Abstract
Background
Galectin-3 has been reported as a mediator of heart failure (HF) development and progression. Most studies, however, have been conducted on patients with chronic HF rather than acute HF (AHF).
The aim of this study was to confirm galectin-3 as a prognostic marker in subjects with AHF and to investigate its possible relationship with left ventricular (LV) remodeling.
Methods
A total of 69 patients hospitalized with a primary diagnosis of AHF were followed up for 18 months. Galectin-3 and echocardiographic parameters were measured at baseline and after 6 months. Survival analysis and exploratory analysis of LV remodeling were performed.
Results
Patients with high baseline galectin-3 values (>16.5 ng/ml) had a significantly worse survival profile over the 18-month follow-up (log-rank test, p = 0.017), with Cox proportional hazards modeling showing a crude hazard ratio (HR) of 4.66 (95% CI = 1.16–18.67; likelihood-ratio test, p = 0.037) for all-cause mortality. Changes in galectin-3 levels (1 SD increase over 6 months) proved to be a significant explanatory factor for HF hospital re-admission in the short term when compared with quasi-stationary galectin-3 levels: worse Kaplan–Meier survival curves (log-rank test, p = 0.001) and a crude HR of 4.44 (95% CI = 1.76–11.18; likelihood-ratio test, p = 0.004). A significant association was found between the pathological evolution of relative wall thickness, LV end-diastolic diameter, LV end-diastolic volume, and increasing levels of galectin-3 in the short term (Cochran–Mantel–Haenszel test, p < 0.01).
Conclusion
Galectin-3 can predict long-term mortality in patients with AHF. The results of our study suggest a possible relation between left ventricular remodeling and increasing galectin-3 levels.
Zusammenfassung
Hintergrund
Galektin-3 wurde als Mediator der Entstehung und Progression einer Herzinsuffizienz beschrieben. Jedoch wurden die meisten Studien eher bei Patienten mit chronischer als mit akuter Herzinsuffizienz durchgeführt. Ziel der vorliegenden Studie war es, Galektin-3 als prognostischen Marker bei akuter Herzinsuffizienz zu bestätigen und seine mögliche Beziehung zum linksventrikulären (LV-)Remodeling zu untersuchen.
Methoden
Insgesamt wurden 69 Patienten mit primärer Diagnose einer akuten Herzinsuffizienz bis zu 18 Monate nachbeobachtet. Zu Beginn und nach 6 Monaten wurden Galektin-3 und echokardiographische Parameter gemessen. Es erfolgte eine Analyse des Überlebens sowie eine exploratorische Analyse des LV-Remodeling.
Ergebnisse
Patienten mit anfänglichen hohen Galektin-3-Werten (>16,5 ng/ml) wiesen ein signifikant schlechteres Überlebensprofil während der 18-monatigen Nachbeobachtung auf (Log-Rank-Test, p = 0,017), dabei ergab das Cox-Proportional-Hazards-Modell eine rohe Hazard Ratio (HR) von 4,66 (95%-Konfidenzintervall, 95%-KI: 1,16–18,67; Likelihood-Ratio-Test, p = 0,037) für die Mortalität aus sämtlichen Ursachen. Die Veränderungen der Galektin-3-Werte (Anstieg von 1 Standardabweichung, SD, über 6 Monate) erwiesen sich auf kurze Sicht als signifikanter erklärender Faktor für die erneute stationäre Aufnahme wegen Herzinsuffizienz im Vergleich zu quasi-stationären Galektin-3-Werten: schlechtere Kaplan-Meier-Überlebenskurven (Log-Rank-Test, p = 0,001) und eine rohe HR von 4,44 (95%-KI: 1,76–11,18; Likelihood-Ratio-Test, p = 0,004). Ein signifikanter Zusammenhang stellte sich kurzfristig zwischen pathologischen Befunden bei der relativen Wanddicke, LV enddiastolischem Durchmesser, LV enddiastolischem Volumen und ansteigenden Galektin-3-Werten heraus (Cochran–Mantel–Haenszel test, p < 0,01).
Schlussfolgerung
Galektin-3 ermöglicht die Vorhersage der Langzeitmortalität bei Patienten mit akuter Herzinsuffizienz. Die Ergebnisse der vorliegenden Studie weisen auf eine mögliche Beziehung zwischen LV-Remodeling und ansteigenden Galektin-3-Werten hin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Heart failure is a complex syndrome characterized by loss of pumping capacity due to myocardial insult, exaggerated neurohormonal activation, hemodynamic loading, and active inflammation that lead to changes in the architecture of the myocardium. Heart failure is responsible for high mortality rates each year and is the leading cause of hospitalization in people older than 65 years. To improve survival and to reduce health-care expenditure it is essential to identify heart failure patients with the highest risk. The challenging task is to assess accurately the prognosis in this burdening disease, and therefore new strategies, such as the biomarker approach, have been developed. A prospective reliable gauge when assessing heart failure patients is the galectin-3 molecule, a beta-galactoside binding protein [1].
Galectin-3 is mainly expressed intracellularly (at the level of the nucleus, cytoplasm, and mitochondria) by inflammatory cells such as macrophages, neutrophils, mast cells, or fibroblasts [2, 3]. It can also be found on the cell surface or extracellularly, where it binds to numerous matrix proteins (tenascin, laminin, fibronectin) thanks to its collagen-specific protein sequence [4], and it performs a whole range of functions, such as cell proliferation and cell cycle progression, or apoptosis, which can be explained by its nuclear expression or by direct interaction with transcription factors [5, 6]. Other functions such as cell differentiation, cell adhesion, chemo-attraction, and pro-inflammatory factor inducement are possible through cell signaling, generated by the interaction of the molecule’s C domain with carbohydrate ligands that are found on the surface of inflammatory cells [6–8]. A major focus has been on the role of galectin-3 in promoting fibrosis, and it has already been proven to be up-regulated in diseases such as liver cirrhosis, renal failure, and idiopathic pulmonary fibrosis, all of which have fibrosis as the underlying pathological mechanism [9–11].
Inflammation and fibrosis are crucial mechanisms in cardiac remodeling and development of heart failure [1, 12], while galectin-3 over-expression by macrophages has been observed in the failing heart [13]. The increased presence of the molecule promotes augmentation of the tumor growth factor (TGF)-beta/Smad3 signaling pathway, which in turn determines cardiac fibroblast activation and proliferation leading to excessive secretion of collagen type I with extracellular deposition and consequent ventricular dysfunction [13, 14]. Clinical studies have reported both the presence of this molecule in high plasma concentrations of patients with chronic heart failure and its baseline level as predictors of mortality [15–17].
The aim of this study was to explore the survival prognostic value of galectin-3 in patients with acute heart failure (AHF). We planned to determine galectin-3 at baseline and again during the follow-up, based on data provided by Chun et al. [18], who described a specific three-phase pattern with a lower rate of events between 4 and 14 months. We aimed to examine the galectin-3 changes as a prognostic factor for those patients who would experience worsening heart failure. In addition, we investigated whether changes in galectin-3 concentrations were associated with left ventricular remodeling parameters assessed echocardiographically and electrocardiographically.
Patients and methods
Patient population and study design
The study included 69 consecutive patients hospitalized in the Cardiology Unit of Arad Clinical Emergency County Hospital, with a primary diagnosis of AHF. Framingham criteria were applied to establish the diagnosis of heart failure, which require either the simultaneous presence of two major factors or a minimum of one major and two minor factors. Patients were included in the study based on the following AHF criteria: (a) dyspnea at rest or minimal effort, (b) pulmonary congestion visible on chest X‑ray, (c) ultrasound signs of interstitial edema or pleural effusion, and (d) need for ≥40 mg intravenous furosemide within 16 h of hospital admission.
Based on the established literature, a prior estimation of the necessary sample size was made, resulting in a total of 102 subjects (hazard ration [HR] = 2.7; 0.25 proportion of exposed subjects; 0.06 baseline event rate; 18-month planned follow-up; 12-month median survival time; 0.1 censoring rate; alpha = 0.05; beta = 0.2). Between March and November 2014, 103 consecutive patients meeting the inclusion criteria were enrolled in the study. Each patient underwent clinical evaluation followed by additional investigations during their period of hospitalization. After discharge, all contact was lost with 34 of them, hence there was no information on the course of their disease. The medical team decided to continue the planned prospective study with the remaining 69 subjects. These early drop-out subjects were not included in the analysis, as no survival data were available for them. The remaining patients were clinically assessed and further re-investigated after a 6-month follow-up, on a scheduled outpatient medical visit. On both occasions, serum galectin-3 was determined. For all 69 patients in the study, the overall follow-up time was 18 months. The primary outcome was all-cause mortality, and the secondary outcome was re-hospitalization due to aggravated heart failure. The study was approved by the hospital’s Medical Ethics Committee and complied with the Helsinki Declaration. Written informed consent was obtained from all patients.
Echocardiography
Transthoracic echocardiography was performed on each patient at both of the medical assessments. Of the total of 69 patients, five died before the planned 6‑month echocardiography. Structural parameters were determined: left ventricular end-diastolic volumes (LVEDV) and diameters (LVEDD), the relative wall thickness index (RWT), and left atrium indexed volume (LAvol). Left ventricular ejection fraction (EF) was determined using the biplane modified Simpson’s method. Diastolic parameters, i. e., transmitral peak velocities (E, A), early deceleration time (DTE), septal proto-diastolic velocity (e′), E/A and E/e′ diastolic ratios, were determined using spectral and tissue Doppler. A Siemens Acuson X300 ultrasound system (Siemens Medical Solutions USA, Inc. Mountain View, CA 94043, USA Model, Made in Korea) was employed and two independent investigators analyzed the echocardiographic data.
Galectin-3 analysis
Blood samples were collected into serum-separating tubes, centrifuged, and stored at −80 °C for later analyses. Serum galectin-3 levels were determined using an optimized enzyme-linked immunosorbent assay kit (Human galectin-3 NBP1-91275; Novus Biologicals, R&D Systems Europe, Germany) and were measured on a Tecan Sunrise (Tecan Austria GmbH Untersbergstr. 1A, A-5082 Grodig, Austria) microplate reader. This immunoassay uses a biotin-conjugated anti-human galectin-3. Calibration and standardization of the assay were performed according to the manufacturer’s protocol.
Statistical analysis
Descriptive statistical analysis was performed as follows: For numerical variables, the Shapiro–Wilk test for normality was applied; descriptive statistics are given as mean ± SD for normally distributed values and median (interquartile range [IQR]) for non-normally distributed data; for categorical variables, the observed frequency counts (percent) are provided. The galectin-3 values were non-normally distributed, and therefore they were described as median (IQR). When comparing categorical variables, a chi-square test was applied (either the asymptotic version or Monte Carlo simulation with 10,000 replicates). For stratified analysis of categorical variables, the Cochran–Mantel–Haenszel test was employed. Further, to enable comparisons and facilitate interpretation, the galectin-3 values were log-transformed and then standardized as z‑values; increasing by 1 SD was considered for the analysis of galectin-3 change over the first 6‑month follow-up. Some of the clinical and echocardiographic indices (numerical variables) were transformed into binary (normal vs. abnormal/pathological) or rank variables (e. g., early deceleration time DTE, or E/A and E/e′ ratios), as appropriate. Survival analysis was conducted to investigate the relationship between elevated levels of galectin-3 and the time to event, scrutinizing the Kaplan–Meier curves and applying the log-rank test. To calculate HRs, Cox proportional hazards (PH) modeling was used, starting with crude models and fitting sequential models with a focus on galectin-3 or its change over time, while controlling for possible confounders related to age, degree of disease, HF etiology, and associated comorbidities. The PH assumptions were evaluated employing both graphic and goodness-of-fit approaches (i. e., both log-log plots and correlation testing for the Schoenfeld residuals to ranked failure times). The regression models were compared using likelihood ratio (LR) statistics and the chi-square test.
All reported probability values were two-tailed and a 0.05 level of significance was considered, while marking the very significant and marginally significant values as well. Statistical analysis was conducted using SPSS v.17 and the R v.3.2.3 software packages (including survival and powerSurvEpi).
Results
Study population
The mean age of the subjects in this study was 64.67 ± 11 years, and 48 (69.6%) were male patients. They were evaluated over a period of 18 months from their discharge and re-assessed at 6 months. The median galectin-3 level was 6 ng/ml (IQR, 2.9–14.5) at baseline and 10.25 ng/ml (IQR, 8.15–13.4) at the 6‑month follow-up.
During the 18-month follow-up period, eight patients reached the primary outcome (i. e., all-cause death) and 23 the secondary outcome (i. e., re-hospitalization for worsening heart failure).
The distribution of galectin-3 values was positively skewed (skewness = 0.835 ± 0.29), with a high degree of heterogeneity between the four groups delimited by the quartiles, especially on the high end, for which the range was 14.5–25 ng/ml (quite large, especially in contrast to the 1.35–2.89 ng/ml range for the low end, i. e., the first group). This issue, combined with the relatively small size cohort, led us to reconsider granularity when dividing the patients according to the levels of galectin-3 [19]. Consequently, we decided to use quintile-defined groups, both for the baseline and the 6‑month re-assessment. The detailed results of the two medical assessments are described in Tables 5 and 6 in the Appendix.
Baseline galectin-3 as prognostic marker
We considered the upper quintile (i. e., the upper two deciles) as being the group of patients potentially at risk. The actual upper quintile cut-off value for baseline galectin-3 was 16.5 ng/ml.
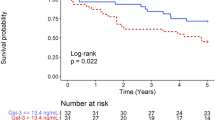
Fig. 1 shows the Kaplan–Meier survival curves for the 18-month follow-up for all-cause mortality. The Cox proportional hazards regression models are presented in Table 1, starting with the crude model and including only the upper quintile status for baseline galectin-3, and subsequently adjusting for potential confounders. Only models found as adding statistically significant information are included in Table 1.
Kaplan–Meier curves for 18-month cumulative survival in relation to the high values of galectin-3, with the log-rank test applied (p = 0.017). The observed survival time (in months) was 13.91 ± 1.81 with four events (i. e., patients deceased) for the group with galectin-3 uppermost quintile values vs. 17.36 ± 0.38 and four events for the rest of the subjects
The high level of baseline galectin-3 was also studied as a potential explanatory marker for the secondary outcome in the study, which is hospital re-admission. The survivor functions and Kaplan–Meier curves showed no difference with regard to the hazard of re-hospitalization: On the contrary, the curves crossed each other multiple times in the course of the 18-month follow-up. No significant results were obtained when investigating the relationship between all-cause mortality and increasing galectin-3 over the 6‑month follow-up.
Increasing galectin-3 level and hospital re-admission
Fig. 2 shows the Kaplan–Meier survival curves on the basis of the galectin-3 1‑SD increase over the 6‑month period. On the left, the curves for the 18-month follow-up can be observed and a plateau in the risk group is evident after a period of approximately 6 months. Cox proportional hazards regression modeling was conducted for the 18-month follow-up (Table 2) and the 6‑month follow-up (Table 3), with a similar approach for the successive regression models, as in the previous section.
Kaplan–Meier curves for cumulative survival free of hospital re-admission in relation to the galectin-3 increase from baseline to the 6‑month follow-up, with log-rank test applied (p = 0.002 and p = 0.001 for 18-month and 6‑month follow-up, respectively). The observed survival time (in months) over the 18-month follow-up was 6.93 ± 2.33 with seven events (i. e., hospital re-admission) in the group of 10 patients at risk for with 1‑SD increase in galectin-3 vs. 13.96 ± 0.89 and 16 events for the rest of the 54 patients. On the other hand, over 6 months the observed survival time (in months) was 3.36 ± 0.7 with the same seven events in the group of 10 patients at risk for with 1‑SD increase in galectin-3 vs. 5.24 ± 0.21 and 13 events for the rest of the 54 subjects. The median survival time for the 10 patients in the risk group was 1.97 months, irrespective of the follow-up time
Possible link to cardiac remodeling
Following the association of the rising galectin-3 values to the hazard of hospital re-admission in the short term, we further investigated whether these values were also related to cardiac remodeling parameters. Table 4 summarizes the results for the stratified analysis of the changes in pathological patterns for QRS, RWT, LVEDD, and LVEDV in the 6 months between the two medical assessments. The strata were defined by a 1-SD increase in galectin-3 level status.
Discussion
In the current study we found that baseline serum galectin-3 levels over 16.5 ng/ml were a significant explanatory factor for all-cause mortality over the subsequent 18-month period in patients admitted with AHF. Repeating galectin-3 measurements at 6 months proved significant in assessing the hazard for the secondary outcome (i. e., re-hospitalization due to heart failure).
Well-established studies such as CORONA and CARE-HF have described the potential utility of galectin-3 in predicting mortality, both of them with reference to patients with chronic stable heart failure. The hazard ratio for cardiovascular mortality was 1.70 (95% CI = 1.19–2.42; p = 0.003) in the former study, and the odds ratio for death was 2.98 (95% CI = 1.43–6.22; p = 0.004) in the latter, with a cut-off value over 30 ng/ml [20, 21]. In the DEAL-HF substudy, plasma galectin-3 was evaluated as a long-term prognostic factor in stable chronic heart failure [16].
Although most studies were carried out in chronic heart failure patients, AHF has also been investigated. Nunez et al. showed the correlation of galectin-3 with long-term mortality and its relation to increased levels of CA125 (above 67 U/ml), a glycoprotein associated with fluid overload and exacerbated inflammatory status [22]. The PRIDE study investigated the association of high galectin-3 levels with mortality for 60-day follow-up (12.9–16.5 ng/ml vs. 9.0–11.6 ng/ml, p = 0.001) [23]. The COACH trial and subsequent investigations by de Boer et al. found elevated levels of galectin-3 associated with a higher risk of death (25.9–66.6 ng/ml vs. 5.0–15.2 ng/ml; HR = 3.34, 95% CI = 2.23–5.01; p < 0.001) [15, 24]. The role of galectin-3 as a complementary biomarker in AHF with comorbidities, such as renal dysfunction [25], has also been investigated. Lower HR values were found by Carasco-Sanchez et al. in a model of AHF patients with preserved ejection fraction, proving that galectin-3 levels in the upper two quartiles are associated with a composite unfavorable outcome over a 1‑year follow-up, when adjusting for comorbidities (HR = 1.43, 95% CI = 1.07–1.91; p = 0.015) [26].
While de Boer et al. [15] claimed that changes in galectin-3 after a 6-month follow-up did not add any prognostic significance to baseline values, van der Velde et al. [27] concluded that a galectin-3 increase over time (either over a threshold of 17.8 ng/ml, or equivalent to more than 15% above the baseline) was associated with high rates of hospital re-admission and mortality in heart failure patients compared with patients with galectin-3 levels that decreased or remained stable over time. The Val-Heft trial for patients with stable chronic heart failure found elevated galectin-3 levels at 4 months (median change of 1 ng/ml from baseline) to be a risk factor for a composite outcome of all-cause mortality, first morbid event, and heart failure admission [28], and the review of Peacock [29] showed the value of elevated galectin-3 at identifying patients with a very high risk for short-term adverse outcomes.
Our present results of survival analysis for baseline galectin-3 levels confirmed previous reports, with values in the upper quintile (i. e., more than 16.5 ng/ml in the study population) signaling a higher risk of all-cause mortality over the 18-month follow-up. The crude hazard ratio (HR = 4.66) was comparable to previously reported values for patients with chronic disease, but when adjusting for age, NYHA class, and comorbidities the value increased considerably to HR = 9.81 (95% CI = 1.67–57.79). At the same time, the large 95% CI in our results certainly leaves room for further, more precise, determination.
Right from the planning phase, we chose not to combine mortality and hospital re-admission as study outcomes, but to consider them separately. On the other hand, increasing galectin-3 levels proved to be of evident prognostic value for re-hospitalization over the short term, with the Kaplan–Meier survival curves displaying important differences between the groups defined by a 1-SD increase status. When scrutinizing the survival curves, the prognostic value is apparent only in the short term: In fact, there were no events in the risk group after the 6‑month second medical assessment, although later events did occur in the group with quasi-stationary galactin-3 levels. Interestingly, events in the risk group were concentrated in the 6‑month time window for which galectin-3 change was assessed, with a survival curve plateau afterwards, thus confirming the findings of Peacock [29]. The high hazard ratio for hospital readmission in the short term (HR = 8.47; 95% CI = 2.77–25.84) after adjusting for age, NYHA class, and comorbidities, is an important argument for repeating the determination of galectin-3 in patients with heart failure.
We found significant differences between the two strata delimited by galectin-3 1‑SD increases regarding the patterns in changes for QRS, RWT, LVEDD, and LVEDV. The relationship between galectin-3 and the parameters of ventricular function and structure might be plausible given the fact that it modulates fibrosis as well as inflammatory and immunological responses, which are crucial underlying pathophysiological mechanisms of cardiac remodeling [1, 13, 24]. At the same time, QRS delay with subsequent intraventricular asynchrony leads to structural remodeling by increasing LVEDV, redistribution of myocardial shortening, and blood flow from the septum to the left ventricular lateral wall [30, 31]. The MADIT-CRT trial investigated the relationship between galectin-3 concentration and medical outcome in heart failure patients undergoing cardiac resynchronization therapy [32]. Other authors have also suggested a relationship between high galectin-3 levels and ventricular remodeling and dysfunction, diastolic parameters, and the first stages of cardiac stiffness [13, 30, 32–38]. The important role of lower galectin-3 concentration in a favorable medical prognosis has also been reported [39, 40].
Galectin-3 dynamics may have important consequences in heart failure pharmacology by the potential targeting of this molecule for pharmacologic inhibition; however, the expression of its fibrotic properties in the pathophysiological chain of heart failure needs to be further explored in experimental studies.
Limitations
The potential limitations of our study are twofold. First, the unexpectedly high rate of drop-out and the subsequent reduction in statistical power restricted the granularity in both the survival analysis and the investigation of the relation between echocardiographic variables and galectin-3 levels. Therefore, the statistical analysis was kept focused on the evidence for rejecting the initial null hypotheses. Second, the echocardiographic follow-up would have benefitted from an extension beyond the 6‑month period, as the remodeling process usually works over longer periods, and thus the profile of its relation to the increase in galectin-3 needs to be confirmed and investigated further.
Conclusion
In this study, we found evidence that galectin-3 can predict long-term mortality in patients with AHF. Serial measurements of galectin-3 are important in the prediction of secondary outcomes, such as hospital re-admission for heart failure. This study and the comprehensive discussion provide new insight into the acute phase and early follow-up of the disease, after the first hospitalization, and suggest a possible relation between left ventricular remodeling and increasing galectin-3 levels.
References
Yang RY, Rabinovich GA, Liu FT (2008) Galectins: structure, function and therapeutic potential. Expert Rev Mol Med 10:e17
Gong HC, Honjo Y, Nangia-Makker P et al (1999) The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res 59:6239–6245
Hughes RC (1997) The galectin family of mammalian carbohydrate-binding molecules. Biochem Soc Trans 25:1194–1198
Kramer F (2013) Galectin-3: clinical utility and prognostic value in patients with heart failure: Res Reports. Clin Cardiol 4:13–22
Balan V, Nangia-Makker P, Raz A (2010) Galectins as cancer biomarkers. Cancers (Basel) 2:592–610
Dagher SF, Wang JL, Patterson RJ (1995) Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci USA 92:1213–1217
Karlsson A, Christenson K, Matlak M et al (2009) Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology 19:16–20
Barboni EA, Bawumia S, Henrick K, Hughes RC (2000) Molecular modeling and mutagenesis studies of the N‑terminal domains of galectin-3: evidence for participation with the C‑terminal carbohydrate recognition domain in oligosaccharide binding. Glycobiology 10:1201–1208
Henderson NC, Mackinnon AC, Farnworth SL et al (2006) Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 103(13):5060–5065
Henderson NC, Mackinnon AC, Farnworth SL et al (2008) Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 172(2):288–298
Mackinnon AC, Gibbons MA, Farnworth SL et al (2012) Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Resp Crit Care Med 185(5):537–546
Oikonomou E, Tousoulis D, Siasos G et al (2011) The role of inflammation in heart failure: new therapeutic approaches. Hellenic J Cardiol 52:30–40
Sharma UC, Pokharel S, van Brakel TJ et al (2004) Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110:3121–3128
Psarras S, Mavroidis M, Sanoudou D et al (2012) Regulation of adverse remodelling by osteopontin in a genetic heart failure model. Eur Heart J 33(15):1954–1963
De Boer RA, Lok DJ, Jaarsma T et al (2011) Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 43(1):60–68. doi:10.3109/07853890.2010.538080
Lok DJ, van der Meer P, de la Porte PW et al (2010) Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 99(5):323–328
Lala RI, Puschita M, Darabantu D, Pilat L (2015) Galectin-3 in heart failure pathology – another brick in the wall? Acta Cardiol 70(3):323–331
Chun S, Tu JV, Wijeysundera HC et al (2012) Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail 5:414–421
Altman DG, Bland JM (1994) Quartiles, quintiles, centiles, and other quantiles. BMJ 309:996
Gullestad L, Ueland T, Kjekshus J et al (2012) The predictive value of galectin-3 for mortality and cardiovascular events in the controlled rosuvastatin multinational trial in heart failure (CORONA). Am Heart J 164(6):878–883
Lopez-Andres N, Rossignol P, Iraqi W et al (2012) Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (cardiac resynchronization in heart failure) trial. Eur J Heart Fail 14(1):74–81
Núñez J, Rabinovich GA, Sandino J et al (2015) Prognostic value of the interaction between Galectin-3 and antigen carbohydrate 125 in acute heart failure. PLOS ONE 10(4):e0122360
Van Kimmenade RR, Januzzi JL Jr, Ellinor PT et al (2006) Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol 48(6):1217–1224
De Boer RA, Voors AA, Muntendam P et al (2009) Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail 11:811–817
Fermann GJ, Lindsell CJ, Storrow AB et al (2012) Galectin 3 complements BNP in risk stratification in acute heart failure. Biomarkers 17(8):706–713
Carrasco-Sánchez FJ, Aramburu-Bodas O, Salamanca-Bautista P et al (2013) Predictive value of serum galectin-3 levels in patients with acute heart failure with preserved ejection fraction. Int J Cardiol 169(3):177–182
van der Velde AR, Gullestad L, Ueland T et al (2013) Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail 6(2):219–226
Anand IS, Rector TS, Kuskowski M et al (2013) Baseline and serial measurements of galectin3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur J Heart Fail 15(5):511–518
Peacock WF (2014) How galectin-3 changes acute heart failure decision making in the emergency department. Clin Chem Lab Med 52(10):1409–1412
Vernooy K, Verbeek XA, Peschar M et al (2005) Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J 26(1):91–98
Iuliano S, Fisher SG, Karasik PE et al (2002) QRS duration and mortality in patients with congestive heart failure. Am Heart J 143:1085–1091
Stolen CM, Adourian A, Meyer TE et al (2014) Plasma galectin-3 and heart failure outcomes in MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy). J Card Fail 20(11):793–799
Shamim W, Francis DP, Yousufuddin M et al (1999) Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int J Cardiol 70:171–178
Lok DJ, Lok SI, Bruggink-André de la Porte PW et al (2013) Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol 102(2):103–110
Zile MR, Baicu CF, Gaasch WH (2004) Diastolic heart failure: abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350:1953–1959
Shah RV, Chen-Tournoux AA, Picard MH et al (2010) Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail 12:826–828
Ho JE, Liu C, Lyass A et al (2012) Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 60:1249–1256
De Boer RA, Edelmann F, Cohen-Solal A et al (2013) Galectin-3 in heart failure with preserved ejection fraction. Eur J Heart Fail 15:1095–1101
Kortekaas KA, Hoogslag GE, de Boer RA et al (2013) Galectin-3 and left ventricular reverse remodelling after surgical mitral valve repair. Eur J Heart Fail 15(9):1011–1018
Zhou K, Zhou Y, Zhao Y et al (2016) The relationship between galectin-3 and different patterns of ventricular geometry remodelling in aortic valve stenosis. Heart Lung Circ 25(4):371–377
Acknowledgements
R.I. Lala was partly supported in his research by the Operational Programme for Human Resources Development (SOPHRD), financed by the European Social Fund and the Romanian Government under contract number POSDRU 141531.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R.I. Lala, D. Lungeanu, D. Darabantiu, L. Pilat, and M. Puschita declare that they have no competing interests.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Lala, R.I., Lungeanu, D., Darabantiu, D. et al. Galectin-3 as a marker for clinical prognosis and cardiac remodeling in acute heart failure. Herz 43, 146–155 (2018). https://doi.org/10.1007/s00059-017-4538-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-017-4538-5