram showed a reduced LV systolic function (EF 30–35%), with no significant valve abnormalities. He underwent on pump coronary artery bypass on a beating heart (left internal mammary artery -LIMA- to LAD, left radial artery Y-conduit from LIMA to diagonal, autologous saphenous vein to obtuse marginal artery of Cx). The intraoperative measurement of coronary grafts flow and the transesophageal echocardiogram were satisfactory, so the patient was transferred to the intensive care unit in good condition and with low dosage of vasoconstrictors (noradrenaline 0.05 mcg/kg/min) and extubated a few hours later. On post-operative day 3, he suddenly developed several episodes of VF requiring DC shock, severe haemodynamic compromise and worsening biventricular dysfunction requiring high doses of inotropic support (enoximone 5 mcg/kg/min, dobutamine 5 mcg/kg/min, noradrenaline 0.1 mcg/kg/min). Despite escalated medical treatment including inotropic support and anti-arrhythmic drugs (amiodarone and lidocaine), his general conditions deteriorated. A coronary angiogram and an electrophysiology study failed to demonstrate early graft failure or a depolarization abnormality. On day 5, due to refractory electrical storm requiring cardiopulmonary resuscitation and emergency reopening of the chest, and in view of the subsequent low cardiac output syndrome and multiorgan failure, we decided to assist the patient with a mechanical circulatory support as a bridge to decision. Initially he was supported with ECLS; the arterial line was connected to the ascending aorta, the venous line was connected to the right femoral vein (VA-ECMO). Heparin was started immediately before implantation (target activated clotting time [ACT] 240 s). Due to ongoing episodes of VF, an Impella CP via right axillary artery was placed to allow an effective unloading of the LV (Fig. 1). The heparin purge fluid was applied and dosed by the Impella controller. The goal ACT was kept at 180–220 s as recommended by the manufacturer. In the following 4 days, the condition of multiorgan failure significantly improved as demonstrated by improved renal function (Fig. 2a), decreased level of lactate (Fig. 2b) and alanine transaminase (Fig. 2c). Several neurological windows showed preserved neurological function, the episodes of ventricular arrhythmias reduced and after 1 week, the ECLS was discontinued while the Impella CP was kept in situ and set to deliver an estimated 3.8–4.0 L/min; however, due to the echocardiographic evidence of residual impaired RV function with tricuspid annular plane excursion (TAPSE) 11 mm, increased central venous pressure (CVP) > 18 mmHg, decreased pulmonary artery pulsatility index—(PAPi) < 2.0 despite inotropic support and inhaled nitric oxide, we decided to provide support to the RV by positioning a ProtekDuo cannula (percutaneosuly inserted through the right jugular vein under fluoroscopy guidance as showed in Fig. 3) which was connected to a centrifugal pump and set to delivery an estimated 3.8–4.0 L/min flow. At this stage, patient was successfully extubated. Impella CP and ProtekDuo were kept up to day 14 and then weaned off once achieved complete stabilization of the haemodynamic parameters with improved cardiac index (> 2.0), decreased CVP (< 14 mmHg), improved PAPi (> 3.0) and laboratory evidence of improved right-sided dysfunction and end organ perfusion. Patient was then moved to our cardiac ward. A repeated TTE showed moderately impaired LV function (EF 40%) and improved RV function with TAPSE 17 mm. On day 24, the patient was discharged from our unit.
Fluoroscopy controlled positioning of ProtekDuo cannula providing drainage of venous blood through the outer 29 Fr. lumen from the right atrium (red arrow) and output through the tip of the inner 16 Fr. cannula into the main pulmonary artery (yellow arrow). It is also evident the Impella CP pump (black asterisk) previously implanted in the LV crossing the aortic valve. LV left ventricle
Discussion
In a contemporary practice, short-term MCS is increasingly employed as a bridge to decision in patients with refractory cardiogenic shock [1, 2]. Subsequently, these patients might be upgraded to durable MCS either as a bridge to recovery or candidacy/transplantation, or as destination therapy. Both central and peripheral ECLS/ECMO in the heart failure setting carries certain limitations and risks such as bleeding, thrombotic or air embolism, extended time on the respirator, difficult mobilization due to the cannulas which are either directly connected to the aorta or are placed into the groin vessels thus potentially leading to limb ischemia [6]. Moreover, infection such as cannulation site infection, pneumonia, sepsis are common complications in ECLS occurring in up to 13% of adult patients [4]. In the setting of a dilated, poorly contracting heart with severe systolic dysfunction, decompression by LV venting might be crucial for the recovery of myocardium. To address LV unloading, the concept of Impella (2.5 or CP) system in combination with ECLS has already been described as ECPELLA [7]. However, other ECLS limitations and complications persist. Some authors have reported their experience of early weaning from VA-ECMO to minimize the risks related to long-term support with ECLS [8]. Ruhparwar and colleagues have recently reported a novel successful approach of biventricular support with Impella 5.0 and ProtekDuo in a series of two patients with severe cardiogenic shock (INTERMACS I) [4]. We believe that our approach of early weaning from VA-ECMO with Impella CP combined to ProtekDuo has proved to be valid and safe. In the clinical scenario, we have described in our report, the prompt use of RVAD, based on the evidence of increased CVP and decreased PAPi [9], as well as echocardiographic evidence of reduced TAPSE, has proved to be a crucial decision for the good outcome of the patient. By continuous biventricular support, we managed to keep haemodynamic stability and to revert the condition of the multiorgan failure syndrome due to the electrical storm. This strategy allowed to minimize ECMO-related complication and to support the patient up to biventricular recovery. The weaning strategy from biventricular mechanical support was driven by improved haemodynamic and clinical parameters such as reduced CVP, reduced systemic lactate level, adequate urine output and increased PAPi. However, we believe that echocardiography is the cornerstone for assessment of cardiac recovery allowing decision-making in favour of or against VAD removal. We decided to go ahead with the weaning off when the following echocardiographic parameters were achieved during temporary interruption of mechanical unloading (“off-pump trials”): LV end-diastolic diameter (LVEDD) < 55 mm and fractional shortening (FS) > 15%, no or ≤ grade I mitral and/or aortic regurgitation, no RV dilation and tricuspid regurgitation ≤ grade II. Firstly, Protek Duo cannula was weaned off, and then Impella CP support was gradually discontinued and finally removed the following day.
To our best knowledge, this is the first report of a similar strategy for the treatment of cardiogenic shock due to postcardiotomy electrical storm.
References
Mohite PN, Sabashnikov A, Koch A, Binu R, Padukone A, Kaul S, Maunz O, García-Sáez D, Zych B, Husain M, De Robertis F, Popov AF, Simon AR. Comparison of temporary ventricular assist devices and extracorporeal life support in post-cardiotomy cardiogenic shock. Interact Cardiovasc Thorac Surg. 2018;27(6):863–9. https://doi.org/10.1093/icvts/ivy185.
den Uil CA, Akin S, Jewbali LS, Dos Reis MD, Brugts JJ, Constantinescu AA, Kappetein AP, Caliskan K. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2017;52(1):14–25. https://doi.org/10.1093/ejcts/ezx088.
Monteagudo-Vela M, Simon A, Riesgo Gil F, Rosenberg A, Dalby M, Kabir T, García Saez D, Panoulas V. Clinical indications of IMPELLA short-term mechanical circulatory support in a tertiary Centre. Cardiovasc Revasc Med. 2019 Dec 6. pii: S1553-8389(19)30806-1. doi: https://doi.org/10.1016/j.carrev.2019.12.010.
Ruhparwar A, Zubarevich A, Osswald A, Raake PW, Kreusser MM, Grossekettler L, Karck M, Schmack B. ECPELLA 2.0-Minimally invasive biventricular groin-free full mechanical circulatory support with Impella 5.0/5.5 pump and ProtekDuo cannula as a bridge-to-bridge concept: a first-in-man method description. J Card Surg. 2020;35(1):195–9. https://doi.org/10.1111/jocs.14283.
Montgomery ML, Oloomi M, El-Eshmawi A, Adams DH. Electrical storm after coronary artery bypass grafting: diagnosing and treating the trigger. J Cardiothorac Vasc Anesth. 2019;33(2):497–500. https://doi.org/10.1053/j.jvca.2018.02.001.
Dalia AA, Lu SY, Villavicencio M, D’Alessandro D, Shelton K, Cudemus G, Essandoh M. Ortoleva J. Extracorporeal cardiopulmonary resuscitation: outcomes and complications at a quaternary referral center. J Cardiothorac Vasc Anesth. 2019 14. pii: S1053-0770(19)31270-4. doi: https://doi.org/10.1053/j.jvca.2019.12.016.
Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Müllerleile K, Colombo A, Sydow K, De Bonis M, Wagner F, Reichenspurner H, Blankenberg S, Zangrillo A, Westermann D. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19(3):404–12. https://doi.org/10.1002/ejhf.668.
Bertoldi LF, Pappalardo F, Lubos E, Grahn H, Rybczinski M, Barten MJ, Bertoglio L, Schrage B, Westermann D, Lapenna E, Reichenspurner H, Bernhardt AM. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: De-escalate and ambulate. J Crit Care. 2020 Jan 10. pii: S0883-9441(19)31374-7. doi: https://doi.org/10.1016/j.jcrc.2019.12.028.
Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2016;35(1):67–73. https://doi.org/10.1016/ì/j.healun.2015.06.009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chivasso, P., Miele, M., Romano, R. et al. Impella CP and ProtekDuo as a bridge to recovery following surgical revascularization complicated by electrical storm. Gen Thorac Cardiovasc Surg 69, 877–881 (2021). https://doi.org/10.1007/s11748-020-01571-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-020-01571-4