Abstract
Thrombectomy is a technique that has completely changed the management of acute stroke and current devices have shown that they can achieve upwards of 90% successful recanalization in selected cohorts. However, despite the effectiveness of these devices, there are a proportion of patients who still fail to achieve reperfusion of the affected vascular territory and an even larger portion of patients who have poor functional outcomes in spite of successful recanalization. There are no guidelines on how to treat these patients when such failures occur. In an effort to understand the underpinnings of how failed thrombectomy occurs, we extensively reviewed the current literature in clot properties, vascular access problems, stroke pathogenic mechanisms, embolic complications, failed procedures and pre-procedural imaging. A short summary of each of these contentious areas are provided and the current state of the art. Together these elements give a cohesive overview of the mechanisms of failed thrombectomy as well as the controversies facing the field. New techniques and devices can then be developed to minimize such factors during stroke thrombectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the rapid advancement of thrombectomy has revolutionized acute stroke care, establishing it as first-line therapy for acute ischemic stroke (AIS) with large vessel occlusion [1,2,3,4]; however, up to 20% of AIS patients treated with mechanical thrombectomy do not have successful recanalization and only around 50% eventually achieve good functional outcomes [5,6,7,8,9,10]. With each failed thrombectomy attempt, reperfusion to the brain is delayed, and each additional pass attempted augments the friction coefficient between the thrombus and the vessel wall with cumulative risks for procedural complications, such as dissection and distal embolization into unaffected territories [7, 9]. This article reviews the factors responsible for failure of thrombectomy that have been described in the recent literature.
Clot Properties
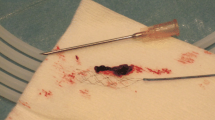
Thrombus composition is a key factor in determining its susceptibility to mechanical thrombectomy methods and the chance of successful recanalization. The precise composition of a thrombus is highly dependent on its source and etiology, but generally consists of fibrin and red blood cells with an additional minor content of white blood cells (WBC) (Fig. 1; [11, 12]).
The proportion of fibrin relative to the red blood cells in a thrombus determines its physical properties and how it responds to thrombectomy. Hypodense, fibrin-rich thrombi demonstrate decreased revascularization rates regardless of the technique employed [13, 14]. Laboratory flow model experiments have echoed similar findings found in clinical practice, with fibrin-rich thrombi that comprise <20% red blood cells having much higher static friction properties and are hence more difficult to mobilize and remove [15]. A mature clot rich in fibrin is also firmer and less deformable when interacting with the struts of a stent retriever, leading the clot compaction which in turn increases friction between the clot and the vessel wall. This leads to each attempt being less effective than the previous one and less likely to remove the clot [15, 16]. Newer generation stent retrievers with increased radial force or which are designed to scoop or capture the clot within its struts rather than penetrate the thrombus, may be more effective in extracting firmer clots rich in fibrin. These newer devices also tend to have a distal net to capture embolized particles.
The amount of time a thrombus has had to mature often determines how “sticky” it is and how difficult it is to be extracted [17]. This is reinforced by the water-hammer effect of systemic blood pressure that squashes and compacts the thrombus making it denser and more resistant to extraction. Finally, as it becomes more compressed, the fibrin-rich clot tends to remain in the same position during the thrombectomy attempt. This is due to the struts of the stent retriever having a greater tendency to “slip” over the dense clot as it is withdrawn, or the clot does not fit securely into the mouth of an aspiration catheter due to its firm characteristics. In contrast, clots rich in red blood cells are soft and easier to remove but tend to be friable and prone to fragmentation, which may lead to distal embolization complications.
The content of WBCs in the thrombus has also been shown to be associated with the ease of recanalization and the length of the procedure [18]. Histological studies have shown that vessel injury leads to platelet attraction, which catalyzes further platelet clumping. The WBCs then migrate to the edges of the platelet clump and fibrin strands form, which serve to trap RBCs giving the thrombus its shape and form [19, 20]. The proportion of WBCs and the degree of migration into the clot are therefore a surrogate marker of the maturity of the thrombus. In more mature clots, the WBCs have had more time to invade the thrombus and this is associated with an increased degree of organization of the thrombus, which make them more resilient and difficult to remove. This organization is closely associated with the stability of the clot and the friction to the vessel wall. More mature clots are therefore more difficult to remove [21]. While there are different types of WBCs, neutrophils specifically catalyze the thrombotic pathway by formation of neutrophil extracellular traps (NETs) [22]. These NETs are created by decondensed deoxyribonucleic acid (DNA) fibers released by neutrophils into the extracellular matrix. The DNA fibers act as supports for red blood cells (RBCs) and platelets to bind to and propagate the clotting cascade [23]. A potential diagnostic use was put forth when an association between NETs and cardioembolic etiology was found [24]. The proportion and location of WBCs and NETs can also determine the underlying etiology of the thrombus; in cardioembolic thrombi a higher proportion of WBCs and NETs are found in the clot, which may be due to the longer time it had to mature in the heart chamber before embolization.
There are other distinct histological features cited in the literature, which may have the potential to determine if clots are resistant to mechanical thrombectomy. Partial endothelialization at the edges of the thrombus is thought to represent early organization and maturation as the body attempts to grow endothelium over the thrombus [25]. It is thought to be associated with mature thrombi that are more difficult to remove. Furthermore, thrombi of atypical origin, such as calcified plaques or neoplastic thrombi can be more resistant to removal [26, 27]. Several cases have been described with failed thrombectomy where the plaque was heavily calcified. After multiple stent retriever attempts were unsuccessful, intracranial balloon angioplasty was required to improve the blood flow. In another case report, a stent retriever was trapped in a dense calcification and could not be withdrawn and finally the stent retriever had to be re-sheathed within the microcatheter [13]. Finally, other plasma constituents, such as von Willebrand factor may contribute to clot resistance. Von Willebrand factor is a large multimeric plasma glycoprotein that links platelets in conjunction with fibrin. A novel study showed that the level of von Willebrand factor correlates with more organized and firmer clots [28, 29]. Despite this level of understanding of how clot composition interacts during thrombectomy, the histological make-up of a clot remains unknown prior to the procedure, and hence all thrombi are currently approached in the same manner. Methods to determine the thrombus type before the procedure are a crucial gap that needs to be addressed and such methods are presently being developed for an in vivo examination of the clot before the first retrieval attempt.
The volume of the thrombus or thrombus burden has a strong association with the location of the occlusion. The thrombus burden tends to be largest in the internal carotid artery and smallest in the distal middle cerebral artery [30]. Even though a larger thrombus burden would have a commensurately larger surface area for thrombus-vessel interaction and therefore increased friction, the literature on thrombus size and successful thrombectomy recanalization has not been able to show such a linear relationship. Higher clot burden scores were associated with better recanalization rates but a longer clot was shown to have poorer functional outcomes in the THERAPY trial [31,32,33,34,35,36,37,38]. Nonetheless, the optimal thrombectomy device is dependent on the thrombus burden. For example, an intermediate catheter that is too small will lead to shearing of the edges of the clot when it is withdrawn into the guide catheter. An AIS that has been proven to be resistant to one thrombectomy method may respond well to a different approach. Traditionally after stent retrievers or aspiration catheters have failed three passes, it is thought to be useful to switch to another method [39]. Currently it appears better to use techniques that optimize all factors, such as the PROTECT-plus technique [40]. In the future, it may be possible to individualize the thrombectomy devices and techniques with respect to different clot locations, clot composition and patient factors.
Access Problems and Stroke Etiology
A frequent reason for failed thrombectomy or abandonment of the procedure is the problem of difficult vascular access. There were two recent large series that looked at factors associated with failed thrombectomy (defined as TICI [thrombolysis in cerebral infarction] 0/1). In the first study, 72 out of 648 consecutive stroke patients had failed thrombectomy. In one fifth of these patients the occlusion could not be reached and in another fifth the thrombus was not passed [41]. In the second series, 63 out of 592 patients had failed thrombectomy, with older age and M2 occlusions being factors associated with failure [42]. In both series, major equally frequent reasons for failed thrombectomy were difficult anatomical access and resistant occlusions [41, 42].
If the patient has calcified or non-patent femoral arteries, an ectatic aortic arch or tortuous neck vessels, it can be difficult to gain access even to the intracranial circulation. An ectatic aorta can be difficult to navigate and other variations such as a bovine arch can render the procedure even more challenging, with the risk of the guiding catheter prolapsing into the aorta during the procedure and the surgeon having to start again [43, 44]. Tortuous, looped or kinked cervical vessels can also prevent smooth navigation as well as take up too much of the catheter length rendering it too short to reach the occlusion [45, 46]. In the light of such challenges, which can often be determined on a preprocedural computed tomography (CT) angiogram, occlusions in certain vessel are sometimes much more easily accessible via an alternative access site; a common example is basilar occlusion for which a radial artery approach can be a superior access point. This will reduce complications and diminish wastage by avoiding changing catheters or wires that are not suitable, as well as cutting down the length of the procedure, ultimately leading to earlier reperfusion of the brain. When the aortic arch or the carotid artery is seen to be challenging in an anterior circulation thrombectomy, a direct common carotid puncture can sometimes help with access as well as to simplify and speed up the procedure; however, there is a shortage of tailored material and risks for carotid wall hematoma formation as well as for postprocedural embolic complications. In addition, there is equipoise regarding closure of the puncture site where both manual compression as well as closure devices and open surgery have been advocated. Because of the risk of a neck hematoma in cases of insufficient closure, most surgeons prefer to treat these patients while they are under general anesthesia to have the airways protected.
In the intracranial circulation, the geometric anatomy of the blood vessel can be a factor leading to the failure of the stent to remove the thrombus. An increase in vessel diameter can cause the clot to fall out of the stent as it is being pulled, e. g. if the stent does not expand to accommodate the wider vessel. A vessel shaped in a hairpin loop will increase the friction of the clot as it is being withdrawn by dragging it against the vessel wall [47, 48]. Similarly, with a hairpin loop or an S‑configuration vessel, avulsion injuries of small adjacent blood vessels are possible during withdrawal of a stent retriever (Fig. 2). This occurs because the pulling force is perpendicular to the direction of the blood vessel. Using an intermediate catheter will improve the vector of force and reduce the risk of avulsion injuries to the vessel or other complications (Fig. 3).
Flow model demonstrating the importance of an intermediate catheter in tortuous anatomy. a Stent retriever without an intermediate catheter in a hairpin turn. b On withdrawal, the friction causes traction and deformation of the vessel without clot removal. c Stent retriever with an intermediate catheter in a hairpin turn. d The change in the direction of force allows withdrawal of the clot without deformation of the vessel (courtesy of Neuravi Inc, Galway, Ireland
Digital subtraction angiography in the lateral views (a, c) and anteroposterior view (b, d). An S‑shaped turn of the vessel and the tortuous path taken by the guidewire. On stent retriever thrombectomy, the high friction between the stent retriever and the wall caused an inadvertent detachment of the stent retriever (arrow in c and d)
An underlying stenotic lesion due to an intracranial atherosclerotic plaque is a possible reason for immediate reocclusion postthrombectomy that results in failure of the thrombectomy procedure [49]. This is more prevalent in Asians and African-Americans compared to the Caucasian population, although typical cardiovascular risk factors such as hypertension and diabetes mellitus remain important for intracranial atherosclerosis in any population [50,51,52]. The location of the occlusion can occasionally suggest an underlying intracranial atherosclerotic stenosis, such as a mid-basilar occlusion with flow in the basilar termination or mid-M1 occlusion with flow in the MCA bifurcation (Fig. 4; [53]). When performing thrombectomy for patients with underlying atherosclerotic lesions, there is often a tendency to require other adjunct devices in addition to the stent retriever, culminating in longer procedure times. This is in contrast to simple cardioembolic occlusions causing AIS, where a stent retriever alone is successful in up to 83.3% of the patients [54]. Other underlying vessel wall pathologies, such as vasculitis or early moyamoya disease can also render the thrombectomy devices ineffective and are rarer causes for failure of thrombectomy [55, 56]. In such cases, without vessel wall magnetic resonance imaging (MRI), it is often difficult to confirm the presence of such a pathology. Therefore, a high degree of clinical suspicion is important to prevent futile repetitive procedures.
In stroke patients with failed mechanical thrombectomy (MT) attempts, approximately 60% of the occlusions can be passed. In such cases, rescue treatment might be considered to improve recanalization and clinical outcome [41]. In these situations, a permanent intracranial artery stent deployment might be a potential solution. Intracranial stenting was first performed in AIS with off-label use of balloon-mounted cardiac stents [57,58,59,60]. Recently, a Korean study demonstrated more favorable outcomes in a group of patients with failed thrombectomy but were rescued with permanent stenting as compared to the non-stented group of patients with failed thrombectomy [5]. A meta-analysis was recently performed on rescue stenting in failed thrombectomy and 8 studies comprising 160 patients were included in which the rate of mTICI 2b-3 was 71%. This led to good outcomes in 43% of the patients and death in 21%. Symptomatic intracerebral hemorrhage was seen in only 12% of the cohort although glycoprotein IIb/IIIa inhibitors were used in 89% of the cases and almost all the patients had antiplatelet therapy after the procedure [61]. The current iteration of intracranial self-expanding stents is based on nitinol and the stents are designed to provide a balance of sufficient outward radial force at body temperatures to open up occluded vessels, yet minimizing the incidence of negative remodeling and in-stent restenosis that have been reported in studies using balloon-mounted stents [62, 63].
Other rescue modalities have also been studied. An interesting study from the North American Solitaire Stent-Retriever Acute Stroke registry looked at failed thrombectomy and intra-arterial tissue plasminogen activator (IA tPA) rescue therapy. There was no significant increase in recanalization, functional outcome, mortality or SICH with IA tPA; however, a sub-group analysis limited to M1 occlusions only and an onset-to-groin puncture ≤8 h, resulted in significantly higher successful revascularization rates with IA tPA rescue therapy (77.8% versus 38.9%; P = 0.02) [64]. A similar study used a low dose of the glycoprotein IIb/IIIa inhibitor tirofiban as rescue treatment in patients with failed thrombectomy. In 154 patients tirofiban was not associated with increased numbers of symptomatic intracranial hemorrhage (SICH) or mortality. A subset of patients with large artery atherosclerosis were associated with a mortality benefit when treated with tirofiban [65].
Distal Emboli and Futile Revascularization
When a thrombectomy procedure is performed, one of the complications that can lead to a worse functional outcome despite good recanalization is embolization from the original occlusive thrombus distally to the same or previously unaffected cerebral territories (Fig. 5; [66, 67]). Embolic complications have been reported with different thrombectomy devices [68,69,70,71]. Many various thrombectomy techniques were used in the MR CLEAN study which reported embolization to new territories in 8.6% of patients, which resulted in 5.6% developing new neurological deficits [1, 72]. In stent retriever studies, 5–22% had distal emboli, with 0–7% of these in new vascular territories [4, 7, 73,74,75,76]. Older thrombectomy devices, such as the MERCI (Concentric medical, Mountain View, CA, USA) device or the initial iteration of the Penumbra system with the separator (Penumbra Inc., Alameda, CA, USA) tended to macerate the clot more with more fragments being generated causing distal emboli. They were shown to have worse outcomes compared to intra-arterial tPA thrombolysis, and therefore are no longer used [72, 77,78,79]. Direct aspiration techniques have been developed with recent iterations having larger lumens and improved trackability. The rates of embolization for these are, however, not yet well described in vivo.
Emboli can be generated during thrombectomy in several different ways: The water-hammer effect of the blood flow can shear off parts of the thrombus. Crossing a clot, especially with larger catheters, such as a 3Max (Penumbra Inc., Alameda, CA, USA), can also potentially cause distal embolization into same arterial territory. Another way emboli are generated is via fragmentation of the thrombus by friction between the vessel wall and the thrombus while withdrawing it. The thrombus can be sheared off the sides of the guide catheter if the lumen is too small as the thrombus is pulled into it. As the thrombus is withdrawn into a larger vessel from a smaller one, there can be a temporary loss of apposition between the stent retriever and the thrombus. Finally, during deployment of stent retrievers, pieces of the thrombus can be forced between the struts of the mesh comprising the thrombectomy device [67, 80].
Factors found to be associated with emboli during thrombectomy include posterior circulation occlusions, the use of conscious sedation and the thrombus length [81]. Several studies have shown that the use of a balloon guide catheter during a thrombectomy to create a state of flow arrest reduces the risk of emboli which results in better functional outcomes [76, 82,83,84]. Posterior circulation occlusions are described to have significantly more distal emboli. This is likely in part due to the lack of flow arrest, as balloon guide catheters are mostly not used for such procedures [85]; however, an aplastic or hypoplastic vertebral artery contralateral to the cannulated vertebral artery for thrombectomy was in a recent study associated with complete recanalization meaning fewer distal emboli. It was suggested that contralateral flow modulation or ipsilateral balloon guide catheter use in patients with bilaterally patent vertebral arteries should be considered and further studied in the future [86].
The incidence of thromboembolic complications into other previously unaffected vessels also increases with the length of the clot. In in vitro experiments the rate of thromboembolic events was 0% with 10-mm length thrombi but increased to 7.4% with 20-mm thrombi and 14.8% with 40-mm thrombi [87]. Finally, procedures using conscious sedation have been reported to have a higher incidence of distal emboli than those using general anesthesia. This may be attributed to movement of the patient during thrombectomy and its attendant difficulties [88]. The actual incidence of emboli during a thrombectomy procedure could be much more than previously thought [77, 89] as during a thrombectomy, a large portion of the thrombus fragments that break off are very small and not visible on standard digital subtracted angiography, as seen in in vitro experiments. These tiny emboli can obstruct collateral flow to the salvageable ischemic penumbra, induce inflammatory injury at the capillary circulation level or even cause infarcts in a previously unaffected vascular territory [73, 90,91,92]. The presence of these fragmented clots has been shown to be associated with worse clinical outcomes [90, 93].
The number of attempts with each device has a strong association with the functional outcome. In a series of 330 patients treated with stent retrievers, recanalization was 46.8% with the first retrieval but only 22.7% with the fifth attempt. The number of passes was an independent negative predictor of good clinical outcome (OR 0.65; 95% CI, 0.435–0.970; P = 0.035) and therefore patients with 1–3 attempts had a significantly better clinical outcome [94]. Further attempts had good recanalization rates, but the rate of favorable clinical outcome did not improve. Another Korean series with 467 patients treated with stent retrievers noted that recanalization rates became lower commensurate with the number of passes [95]. In their multivariable analysis, 1–4 passes were significantly associated with better outcomes compared to patients without recanalization; however, 5 or more passes was not associated with better outcomes. The recanalization rate for ≥5 passes of the stentretriver (SR) was 5.5% and the authors concluded that it was futile for both recanalizations as well as for good functional outcome. Finally, in sub-studies from the ARISE-II dataset, the first-pass effect, i. e. removal of the thrombus with TICI 2b-3 recanalization with only one attempt, was more important for good clinical outcome, SICH and mortality than a final equivalent recanalization [96, 97].
Imaging to Predict Thrombectomy Failure
Preprocedural imaging, whether CT-angiography (CTA) or MR-angiography (MRA), is crucial for the operator to quickly plan the thrombectomy strategy. It can predetermine the best site for vascular access as well as anticipate any problems the surgeon is likely to face. Ideally, it can also determine which are the ideal tools and technique to use and prognosticate the chance of a successful thrombectomy.
The hyperdense middle cerebral artery (MCA) sign scan be seen on a non-contrasted CT scan and is strongly associated with a thrombus containing a higher RBC content (Fig. 6; [98]). Despite this predilection towards RBCs having higher Hounsfield units, fibrin, RBCs and WBCs all affect the density of a clot on a CT-scan [13]. The IV tPA (intravenous tissue plasminogen activator) has a higher rate of recanalization with RBC-rich clots compared as to fibrin-rich clots [99], whereas the literature available on CT thrombus density and endovascular thrombectomy is still uncertain. The older thrombectomy devices do not appear to perform better with certain types of clots, although stent retrievers appear to show greater success with increased thrombus density [32, 33, 100, 101]. The RBC-rich thrombi can also be identified using MRI scans, where the iron in the RBC of the thrombus induces a blooming artifact on Gradient echo (GRE) and susceptibility weighted imaging (SWI) sequences [102, 103].
Larger and more proximally located thrombi have been shown to have lower recanalization rates with intravenous thrombolysis and are therefore associated with worse clinical outcomes and higher complication rates [104, 105]. Conversely, thrombi which are longer have been shown to have better recanalization rates with aspiration thrombectomy when compared to IV tPA. It is therefore important to identify and know the thrombus burden as it affects the therapeutic decision making. A method of measuring the thrombus length is by measuring the filling defect on CTA. On a single phase CTA, the arterial filling defect depends on imaging acquisition timing after the contrast bolus and the presence of collateral circulation which will allow the contrast to reach past the occlusion and showcase the distal end of the thrombus [106, 107]. In newer techniques, such as the dynamic CTA or multiphasic CTA, this is less of a problem as the delayed scans will allow the contrast time to fill the entire gap and reflect the thrombus boundaries [108]. A less precisely validated alternative is the clot burden score which is a semiquantitative validated method of measuring the clot burden using prespecified vessel segments that fail to be opacified by contrast on a single phase CTA [109]. Longer and curved susceptibility vessel signs on MRI have also been associated with reduced effectiveness of reperfusion therapies [102, 103].
A good collateral circulation is associated with a better chance of recanalization with intravenous thrombolysis but may also potentially aid in removing the thrombus in mechanical thrombectomy [110]. Theoretically, a good collateral circulation allows the intravenous thrombolytics to act on both ends of the clot and using the increased surface area enhances the penetration of the clot thereby softening it. A good collateral circulation also reduces the water-hammer effect from the systolic blood pressure in the original vessel and in doing so reduces the pressure gradient across the clot allowing easier withdrawal of the clot, either by direct aspiration or by a stent retriever.
The perviousness of thrombus to contrast agents on the CT scan can be determined by the change in Hounsfield units (HU) precontrast and postcontrast administration. Fibrin-rich clots seem to have a characteristic of increased perviousness as compared to RBC-rich clots with a lower initial HU, but with a higher increase in HU after contrast is administered [111]. This has clinical implications as some recent literature has suggested that more pervious clots with an increase in HU of 23 or more after contrast administration was associated with smaller infarcts, superior recanalization, and better functional outcomes after IV thrombolysis [99, 108, 112]. This method can be used to predetermine the thrombus make-up and select devices more likely to successfully remove usually difficult fibrin-rich clots, although more research will need to be done in this aspect.
The clot gap on CTA has also been suggested to have the potential to determine the underlying pathology of the occlusion. An occlusion involving the MCA bifurcation in the sylvian fissure was defined as a branching type occlusion and was more likely to be a thrombus of embolic origin. Conversely a clot in the trunk of the artery with opacification of all distal MCA branches from the bifurcation was associated with an underlying intracranial atherosclerotic lesion, likely with a thrombus forming on it (Fig. 7). While truncal type occlusions comprise only 12% of all occlusions, they are associated with poorer recanalization rates of 18.2% with a tendency to immediately re-occlude after the stent retriever is withdrawn. They therefore have longer procedural times, worse clinical outcomes and more frequent additional device use. This is in comparison to branching type occlusions which have been reported to have a 4 in 5 chance of successful recanalization [113].
Artificial intelligence (AI) for analysis of imaging is now creeping into the field. A study of the clot characteristics on CT and CTA was done in 67 patients with proximal anterior circulation occlusions treated with IV alteplase. A total of 326 radiomics features were extracted from each thrombus and used to train a linear support vector machine classifier. When ROC curves were constructed, the combination of radiomics features was significantly better at predicting early recanalization with IV alteplase than any other single radiological feature from CT or CTA (area under the curve = 0.85) [114].
Finally, there are angiographic features of poorer outcomes with thrombectomy. While poor or failed recanalization is a well-known and instinctively understood marker of poor functional outcomes, there are several more subtle signs which are indicative of poorer prognosis despite good recanalization. Early venous shunting can be associated with already infarcted tissue especially involving the basal ganglia (Fig. 8; [115]). Similarly, leptomeningeal collaterals which persist longer than the venous phase are also associated with the area of infarction and poor functional outcomes (Fig. 9; [116]).
Conclusion
Failed thrombectomy in AIS is associated with poor outcomes, with a high incidence of functional dependency. This article describes the key problems and challenges that interventionists commonly face today. Future research efforts need to be focused on these areas to develop techniques and devices that make it possible to reach and remove even difficult clots in a difficult anatomy in a timely fashion.
References
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t‑PA vs. t‑PA alone in stroke. N Engl J Med. 2015;372:2285–95.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30.
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20.
Baek JH, Kim BM, Kim DJ, Heo JH, Nam HS, Yoo J. Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke. 2016;47:2360–3.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS; TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–40.
Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, Meschia JF, Ovbiagele B, Yavagal DR; American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–35.
Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO; SWIFT Trialists. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9.
Song D, Cho AH. Previous and recent evidence of endovascular therapy in acute ischemic stroke. Neurointervention. 2015;10:51–9.
Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, Vespa PM, Selco S, Rajajee V, Kim D, Sanossian N, Saver JL. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–93.
Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK, Shah SH, Towfighi A, Ovbiagele B, Kidwell CS, Tateshima S, Jahan R, Duckwiler GR, Viñuela F, Salamon N, Villablanca JP, Vinters HV, Marder VJ, Saver JL. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–43.
Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie CBLM, Dippel DWJ, Siddiqui AH, Khatri P, Baxter B, Nogeuira R, Gounis M, Jovin T, Kallmes DF. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg. 2017;9:529–34.
Cline B, Vos J, Carpenter J, Rai1 A. O‑027 Pathological analysis of extracted clots in embolectomy patients with acute ischaemic stroke. J Neurointerv Surg. 2013;5:A15–A6.
Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. 2018;10:34–8.
Duffy S, Farrell M, McArdle K, Thornton J, Vale D, Rainsford E. Novel methodology to replicate clot analogs with diverse composition in acute ischemic stroke. J Neurointerv Surg. 2017;9:486–91.
Pikija S, Magdic J, Trkulja V, Unterkreuter P, Mutzenbach JS, Novak HF, Weymayr F, Hauer L, Sellner J. Intracranial thrombus morphology and composition undergoes time-dependent changes in acute Ischemic stroke: a CT densitometry study. Int J Mol Sci. 2016;17(11):E1959.
Boeckh-Behrens T, Schubert M, Förschler A, Prothmann S, Kreiser K, Zimmer C, Riegger J, Bauer J, Neff F, Kehl V, Pelisek J, Schirmer L, Mehr M, Poppert H. The impact of histological clot composition in embolic stroke. Clin Neuroradiol. 2016;26:189–97.
French JE. The structure of natural and experimental thrombi. Ann R Coll Surg Engl. 1965;36:191–200.
Welch WH. The structure of white thrombi. Trans Path Soc. 1887;13:281.
Yuki I, Kan I, Vinters HV, Kim RH, Golshan A, Vinuela FA, Sayre JW, Murayama Y, Vinuela F. The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. AJNR Am J Neuroradiol. 2012;33:643–8.
Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–76.
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–5.
Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, Vanhoorelbeke K, De Meyer SF. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–32.
Almekhlafi MA, Hu WY, Hill MD, Auer RN. Calcification and endothelialization of thrombi in acute stroke. Ann Neurol. 2008;64:344–8.
Dobrocky T, Piechowiak E, Cianfoni A, Zibold F, Roccatagliata L, Mosimann P, Jung S, Fischer U, Mordasini P, Gralla J. Thrombectomy of calcified emboli in stroke: does histology of thrombi influence the effectiveness of thrombectomy? J Neurointerv Surg. 2018;10:345–50.
Judge C, Mello S, Bradley D, Harbison J. A systematic review of the causes and management of ischaemic stroke caused by nontissue emboli. Stroke Res Treat. 2017;2017:7565702.
Denorme F, Langhauser F, Rottenschneider H, Plaimauer B, Scheiflinger F, Kleinschnitz C, Vanhoorelbeke K, De Meyer SF. ADAMTS13 destabilizes thrombi in a mouse model of thrombotic focal cerebral ischemia. J Thromb Haemost. 2015;13(Suppl 2):694–5.
De Meyer SF, Stoll G, Wagner DD, Kleinschnitz C. Von Willebrand factor: an emerging target in stroke therapy. Stroke. 2012;43:599–606.
Kim EY, Yoo E, Choi HY, Lee JW, Heo JH. Thrombus volume comparison between patients with and without hyperattenuated artery sign on CT. AJNR Am J Neuroradiol. 2008;29:359–62.
Treurniet KM, Yoo AJ, Berkhemer OA, Lingsma HF, Boers AM, Fransen PS, Beumer D, van den Berg LA, Sprengers ME, Jenniskens SF, Lycklama À Nijeholt GJ, van Walderveen MA, Bot JC, Beenen LF, van den Berg R, van Zwam WH, van der Lugt A, van Oostenbrugge RJ, Dippel DW, Roos YB, Marquering HA, Majoie CB; MR CLEAN Investigators. Clot burden score on baseline computerized tomographic angiography and intra-arterial treatment effect in acute ischemic stroke. Stroke. 2016;47:2972–8.
Spiotta AM, Vargas J, Hawk H, Turner R, Chaudry MI, Battenhouse H, Turk AS. Hounsfield unit value and clot length in the acutely occluded vessel and time required to achieve thrombectomy, complications and outcome. J Neurointerv Surg. 2014;6:423–7.
Mokin M, Morr S, Natarajan SK, Lin N, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2015;7:104–7.
Jindal G, Miller T, Shivashankar R, Mitchell J, Stern BJ, Yarbrough K, Gandhi D. Relationship of thrombus length to number of stent retrievals, revascularization, and outcomes in acute ischemic stroke. J Vasc Interv Radiol. 2014;25:1549–57.
Soize S, Batista AL, Rodriguez Regent C, Trystram D, Tisserand M, Turc G, Serre I, Ben Hassen W, Zuber M, Calvet D, Mas JL, Meder JF, Raymond J, Pierot L, Oppenheim C, Naggara O. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. Eur J Neurol. 2015;22:967–72.
Angermaier A, Michel P, Khaw AV, Kirsch M, Kessler C, Langner S. Intravenous thrombolysis and passes of thrombectomy as predictors for endovascular revascularization in ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:2488–95.
Mokin M, Levy EI, Siddiqui AH, Goyal M, Nogueira RG, Yavagal DR, M Pereira V, Saver JL. Association of clot burden score with radiographic and clinical outcomes following Solitaire stent retriever throm-bectomy: analysis of the SWIFT PRIME trial. J Neurointerv Surg. 2017;9:929–32.
Yoo AJ, Khatri P, Mocco J, Zaidat OO, Gupta R, Frei D, Lopes D, Shownkeen H, Berkhemer OA, Meyer D, Hak SS, Kuo SS, Buell H, Bose A, Sit SP, von Kummer R, THERAPY Trial Investigators. Impact of thrombus length on outcomes after intra-arterial aspiration thrombectomy in the THERAPY trial. Stroke. 2017;48:1895–900.
Endovascular Treatment in Ischemic Stroke (ETIS) Research Investigators, Gory B, Mazighi M, Blanc R , Labreuche J, Piotin M, Turjman F, Lapergue B. Mechanical thrombectomy in basilar artery occlusion: influence of reperfusion on clinical outcome and impact of the first-line strategy (ADAPT vs stent retriever). J Neurosurg. 2018;129:1482-91.
Maegerlein C, Berndt MT, Mönch S, Kreiser K, Boeckh-Behrens T, Lehm M, Wunderlich S, Zimmer C, Friedrich B. Further development of combined techniques using stent retrievers, aspiration catheters and BGC: the PROTECT(PLUS) technique. Clin Neuroradiol. 2018 Nov 9. [Epub ahead of print]
Leischner H, Flottmann F, Hanning U, Broocks G, Faizy TD, Deb-Chatterji M, Bernhardt M, Brekenfeld C, Buhk JH, Gellissen S, Thomalla G, Gerloff C, Fiehler J. Reasons for failed endovascular recanalization attempts in stroke patients. J Neurointerv Surg. 2018 Nov 24. [Epub ahead of print]
Kaesmacher J, Gralla J, Mosimann PJ, Zibold F, Heldner MR, Piechowiak E, Dobrocky T, Arnold M, Fischer U, Mordasini P. Reasons for reperfusion failures in stent-retriever-based thrombectomy: registry analysis and proposal of aclassification system. AJNR Am J Neuroradiol. 2018;39:1848–53.
Burzotta F, Nerla R, Pirozzolo G, Aurigemma C, Niccoli G, Leone AM, Saffioti S, Crea F, Trani C. Clinical and procedural impact of aortic arch anatomic variants in carotid stenting procedures. Catheter Cardiovasc Interv. 2015;86:480–9.
Layton KF, Kallmes DF, Cloft HJ, Lindell EP, Cox VS. Bovine aortic arch variant in humans: clarification of a common misnomer. AJNR Am J Neuroradiol. 2006;27:1541–2.
Han HC. Twisted blood vessels: symptoms, etiology and biomechanical mechanisms. J Vasc Res. 2012;49:185–97.
Kaymaz ZO, Nikoubashman O, Brockmann MA, Wiesmann M, Brockmann C. Influence of carotid tortuosity on internal carotid artery access time in the treatment of acute ischemic stroke. Interv Neuroradiol. 2017;23:583–8.
Schwaiger BJ, Gersing AS, Zimmer C, Prothmann S. The curved MCA: influence of vessel anatomy on recanalization results of mechanical thrombectomy after acute ischemic stroke. AJNR Am J Neuroradiol. 2015;36:971–6.
Yamamoto S, Yamagami H, Todo K, Kuramoto Y, Ishikawa T, Imamura H, Ueno Y, Adachi H, Kohara N, Sakai N. Correlation of middle cerebral artery tortuosity with successful recanalization using the Merci retrieval system with or without adjunctive treatments. Neurol Med Chir. 2014;54:113–9.
Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke. 2014;16:27–35.
Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12:1106–14.
Kim JS, Bonovich D. Research on intracranial atherosclerosis from the east and west: why are the results different? J Stroke. 2014;16:105–13.
Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–9.
van der Kolk AG, Zwanenburg JJ, Brundel M, Biessels GJ, Visser F, Luijten PR, Hendrikse J. Distribution and natural course of intracranial vessel wall lesions in patients with ischemic stroke or TIA at 7.0 Tesla MRI. Eur Radiol. 2015;25:1692–700.
Matias-Guiu JA, Serna-Candel C, Matias-Guiu J. Stroke etiology determines effectiveness of retrievable stents. J Neurointerv Surg. 2014;6:e11.
Jia B, Feng L, Liebeskind DS, Huo X, Gao F, Ma N, Mo D, Liao X, Wang C, Zhao X, Pan Y, Li H, Liu L, Wang Y, Wang Y, Miao ZR; EAST Study Group. Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J Neurointerv Surg. 2018;10:746–50.
Lee JS, Hong JM, Lee KS, Suh HI, Choi JW, Kim SY. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke. 2016;18:96–101.
Levy EI, Ecker RD, Horowitz MB, Gupta R, Hanel RA, Sauvageau E, Jovin TG, Guterman LR, Hopkins LN. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery. 2006;58:458–63.
Ramee SR, Subramanian R, Felberg RA, McKinley KL, Jenkins JS, Collins TJ, Dawson RC, White CJ. Catheter-based treatment for patients with acute ischemic stroke ineligible for intravenous thrombolysis. Stroke. 2004;35:e109–11.
Gupta R, Vora NA, Horowitz MB, Tayal AH, Hammer MD, Uchino K, Levy EI, Wechsler LR, Jovin TG. Multimodal reperfusion therapy for acute ischemic stroke: factors predicting vessel recanalization. Stroke. 2006;37:986–90.
Sauvageau E, Samuelson RM, Levy EI, Jeziorski AM, Mehta RA, Hopkins LN. Middle cerebral artery stenting for acute ischemic stroke after unsuccessful Merci retrieval. Neurosurgery. 2007;60:701–6.
Wareham J, Flood R, Phan K, Crossley R, Mortimer A. A systematic review andmeta-analysis of observational evidence for the use of bailout self-expandable stents following failed anterior circulation stroke thrombectomy. J Neurointerv Surg. 2018 Nov 10. [Epub ahead of print]
Levy EI, Mehta R, Gupta R, Hanel RA, Chamczuk AJ, Fiorella D, Woo HH, Albuquerque FC, Jovin TG, Horowitz MB, Hopkins LN. Selfexpanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007;28:816–22.
Henkes H, Miloslavski E, Lowens S, Reinartz J, Liebig T, Kühne D. Treatment of intracranial atherosclerotic stenoself-expandable stent with balloon dilatation and self-expanding stent deployment (WingSpan). Neuroradiology. 2005;47:222–8.
Zaidi SF, Castonguay AC, Jumaa MA, Malisch TW, Linfante I, Marden FA, Abraham MG, Chebl AB, Novakovic R, Taqi MA, Nogueira RG, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Dabus G, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Nguyen TN, Yoo AJ, Shaltoni H, Janardhan V, Chen PR, Britz GW, Kaushal R, Nanda A, Gupta R, Zaidat OO. Intraarterial thrombolysis as rescue therapy for large vessel occlusions. Stroke. 2019 Feb 22. [Epub ahead of print]
Zhang S, Hao Y, Tian X, Zi W, Wang H, Yang D, Zhang M, Zhang X, Bai Y, Li Z, Sun B, Li S, Fan X, Liu X, Xu G. Safety of intra-arterial tirofiban administration in Ischemic stroke patients after unsuccessful mechanical thrombectomy. J Vasc Interv Radiol. 2019;30:141–7.
Chueh JY, Kühn AL, Puri AS, Wilson SD, Wakhloo AK, Gounis MJ. Reduction in distal emboli with proximal flow control during mechanical thrombectomy: a quantitative in vitro study. Stroke. 2013;44:1396–401.
Mordasini P, Frabetti N, Gralla J, Schroth G, Fischer U, Arnold M, Brekenfeld C. In vivo evaluation of the first dedicated combined flow-restoration and mechanical thrombectomy device in a swine model of acute vessel occlusion. AJNR Am J Neuroradiol. 2011;32:294–300.
Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, Spiotta A, Mokin M, Dewan M, Quarfordt S, Battenhouse H, Turner R, Chaudry I. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg. 2014;6:260–4.
Tomsick TA, Khatri P, Jovin T, Demaerschalk B, Malisch T, Demchuk A, Hill MD, Jauch E, Spilker J, Broderick JP; IMS III Executive Committee. Equipoise among recanalization strategies. Neurology. 2010;74:1069-76.
Mokin M, Kass-Hout T, Levy EI. Solitaire FR—a promising new device for acute ischemic stroke treatment. World Neurosurg. 2012;78:557–8.
Dorn F, Stehle S, Lockau H, Zimmer C, Liebig T. Endovascular treatment of acute intracerebral artery occlusions with the solitaire stent: single-centre experience with 108 recanalization procedures. Cerebrovasc Dis. 2012;34:70–7.
Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra-arterial prourokinase for acute ischemic stroke. the PROACT II study: a randomized controlled trial prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003–11.
Akins PT, Amar AP, Pakbaz RS, Fields JD; SWIFT Investigators. Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and merci devices. AJNR Am J Neuroradiol. 2014;35:524–8.
Gascou G, Lobotesis K, Machi P, Maldonado I, Vendrell JF, Riquelme C, Eker O, Mercier G, Mourand I, Arquizan C, Bonafé A, Costalat V. Stent retrievers in acute ischemic stroke: complications and failures during the perioperative period. AJNR Am J Neuroradiol. 2014;35:734–40.
Nguyen TN, Malisch T, Castonguay AC, Gupta R, Sun CH, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Linfante I, Dabus G, Marden FA, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Taqi M, Abraham MG, Janardhan V, Shaltoni H, Novakovic R, Yoo AJ, Abou-Chebl A, Chen PR, Britz GW, Kaushal R, Nanda A, Issa MA, Masoud H, Nogueira RG, Norbash AM, Zaidat OO. Balloon guide catheter improves revascularization and clinical outcomes with the Solitaire device: analysis of the North American Solitaire Acute Stroke Registry. Stroke. 2014;45:141–5.
Mazur MD, Kilburg C, Park MS, Taussky P. Patterns and clinical impact of angiographically visible distal emboli during thrombectomy with solitaire for acute ischemic stroke. Neurosurgery. 2016;78:242–50.
Penumbra Pivotal Stroke Trial Investigators.. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–8.
Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S, Gobin YP; Multi MERCI Investigators, Frei D, Grobelny T, Hellinger F, Huddle D, Kidwell C, Koroshetz W, Marks M, Nesbit G, Silverman IE. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–12.
Ogawa A, Mori E, Minematsu K, Taki W, Takahashi A, Nemoto S, Miyamoto S, Sasaki M, Inoue T; MELT Japan Study Group. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the middle cerebral artery embolism local fibrinolytic intervention trial (MELT) Japan. Stroke. 2007;38:2633–9.
Yoo AJ, Andersson T. Thrombectomy in acute Ischemic stroke: challenges to procedural success. J Stroke. 2017;19:121–30.
Yeo LLL, Holmberg A, Mpotsaris A, Söderman M, Holmin S, Kuntze Söderqvist A, Ohlsson M, Bhogal P, Gontu V, Andersson T, Brouwer PA. Posterior circulation occlusions May be associated with distal emboli during thrombectomy : factors for distal embolization and a review of the literature. Clin Neuroradiol. 2018 Mar 22. [Epub ahead of print]
Gralla J, Schroth G, Remonda L, Fleischmann A, Fandino J, Slotboom J, Brekenfeld C. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol. 2006;27:1357–61.
Spiotta AM, Chaudry MI, Hui FK, Turner RD, Kellogg RT, Turk AS. Evolution of thrombectomy approaches and devices for acute stroke: a technical review. J Neurointerv Surg. 2015;7:2–7.
Brinjikji W, Starke RM, Murad MH, Fiorella D, Pereira VM, Goyal M, Kallmes DF. Impact of balloon guide catheter on technical and clinical outcomes: a systematic review and meta-analysis. J Neurointerv Surg. 2018;10:335–9.
Yeo LLL, Holmberg A, Mpotsaris A, Söderman M, Holmin S, Kuntze Söderqvist A, Ohlsson M, Bhogal P, Gontu V, Andersson T, Brouwer PA. Posterior circulation occlusions may be associated with distal emboli during thrombectomy. Clin Neuroradiol. 2018 Mar 22. [Epub ahead of print]
Boeckh-Behrens T, Pree D, Lummel N, Friedrich B, Maegerlein C, Kreiser K, Kirschke J, Berndt M, Lehm M, Wunderlich S, Mosimann PJ, Fischer U, Zimmer C, Kaesmacher J. Vertebral artery patency and thrombectomy in basilar artery occlusions. Stroke. 2019;50:389–95.
Gralla J, Burkhardt M, Schroth G, El-Koussy M, Reinert M, Nedeltchev K, Slotboom J, Brekenfeld C. Occlusion length is a crucial determinant of efficiency and complication rate in thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol. 2008;29:247–52.
Maegerlein C, Mönch S, Boeckh-Behrens T, Lehm M, Hedderich DM, Berndt MT, Wunderlich S, Zimmer C, Kaesmacher J, Friedrich B. PROTECT: PRoximal balloon Occlusion TogEther with direCt Thrombus aspiration during stent retriever thrombectomy—evaluation of a double embolic protection approach in endovascular stroke treatment. J Neurointerv Surg. 2018;10:751–5.
Chueh JY, Wakhloo AK, Gounis MJ. Effectiveness of mechanical endovascular thrombectomy in a model system of cerebrovascular occlusion. AJNR Am J Neuroradiol. 2012;33:1998–2003.
Kurre W, Vorlaender K, Aguilar-Perez M, Schmid E, Bazner H, Henkes H. Frequency and relevance of anterior cerebral artery embolism caused by mechanical thrombectomy of middle cerebral artery occlusion. AJNR Am J Neuroradiol. 2013;34:1606–11.
Soize S, Barbe C, Kadziolka K, Estrade L, Serre I, Pierot L. Predictive factors of outcome and hemorrhage after acute ischemic stroke treated by mechanical thrombectomy with a stent-retriever. Neuroradiology. 2013;55:977–87.
Todo A, Minaeian A, Sahni R, Chao KH. Incidence and outcome of procedural distal emboli using the Penumbra thrombectomy for acute stroke. J Neurointerv Surg. 2013;5:135–8.
Costalat V, Lobotesis K, Machi P, Mourand I, Maldonado I, Heroum C, Vendrell JF, Milhaud D, Riquelme C, Bonafé A, Arquizan C. Prognostic factors related to clinical outcome following thrombectomy in ischemic stroke (RECOST study). 50 patients prospective study. Eur J Radiol. 2012;81:4075–82.
Flottmann F, Leischner H, Broocks G, Nawabi J, Bernhardt M, Faizy TD, Deb-Chatterji M, Thomalla G, Fiehler J, Brekenfeld C. Recanalization rate per retrieval attempt in mechanical thrombectomy for acute Ischemic stroke. Stroke. 2018;49:2523–5.
Baek JH, Kim BM, Heo JH, Nam HS, Kim YD, Park H, Bang OY, Yoo J, Kim DJ, Jeon P, Baik SK, Suh SH, Lee KY, Kwak HS, Roh HG, Lee YJ, Kim SH, Ryu CW, Ihn YK, Kim B, Jeon HJ, Kim JW, Byun JS, Suh S, Park JJ, Lee WJ, Roh J, Shin BS. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke. 2018;49:2088–95.
Yoo AJ, Andersson T, Ribo M, Bozorgchami H, Liebeskind D, Jadhav A, Saver JL, Mattle HP, Zaidat OO. Abstract TP41: the importance of first pass substantial reperfusion in the ARISE II study. Stroke. 2019;50:ATP41. https://doi.org/10.1161/str.50.suppl_1.TP41.
Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Dabus G, Malisch TW, Marden FA, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Nguyen TN, Taqi MA, Abraham MG, Yoo AJ, Janardhan V, Shaltoni H, Novakovic R, Abou-Chebl A, Chen PR, Britz GW, Sun CJ, Bansal V, Kaushal R, Nanda A, Nogueira RG. First pass effect: a new measure for stroke thrombectomy devices. Stroke. 2018;49:660–6.
Kirchhof K, Welzel T, Mecke C, Zoubaa S, Sartor K. Differentiation of white, mixed, and red thrombi: value of CT in estimation of the prognosis of thrombolysis phantom study. Radiology. 2003;228:126–30.
Santos EM, Dankbaar JW, Treurniet KM, Horsch AD, Roos YB, Kappelle LJ, Niessen WJ, Majoie CB, Velthuis B, Marquering HA; DUST Investigators. Permeable thrombi are associated with high¬er intravenous recombinant tissue-type plasminogen activator treatment success in patients with acute ischemic stroke. Stroke. 2016;47:2058–65.
Moftakhar P, English JD, Cooke DL, Kim WT, Stout C, Smith WS, Dowd CF, Higashida RT, Halbach VV, Hetts SW. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44:243–5.
Froehler MT, Tateshima S, Duckwiler G, Jahan R, Gonzalez N, Vinuela F, Liebeskind D, Saver JL, Villablanca JP; UCLA Stroke Investigators. The hyperdense vessel sign on CT predicts successful recanalization with the Merci device in acute ischemic stroke. J Neurointerv Surg. 2013;5:289–93.
Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol. 2015;42:86–92.
Denorme F, Langhauser F, Desender L, Vandenbulcke A, Rottensteiner H, Plaimauer B, François O, Andersson T, Deckmyn H, Scheiflinger F, Kleinschnitz C, Vanhoorelbeke K, De Meyer SF. ADAMTS13-mediated thrombolysis of t‑PA-resistant occlusions in ischemic stroke in mice. Blood. 2016;127:2337–45.
Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke. 2016;47:2409–12.
Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, Lopes D, Frei D, Shownkeen H, Budzik R, Ajani ZA, Grossman A, Altschul D, McDougall C, Blake L, Fitzsimmons BF, Yavagal D, Terry J, Farkas J, Lee SK, Baxter B, Wiesmann M, Knauth M, Heck D, Hussain S, Chiu D, Alexander MJ, Malisch T, Kirmani J, Miskolczi L, Khatri P; THERAPY Trial Investigators*. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47:2331–8.
Mortimer AM, Little DH, Minhas KS, Walton ER, Renowden SA, Bradley MD. Thrombus length estimation in acute ischemic stroke: a potential role for delayed contrast enhanced CT. J Neurointerv Surg. 2014;6:244–8.
Kamalian S, Morais LT, Pomerantz SR, Aceves M, Sit SP, Bose A, Hirsch JA, Lev MH, Yoo AJ. Clot length distribution and predictors in anterior circulation stroke: implications for intra-arterial therapy. Stroke. 2013;44:3553–6.
Mishra SM, Dykeman J, Sajobi TT, Trivedi A, Almekhlafi M, Sohn SI, Bal S, Qazi E, Calleja A, Eesa M, Goyal M, Demchuk AM, Menon BK. Early reperfusion rates with IV tPA are determined by CTA clot characteristics. AJNR Am J Neuroradiol. 2014;35:2265–72.
Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, Martin M, Symons SP, Fox AJ, Aviv RI. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–31.
Yeo LLL, Paliwal P, Teoh HL, Seet RC, Chan BP, Ting E, Venketasubramanian N, Leow WK, Wakerley B, Kusama Y, Rathakrishnan R, Sharma VK. Assessment of intracranial collaterals on CT-Angiography in anterior circulation acute ischemic stroke. AJNR Am J Neuroradiol. 2015;36:289–94.
Borggrefe J, Kottlors J, Mirza M, Neuhaus VF, Abdullayev N, Maus V, Kabbasch C, Maintz D, Mpotsaris A. Differentiation of clot composition using conventional and dual-energy computed tomography. Clin Neuroradiol. 2018;28:515–22.
Santos EM, Marquering HA, den Blanken MD, Berkhemer OA, Boers AM, Yoo AJ, Beenen LF, Treurniet KM, Wismans C, van Noort K, Lingsma HF, Dippel DW, van der Lugt A, van Zwam WH, Roos YB, van Oostenbrugge RJ, Niessen WJ, Majoie CB; MR CLEAN Investigators. Thrombus permeability is associated with improved functional outcome and recanalization in patients with ischemic stroke. Stroke. 2016;47:732–41.
Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA; IMS I and II Investigators. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–72.
Qiu W, Kuang H, Nair J, Assis Z, Najm M, McDougall C, McDougall B, Chung K, Wilson AT, Goyal M, Hill MD, Demchuk AM, Menon BK. Radiomics-based Intracranial thrombus features on CT and CTA predict recanalization with intravenous alteplase in patients with acute Ischemic stroke. AJNR Am J Neuroradiol. 2019;40:39-44.
Fritzsch D, Reiss-Zimmermann M, Lobsien D, Quäschling U, Hoffmann KT. Arteriovenous shunts and capillary blush as an early sign of basal ganglia infarction after successful mechanical intra-arterial thrombectomy in ischaemic stroke. Eur Radiol. 2015;25:3060–5.
Yeo LLL, Cervo A, Gopinathan A, Cunli Y, Holmberg A, Söderman M, Holmin S, Bhogal P, Gontu V, Mpotsaris A, Andersson T, Cornelissen SA. Very late leptomeningeal collaterals-potential new way to subdivide modified thrombolysis in cerebral Ischemia (mTICI) 2B. Clin Neuroradiol. 2018 Nov 26. [Epub ahead of print]
Acknowledgements
The authors gratefully acknowledge David Vale and Michael Gilvarry of Neuravi/Cerenovus Inc. for the use of figures and videos from their in vitro modeling.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L.L.L. Yeo has received substantial grant funding from the National Medical Research Council (NMRC), Singapore, substantial grant funding from the Ministry of Health (MOH), Singapore and a moderate grant funding from I2R, A‑STAR, Singapore. P. Bhogal is consultant for Neurvana Medical and Phenox. T. Andersson is a consultant for Ablynx, Amnis Therapeutics, Medtronic, Neuravi/J&J, Rapid Medical and Stryker. A. Gopinathan, Y. Cunli and B. Tan declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yeo, L.L.L., Bhogal, P., Gopinathan, A. et al. Why Does Mechanical Thrombectomy in Large Vessel Occlusion Sometimes Fail?. Clin Neuroradiol 29, 401–414 (2019). https://doi.org/10.1007/s00062-019-00777-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-019-00777-1