Abstract
Purpose
Endovascular mechanical thrombectomy (mTE) in acute ischemic stroke due to large cerebral artery occlusion is effective and safe. The procedure is currently offered by specialized hospitals. Physicians from smaller hospitals need to refer patients to stroke centers. Secondary referrals involve delays for transportation. Little is known about effects of distant referrals on outcome and complications as compared to direct admittance.
Methods
To evaluate the effects of referral patterns on outcome and safety, we analyzed 941 patients with anterior circulation stroke receiving mTE between January 2010 and December 2015. Patients were divided into three groups: directly admitted patients (DAP), inner-city transfers (ICT) and long-distance referrals (LDR). We assessed (1) procedural parameters (2) frequency of good functional outcome (mRS ≤2 at 3 months) and (3) mortality rates.
Results
Referrals had a significantly longer imaging-to-groin time compared to DAP (median 150 min vs. 85 min, p <0.001), the same was true for LDR vs. ICT (median 157 min vs. 133.5 min, p <0.001). Time to recanalization was significantly longer for referrals compared to DAP (median 348 min vs. 260 min, p <0.001). There was no significant difference in the frequency of good functional outcome (DAP 39.5%, ICT 35.1%, LDR 37.0%; p =0.709), all-cause mortality at day 90 (DAP 31.5%, ICT 23.0%, LDR 27.0%; p =0.212) and the rate of symptomatic intracranial hemorrhage (p =0.834).
Conclusion
Timing remains a critical factor in acute ischemic stroke treatment by endovascular means. Long distance referral to specialized neurovascular centers with high recanalization rates, however, does allow for a good functional outcome in a significant number of patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2015 randomized controlled multicenter trials proved superiority for mechanical thrombectomy (mTE) in addition to intravenous thrombolysis (IVT) compared to IVT alone in acute ischemic stroke caused by embolic large vessel occlusion [1–5]. Consequently, international guidelines were modified and a consensus statement with specific recommendations for implementation of mTE was published [6]; however, mTE was and still is mainly available in specialized neurovascular centers in urban areas [7, 8]. Primary stroke centers and community hospitals are mostly unable to provide endovascular stroke service on a 24/7 basis. In order to offer a beneficial revascularization therapy to as many people as possible, neurovascular networks were created [9].
Whether endovascular thrombectomy after referral is as safe and effective as in directly admitted patients is a matter of debate and highly relevant for the future organization of acute stroke care facing new strategies of centralization versus decentralization regarding endovascular service infrastructure [10, 11].
Methods
Data Collection
We performed a non-interventional, register-based single center study. All data were collected prospectively according to the approval by the local ethics committee (Board of Physicians of Baden-Wuerttemberg). Consecutive patients treated with mTE and/or aspiration thrombectomy (aTE) between January 2010 and December 2015 were included. They were either directly admitted or secondarily transferred. A population of approximately 2.4 million people is covered [12]. Information on age, sex, medical history, onset of stroke, baseline National Institutes of Health Stroke Scale score (NIHSS) and imaging findings were documented according to referral letters or our own admission notes. Imaging times were recorded by the computed tomography (CT) or magnetic resonance imaging (MRI) scanners and stored in our picture archiving and communication system (PACS). Periprocedural information as well as the Thrombolysis in Cerebral Infarction Score (TICI) were documented by the neuroradiologist in charge. Modified Rankin Scale score (mRS) at discharge and information on stroke etiology were drawn from the discharge papers. The follow-up data were collected by a stroke and study nurse via telephone calls and through direct examination.
Study Population
We included patients with an anterior circulation stroke and a proven occlusion of either the internal carotid artery (ICA), the ICA bifurcation, an M1 or M2 branch of the middle cerebral artery (MCA). Enrolment was based on the initial intention to treat patients by endovascular means. Once this decision was made either by us or in the referring hospital, there was no secondary triage or selection procedure. Patients in the ICT and LDR group underwent endovascular treatment without another CT or MR imaging in our hospital and irrespective of clinical improvement or deterioration during transportation. Only those patients with an initial large vessel occlusion, which was found recanalized (either spontaneously or after intravenous thrombolysis) angiographically were excluded from further analysis. In order to show the whole spectrum of endovascular therapy, we also included patients that did not meet current recommendations for mTE (e. g., due to a delayed treatment onset) [6]. We did exclude patients undergoing primary stenting without mTE because of high-grade intracranial or extracranial stenosis or dissection due to anticipated differences in clinical outcome. To increase comparability with published data only new generation stent retrievers and aspiration systems used in recent randomized controlled trials were included (Solitaire FR, Medtronic, Dublin, Ireland; pREset, phenox, Bochum, Germany; ACE aspiration catheters, Penumbra, Alameda, CA; Sofia, MicroVention, Tustin, CA). Data sets without a 3-month follow-up as well as inconsistent data that could not be verified or traced back were excluded. We did include incomplete data sets and removed the respective information from subgroup analysis.
All remaining patients were classified into three groups: (1) patients directly admitted to our endovascular center (directly admitted patients, DAP), (2) patients transferred from any hospital within the city of Stuttgart (inner-city transfer patients, ICT) and (3) patients transferred from hospitals outside Stuttgart (long distance referral patients, LDR). Up to 8 June 2015, when a joint neurovascular center providing centralized stroke care including endovascular therapy was established in our institution, the neurology department including stroke unit care used to be spatially separated from the neuroradiology department. Transfer within the complex for those patients primarily seen in the previous neurology department was inevitable. For our analysis these patients were treated the same as ICT patients.
Outcome and Safety Measures
Primary outcome measure was the modified Rankin Scale score (mRS) at day 90 with mRS 0–2 indicating good functional outcome. Secondary outcome measures were: (1) development of a symptomatic intracranial hemorrhage (sICH) defined according to the Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS-MOST) criteria as a parenchymal hemorrhage type 2 (PH2) 22–36 h after treatment with a deterioration in the NIHSS ≥ 4 points or leading to death [13, 14], (2) in-hospital mortality and (3) all-cause mortality after 3 months.
Statistical Analysis
Numerical baseline characteristics, such as age or time periods were described as medians (quartiles) or means (standard deviation). Frequencies were used to describe categorical baseline parameters. Baseline characteristics and outcome parameters were analyzed using Fisher’s exact test or the Mann-Whitney U‑test as appropriate. For direct comparison of groups, Fisher’s exact test, the Kruskal-Wallis test or the χ2-test were used. Dichotomized outcome (mRS at day 90) was analyzed in a univariate logistic regression model adjusting for possible confounders (based on literature research; baseline NIHSS score, age, duration of treatment, ICA occlusion, atrial fibrillation and rt-PA therapy). In a multivariate logistic regression model we tried to identify predictors for good clinical outcome. A p-value <0.05 was considered statistically significant. We used Stata/IC 13.1 for Windows (StataCorp LP, College Station, TX) for statistical analysis.
Results
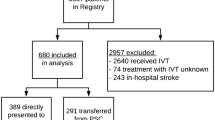
Between January 2010 and December 2015 a total of 1572 consecutive patients were initially considered and transferred for endovascular thrombectomy (Fig. 1). Of the patients 32 patients did not receive endovascular treatment (DAP 2 patients, ICT 4 patients and LDR 28 patients), 14 of those due to recanalization (spontaneous or after IVT), 7 did not have a large vessel occlusion on initial imaging, in 4 cases there was a major demarcation of infarcted tissue in plain CT and 7 patients did not get treatment due to other reasons (e. g., refusal by the patient). A total of 599 patients (38.9%) out of the remaining 1540 patients did not meet the predefined inclusion criteria so that 941 patients (61.1%) could be included in this analysis. Out of those 124 (13.2%) were categorized as DAP, 239 (25.4%) as ICT (average distance 2.7 km or 1.7 miles) and 578 (61.4%) came from distant locations (average distance 33.9 km or 21.1 miles).
The distance and number of enrolled patients from the five main hospitals in the LDR group were as follows: Klinikum Sindelfingen-Böblingen (20 km, n = 156), Klinikum Esslingen (17.7 km, n = 120), Winnenden (Rems-Murr-Kliniken/Klinikum Schloss Winnenden, 24.7 km, n = 80), Stauferklinikum Schwäbisch Gmünd (58.2 km, n = 70) and Rems-Murr-Kliniken Schorndorf (31.6 km, n = 39). All names of the referring hospitals, the patient count, distance to our institution and geographical distribution are shown in supplementary Table 4 and supplementary Fig. 1.
The baseline characteristics are shown in Table 1. There were no differences in sex, age, baseline NIHSS score and most cardiovascular risk factors between directly admitted as compared to transferred patients; however, atrial fibrillation was significantly more present in LDR compared to ICT (66.1% vs. 57.8%; p = 0.039), the rate of coronary artery disease was higher in DAP compared to referrals (39.5% vs. 28.1%; p = 0.046) and MRI was available more often in external patients (29.8% vs. 16.1%; p < 0.001). Subgroup analysis showed that this effect was mainly due to ICT patients having significantly more MRI scans compared to LDR (44.4% vs. 23.7%; p < 0.001) and 21.5% of external referrals received bridging therapy with IVT. A significantly lower number of DAP received IVT (6.5%; p < 0.001). The same difference with a p-value of <0.001 was seen comparing ICT and LDR (8.4% vs. 27.0%). There was no significant difference in the rate of successful recanalization between the three groups (TICI 2b/3; DAP: 87.8%, ICT: 89.5%, LDR: 88.6%; p = 0.848, see Table 2).
There was no significant difference in the frequency of good functional outcome (mRS ≤2) between all groups (DAP 39.5%, ICT 35.1%, LDR 37.0%; p = 0.709, Table 2). Looking at a head-to-head comparison in univariate analysis adjusted for the abovementioned confounders, results did not change (DAP vs. referrals: OR 0.87, 95% CI 0.46–1.64; ICT vs. LDR: OR 1.45, 95% CI 0.92–2.27 see supplementary Table 2). Equally, the rates of in-hospital mortality (DAP 23.4%, ICT 18.4%, LDR 17.6%; p = 0.322) and all-cause mortality at day 90 (DAP 31.5%, ICT 23.0%, LDR 27.0%; p = 0.212) did not significantly differ. In univariate analysis for all-cause mortality DAP vs. referrals (OR 0.76, 95% CI 0.50–1.14) and ICT vs. LDR (OR 1.24, 95% CI 0.87–1.76, supplementary Table 6) did not significantly differ.
Looking at safety aspects, the rate of sICH was alike in all groups (6.8% for DAP, 5.5% for ICT and 6.7% for LDR, p = 0.834, Table 2). Similarly, the rates of subarachnoid hemorrhage (SAH) and PH did not differ. As shown in Table 2, onset-to-groin time was in median 169 min for DAP compared to 222 min for ICT and 239.5 min for LDR (p < 0.001). The same time delay between groups was shown for imaging-to-groin time: with a median of 85 min, DAP could be treated significantly faster (p < 0.001). Patients from remote areas lost in median 23.5 min compared to patients transferred within the city (157 min vs. 133.5 min, p < 0.001). Due to shorter symptom-to-imaging intervals (median 77 min vs. 85 min, p = 0.066) and a trend towards a shorter duration of treatment (89.5 min vs. 94 min, p = 0.137) for LDR, both external groups had a comparable time to recanalization (ICT 349 min, LDR 347.5 min; p = 0.751). With 260 min in median, DAP had a significantly shorter time to recanalization (p < 0.001). Within the ICT group, patients referred to our “old” neurology department (ICTa, up to June 2015) vs. patients initially referred to another hospital within Stuttgart (ICTb) were compared. The symptom-to-groin time was 222 min (ICTa) vs. 230.5 min (ICTb) (median, p = 0.738) and time to recanalization was 352 min (ICTa) vs. 348 min (ICTb) (median, p = 0.970).
We performed receiver operating characteristics (ROC) analysis for imaging-to-groin time, symptom-to-groin time as well as time to recanalization. With an area under the ROC curve below 0.7 for all of them, good functional outcome could not be predicted (imaging-to-groin: 0.52, 95% CI 0.48–0.56, symptom-to-groin: 0.58, 95% CI 0.54–0.62, time to recanalization: 0.64, 95% CI 0.60–0.68; see supplementary Fig. 2).
After categorizing time-to-recanalization, there was an effect of time (Table 3). We chose a time to recanalization of below 4 h as a reference group (54.8% of those patients had mRS ≤2 in follow-up). Above 5 h, 36.7% of patients presented with mRS ≤2 at day 90 (OR 0.49, 95% CI 0.29–0.81). Above 9 h, 28.6% had a good functional outcome (OR 0.33, 95% CI 0.18–0.62). As can be seen in Table 3, the distribution of patients within these time periods differed significantly (p < 0.001).
In a multivariate logistic regression model we tried to identify prognostic factors for good clinical outcome (supplementary Table 7). TICI 2b/3 (OR 3.14, 95% CI 1.38–7.14) and M2 occlusions (OR 2.33, 95% CI 1.20–4.50) showed a positive correlation. Baseline NIHSS (OR 0.88, 95% CI 0.84–0.91) and age (OR 0.95, 95% CI 0.94–0.97) were negatively correlated to good clinical outcome. The same was true for time to recanalization (OR 0.63, 95% CI 0.50–0.79); however, we could only include 724 out of the 941 data sets in this analysis due to missing data (see supplementary Table 5 for a list of missing data per group).
Endovascular techniques and operator experience have developed during the last decade. In our hospital, Solitaire as a stent-retriever was first used in March 2008. For a longitudinal analysis we tabulated all patients treated with endovascular therapy that could be included in this analysis, the rate of efficient recanalization and the percentage of good functional outcome (Table 4).
Discussion
With mTE/aTE being high on the agenda, the question of how many endovascular centers are needed is a matter of debate. On the one hand, offering a mTE/aTE service 24/7 is expensive, training is long and time-consuming and we expect that high-volume centers performing mTE/aTE on a regular basis provide a better clinical outcome [15–18]. On the other hand, there is a substantial time delay due to secondary transport to thrombectomy centers. This influences early revascularization rates, which seem to be crucial in facilitating good functional outcome [19, 20]. In part, the interval between symptom onset and endovascular treatment could be shortened by ultrasound examinations or CT imaging in the ambulance, with direct transportation of all patients eligible for mTE/aTE (e. g., acute proximal large vessel occlusion and no intracranial hemorrhage) to specialized neurointerventional centers [21]. Protocols like this could replace the current practice of primary admission to local hospitals, followed by a “drip and ship” transfer. Imaging protocols in this series significantly differ between hospitals. The great majority of enrolled patients had either CT/CTA imaging or MRI/MRA examinations, performed in the referring institutions. We performed on a regular basis CT/CTA and CT perfusion studies in the DAP group. Patients in the ICT and LDR group did not undergo reimaging after arrival. We did, however, not use any imaging based parameter or anatomical features, such as the presence of leptomeningeal collaterals for patient selection prior to mTE/aTE.
Imaging-to-groin time including decision-making and secondary transportation can be seen as a marker for functionality of an infrastructure and is said to influence outcome [22]. Not surprisingly, DAP had a significantly shorter imaging-to-groin time compared to referrals. The 85 min on average reported here for DAP as well as the median 150 min for external patients are comparable to recently published data [2–4, 11, 19, 22]. Symptom-to-groin time was 169 min on average for DAP and 234 min for referrals (ICT: 222 min, LDR: 239.5 min), again in line with real world data [11, 19, 22, 23]. As there were remarkable distances to cover, this moderate loss of time for LDR compared to ICT indicates that a functional drip-and-ship concept can cope with long distance transfer. With a median of 260 min, DAP were recanalized 88 min earlier compared to secondary referrals (348 min); however, despite this time delay, we could not detect any differences in the frequency of good functional outcome between DAP and referrals. Outcome in patients with secondary transfer for mTE/aTE from remote areas did not differ from outcome in patients with short distance transfer. In addition, there were no differences in mortality rates, occurrence of sICH or in successful recanalization rates (TICI 2b/3). Recently, Pfaff et al. analyzed a small cohort of external patients sent for mTE dividing them in two groups by using the marathon distance as a cut-off [19]. Like in our data, no difference in outcome was detected. A possible explanation for the lack of relevance for clinical outcome might be selection bias [10]. Indeed, there is a possibility that highly disabled patients from remote areas will not reach a hospital providing proper stroke care. Also, we do not know in which case the referring hospitals did not opt for mTE/aTE. In our cohort, the city population seems to be predominantly selected; most patients received an MRI scan and transportation times were only moderately faster compared to LDR but the outcome was similar to DAP (treated faster) and LDR.
A multivariate logistic regression model showed an association between time-to-recanalization and good functional outcome. After categorizing time-to-recanalization, individual patients waiting 5 h or more had significantly lower rates of good functional outcome compared to a reference group where recanalization took place within 4 h. Indeed, as brain tissue not supplied with oxygen dies over time, a time dependency of good functional outcome is reasonable. Recently, it was shown that occlusion time is the critical determinant for good functional outcome in transferred patients [24] but looking at the significant difference in the distribution of our patients in the categorized analysis of time-to-recanalization one would expect a noticeable difference in outcome. For example, 3.4% of DAP were finally recanalized beyond 9 h compared to 11.8% of external patients. In addition, an area under the ROC curve of 0.64 for time-to-recanalization suggested only moderate predictability. There must be other factors with a major influence on clinical outcome.
Different degrees of leptomeningeal collateralization might play an important role for the clinical outcome [25, 26]. It has been shown recently that CT perfusion mismatch in acute ischemic stroke is not dependent on time but on the collateral status [27]. Brain tissue in patients without sufficient early collaterals might be highly dependent on time whereas efficacious leptomeningeal collateralization can secure brain tissue over hours [28]; therefore, collateral supply status might introduce selection bias in general in patients with good functional outcome. Selection bias of patients sent for mTE might have occurred on the basis of MRI (more frequently used in transferred patients).
One of the main differences within our cohort was the rate of IVT. Stuttgart in general was quite reluctant (6.5% of DAP, 8.4% of ICT) whereas significantly more LDR patients received bridging therapy (27.0%). This was not because of possible contraindications but prior to the recent successful randomized controlled trial (RCT) uncertainties concerning thrombus fragmentation and additional loading with platelet aggregation inhibitors in case of primary stenting. An IVT prior to mTE might be associated with higher rates of recanalization, as shown recently [29]. Final recanalization status itself seems to be the strongest positive predictor for good clinical outcome [19, 30, 31] although IVT might add an additional beneficial effect. No increase in sICH or aICH was observed.
This study has several limitations. First there is an imbalance of group sizes leading to a relatively small number of DAP and a large number of patients in the LDR arm. This was due to our infrastructure that only changed in 2015 as well as population figures with the majority of people covered living in remote areas. Another limitation is the fairly high amount of baseline characteristics missing in the DAP which might introduce information bias. Selection criteria for mTE were identical in the DAP and ICTa group. Within the LDR group the decision to transfer a patient for mTE was based on the discretion of the responsible physician and a certain level of inconsistency of this decision-making can the assumed. The strength of this series is the sample size. Instead of sticking to current inclusion or exclusion criteria we wanted to show real world clinical outcome data.
Conclusion
We describe a set-up of centralized endovascular thrombectomy in a specialized, high-volume hospital accepting selected stroke patients with large vessel occlusion of the anterior cerebral circulation from several peripheral primary stroke centers. In this setting referral leads to longer symptom-to-groin times but did not lead to lower rates of recanalization, higher rates of intracranial bleeding or mortality. Time remains a critical factor in the scenario of acute ischemic stroke treatment by means of thrombectomy. Long distance referral to specialized neurovascular centers with high recanalization rates may, however, enable a good functional outcome in a significant number of patients. Professionalized networks and efficacious referral logistics may further improve the outcome after thrombectomy. The future will show if the implementation of low volume neuroendovascular units and aTE/mTE procedures performed by neurovascular lay persons will allow acceptable recanalization and outcome rates. There is evidence that good functional outcome is not exclusively dependent on time to recanalization. An IVT prior to aTE/mTE is safe and might add some additional outcome benefit.
References
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW, MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R, SWIFT PRIME Investigators. Stent-Retriever thrombectomy after Intravenous t‑PA vs. t‑PA alone in stroke. New Engl J Med. 2015;372:2285–95.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM, EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A, REVASCAT Trial Investigators. Thrombectomy within 8 h after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306.
Wahlgren N, Moreira T, Michel P, Steiner T, Jansen O, Cognard C, Mattle HP, van Zwam W, Holmin S, Tatlisumak T, Petersson J, Caso V, Hacke W, Mazighi M, Arnold M, Fischer U, Szikora I, Pierot L, Fiehler J, Gralla J, Fazekas F, Lees KR, ESO-KSU, ESO, ESMINT, ESNR, EAN. Mechanical thrombectomy in acute ischemic stroke: Consensus statement by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int J Stroke. 2016;11:134–47.
Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO, SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9.
Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS, TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–40.
Alberts MJ, Latchaw RE, Selman WR, Shephard T, Hadley MN, Brass LM, Koroshetz W, Marler JR, Booss J, Zorowitz RD, Croft JB, Magnis E, Mulligan D, Jagoda A, O’Connor R, Cawley CM, Connors JJ, Rose-DeRenzy JA, Emr M, Warren M, Walker MD. Brain attack coalition. Recommendations for comprehensive stroke centers: A consensus statement from the brain attack coalition. Stroke. 2005;36:1597–616.
Park MS, Yoon W, Kim JT, Choi KH, Kang SH, Kim BC, Lee SH, Choi SM, Kim MK, Lee JS, Lee EB, Cho KH. Drip, ship, and on-demand endovascular therapy for acute ischemic stroke. PLOS ONE. 2016;1:e0150668.
Weber R, Reimann G, Weimar C, Winkler A, Berger K, Nordmeyer H, Hadisurya J, Brassel F, Kitzrow M, Krogias C, Weber W, Busch EW, Eyding J. Neurovascular Net Ruhr. Outcome and periprocedural time management in referred versus directly admitted stroke patients treated with thrombectomy. Ther Adv Neurol Disord. 2016;9:79–84.
Brachart-Schwarz W. Baden-Württemberg – das Land der kleinen und mittleren Gemeinden 2016. www.statistik.baden-wuerttemberg.de/Service/Veroeff/Monatshefte/PDF/Beitrag16_04_01.pdf. Accessed August 27, 2016.
Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G, SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis In Stroke – Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–2822.
Gumbinger C, Gruschka P, Böttinger M, Heerlein K, Barrows R, Hacke W, Ringleb P. Improved prediction of poor outcome after thrombolysis using conservative definitions of symptomatic hemorrhage. Stroke. 2012;43:240–2.
Meyers PM, Schumacher HC, Alexander MJ, Derdeyn CP, Furlan AJ, Higashida RT, Moran CJ, Tarr RW, Heck DV, Hirsch JA, Jensen ME, Linfante I, McDougall CG, Nesbit GM, Rasmussen PA, Tomsick TA, Wechsler LR, Wilson JR, Zaidat OO. Performance and training standards for endovascular acute ischemic stroke treatment. Neurology. 2012;79:234–8.
Ernst M, Kriston L, Romero JM, Frölich AM, Jansen O, Fiehler J, Buhk JH. Quantitative evaluation of performance in interventional neuroradiology: An integrated curriculum featuring theoretical and practical challenges. PLOS ONE. 2016;11:e0148694.
Tatlisumak T. Implication of the recent positive endovascular intervention trials for organizing acute stroke care: European perspective. Stroke. 2015;46:1468–73.
Gupta R, Horev A, Nguyen T, Gandhi D, Wisco D, Glenn BA, Tayal AH, Ludwig B, Terry JB, Gershon RY, Jovin T, Clemmons PF, Frankel MR, Cronin CA, Anderson AM, Hussain MS, Sheth KN, Belagaje SR, Tian M, Nogueira RG. Higher volume endovascular stroke centers have faster times to treatment, higher reperfusion rates and higher rates of good clinical outcomes. J Neurointerv Surg. 2013;5:294–7.
Pfaff J, Pham M, Herweh C, Wolf M, Ringleb PA, Schönenberger S, Bendszus M, Möhlenbruch M. Clinical Outcome after mechanical thrombectomy in non-elderly patients with acute ischemic stroke in the anterior circulation: primary admission versus patients referred from remote hospitals. Clin Neuroradiol. 2015 Sep 2. [Epub ahead of print]
Saver JL. Time is brain – quantified. Stroke. 2006;37:263–6.
Wendt M, Ebinger M, Kunz A, Rozanski M, Waldschmidt C, Weber JE, Winter B, Koch PM, Freitag E, Reich J, Schremmer D, Audebert HJ, STEMO Consortium. Improved prehospital triage of patients with stroke in a specialized stroke ambulance: results of the pre-hospital acute neurological therapy and optimization of medical care in stroke study. Stroke. 2015;46(3):740–5.
Sun CH, Nogueira RG, Glenn BA, Connelly K, Zimmermann S, Anda K, Camp D, Frankel MR, Belagaje SR, Anderson AM, Isakov AP, Gupta R. “Picture to puncture”: A novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013;127:1139–48.
Gratz P, Jung S, Schroth G, Gralla J, Mordasini P, Hsieh K, Heldner MR, Mattle HP, Mono ML, Fischer U, Arnold M, Zubler C. Outcome of standard and high-risk patients with acute anterior circulation stroke after stent retriever thrombectomy. Stroke. 2014;45:152–8.
Prothmann S, Schwaiger BJ, Gersing AS, Reith W, Niederstadt T, Felber A, Kurre W. Acute recanalization of thrombo-embolic ischemic stroke with pREset (ARTESp): the impact of occlusion time on clinical outcome of directly admitted and transferred patients. J Neurointerv Surg. 2016 Aug 16. [Epub ahead of print]
Lee SU, Hong JM, Kim SY, Bang OY, Demchuk AM, Lee JS. Differentiating carotid terminus occlusions into two distinct populations based on Willisian collateral status. J Stroke. 2016;18:179–86.
Kleine JF, Beller E, Zimmer C, Kaesmacher J. Lenticulostriate infarctions after successful mechanical thrombectomy in middle cerebral artery occlusion. J Neurointerv Surg. 2016 Mar 3. [Epub ahead of print]
von Baumgarten L, Thierfelder KM, Beyer SE, Baumann AB, Bollwein C, Janssen H, Reiser MF, Straube A, Sommer WH. Early CT perfusion mismatch in acute stroke is not time-dependent but relies on collateralization grade. Neuroradiology. 2016;58:357–65.
Maurer CJ, Egger K, Dempfle AK, Reinhard M, Meckel S, Urbach H. Facing the time window in acute ischemic stroke: the infarct core. Clin Neuroradiol. 2016;26:153–8.
Angermaier A, Michel P, Khaw AV, Kirsch M, Kessler C, Langner S. Intravenous thrombolysis and passes of thrombectomy as predictors for endovascular revascularization in ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:2488–95.
Lin M, Tsivgoulis G, Alexandrov A, Chang J. Factors affecting clinical outcome in large- vessel occlusive ischemic strokes. Int J Stroke. 2015;10:479–84.
Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: Pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and multi MERCI trials. Stroke. 2009;40:3777–83.
Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, Sacco RL, Connolly SJ, Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014;13:429–38.
Acknowledgements
Statistician Hiltrud Niggemann joined us in conducting the statistical analysis. Casjupea Knispel, stroke and study nurse of the Neuroradiological Clinic, participated in data collection and ran the follow-up telephone interviews.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. H. Nolte received honoraria for speeches from Pfizer, BMS, Sanofi and Boehringer-Ingelheim as well as travel support for congress participation from Bayer, Sanofi and Boehringer-Ingelheim. M. A. Pérez serves as a proctor and consultant for phenox. H. Henkes is co-founder and shareholder of phenox. P. Bücke, E. Schmid and H. Bäzner declare that they have no competing interests.
Ethical standards
All studies on humans described in this manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration from 1964 (in its present revised form). Informed consent was obtained from all participants in the studies.
Caption Electronic Supplementary Material
62_2017_558_MOESM1_ESM.pdf
In the online supplement we provide an extended version of table 1 including missing data, head-to-head comparison of groups for all outcome parameters as well as information on all hospitals referring patients on a regular basis, their distances to our institution and their respective patient count
Rights and permissions
About this article
Cite this article
Bücke, P., Pérez, M.A., Schmid, E. et al. Endovascular Thrombectomy in Acute Ischemic Stroke: Outcome in Referred Versus Directly Admitted Patients. Clin Neuroradiol 28, 235–244 (2018). https://doi.org/10.1007/s00062-017-0558-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-017-0558-z