Abstract
Background
In advanced congestive heart failure (CHF), intravenous (i.v.) inotropic agents, i.v. diuretics, ultrafiltration, and hemodialysis have been shown to not yield better clinical outcomes. In this scenario, the simultaneous administration of hypertonic saline solution (HSS) and furosemide may offer a more effective therapeutic option with a good safety profile.
Methods
Therefore, a meta-analysis was performed to compare combined therapy, consisting of i.v. furosemide plus concomitant administration of HSS, with i.v. furosemide alone for acute decompensated heart failure (ADHF). The outcomes we chose were all-cause mortality, risk of re-hospitalization for ADHF, length of hospital stay, weight loss, and variation of serum creatinine.
Results
Based on five randomized controlled trials (RCTs) involving 1,032 patients treated with i.v. HSS plus furosemide vs. 1,032 patients treated with i.v. furosemide alone, a decrease in all-cause mortality in patients treated with HSS plus furosemide was proven [RR = 0.57; 95 % confidence interval (CI) = 0.44–0.74, p = 0.0003]. Likewise, combined therapy with HSS plus furosemide was shown to be associated with a reduced risk of ADHF-related re-hospitalization (RR = 0.51; 95 % CI = 0.35–0.75, p = 0.001). Besides, combined therapy with HSS plus furosemide was found to be associated with a reduced length of hospital stay (p = 0.0002), greater weight loss (p < 0.00001), and better preservation of renal function (p < 0.00001).
Conclusion
HSS as an adjunct to i.v. furosemide for diuretic-resistant CHF patients led to a better renal safety profile and improved clinical endpoints such as mortality and heart failure-related hospitalizations.
Zusammenfassung
Hintergrund
Bei fortgeschrittener kongestiver Herzinsuffizienz (CHF) haben intravenös (i.v.) applizierte inotrope Substanzen, i.v.-Diuretika, Ultrafiltration und Hämodialyse nachgewiesenermaßen nicht zu besseren klinischen Ergebnissen geführt. Im vorliegenden Szenario stellen die gleichzeitige Gabe von hypertoner Kochsalzlösung (HSS) und Furosemid möglicherweise eine wirksamere therapeutische Option mit gutem Sicherheitsprofil dar.
Methoden
Daher wurde eine Metaanalyse durchgeführt mit dem Ziel, die kombinierte Therapie aus Furosemid i.v. plus begleitender Gabe von HSS mit der Gabe von Furosemid i.v. allein bei akut dekompensierter Herzinsuffizienz (ADHF) zu vergleichen. Die gewählten Endpunkte waren Mortalität aus sämtlichen Ursachen, Risiko der Wiederaufnahme ins Krankenhaus wegen ADHF, Krankenhausverweildauer, Gewichtsabnahme und Veränderung des Serumkreatinins.
Ergebnisse
Auf der Grundlage von 5 randomisierten kontrollierten Studien mit 1032 Patienten, die mit HSS plus Furosemid i.v. behandelt wurden, versus 1032 Patienten, die mit Furosemid i.v. allein behandelt wurden, wurde eine Abnahme der Mortalität aus sämtlichen Ursachen bei Patienten mit der Gabe von HSS plus Furosemid nachgewiesen (RR = 0,57; 95%-Konfidenzintervall, 95%-KI: 0,44–0,74; p = 0,0003). Gleichermaßen wurde gezeigt, dass die kombinierte Therapie aus HSS plus Furosemid mit einem verminderten Risiko einer stationären Wiederaufnahme wegen ADHF einherging (RR = 0,51; 95%-KI: 0,35–0,75; p = 0,001). Außerdem stellte sich heraus, dass die kombinierte Therapie aus HSS plus Furosemid mit einer geringeren Krankenhausverweildauer (p = 0,0002), größerer Gewichtsabnahme (p < 0,00001) und besserer Aufrechterhaltung der Nierenfunktion (p < 0,00001) assoziiert war.
Schlussfolgerung
Mit HSS zusätzlich zu Furosemid bei diuretikaresistenten CHF-Patienten wurde eine Verbesserung des renalen Sicherheitsprofils und klinischer Endpunkte wie Mortalität und herzinsuffizienzbedingte stationäre Aufnahme wahrscheinlich.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hypertonic saline solution (HSS) has been proposed for a long time as an adjuvant therapy to intravenous (i.v.) loop diuretics, in order to enhance or retrieve their original pharmacological efficacy [1]. The rationale for using HSS is based on its property of exerting a quick osmotic recall of free water contained in the interstitial spaces, so as to promote an optimal refilling of the intravascular compartment during i.v. diuretic therapy [2, 3]. The supplementation of i.v. diuretic therapy by means of an i.v. injection of small volumes of HSS is able to prevent the possible decline in effective arterial circulating volume relating to i.v. diuretics [4, 5], as well as to avoid the consequent possible critical decrease in renal blood flow and glomerular filtration rate that is implicated in worsening renal function (WRF), usually defined by a serum creatinine increase of more than 0.3 mg/dl from baseline and found to be frequently associated with too drastic or overzealous diuretic therapy in chronic heart failure (CHF) patients [6, 7, 8, 9]. Besides, HSS has been proven to keep an adequate Na+ delivery at the level of Henle’s loop of the nephron, i.e., the site of action of loop diuretics, thereby enabling them to better exert their pharmacodynamic effect, consisting in the inhibition of re-absorption of the sodium and water bound to this ion inside the renal tubular lumen [10].
Before being adopted in heart failure, hypertonic saline solutions had been used at various concentrations in the setting of hypovolemic-hemorrhagic shock for resuscitative purposes since 1917 [11]. Furthermore, data from experimental shock models demonstrate that the infusion of 7.5 % NaCl produces vasodilatation and increased regional blood flow to coronary [12], renal [13], intestinal, and skeletal muscle [14] circulation.
Numerous contributions have subsequently highlighted the clinical improvement achievable by acutely administered HSS under conditions of shock and low flow (particularly hemorrhagic and septic shock) [15, 16, 17]. Notably, HSS improves myocardial contractility, a finding that is attributed to a direct cardiac inotropic effect induced by hypertonicity [18, 19].
Subsequently, HSS at various concentrations has been introduced by several authors as adjuvant routine therapy to be used for supplementing diuretic therapy in patients with CHF [2, 3], mostly in cases of diuretic resistance, in order to corroborate and/or restore the effect of loop diuretics already involved in the therapy but tending toward a progressive pharmacodynamic weakening (the“braking phenomenon”) [20] in patients with so-called refractory heart failure.
The present study aimed to assess the efficacy and safety of HSS in heart failure, through the systematic review and meta-analysis of studies that made a comparison between HSS plus i.v. loop diuretics as a combined approach and i.v. loop diuretics alone. In the present study, the evaluation of qualitative findings from interventional studies (randomized controlled trials, RCTs) was completed whenever possible by quantitative analysis (meta-analysis), by adopting as a criterion a number of efficacy and safety endpoints, chosen among the most used outcomes from the relevant studies available in the literature.
Methods
Study selection
According to the design of the present systematic review and meta-analysis, the studies to be included in the search should have investigated the use of HSS with furosemide for therapy of acute decompensated heart failure (ADHF).
Thus, a systematic search was conducted by adopting as search terms the keywords“hypertonic saline” and“heart failure,” in order to retrieve all of the relevant data through consultation of the PubMed and Embase electronic archives from 1950 to April 2013.
Studies were included if they met the following criteria: (a) the intervention group should have included patients with ADHF treated with HSS plus furosemide; (b) the control group should have included patients with ADHF treated with i.v. furosemide alone.
The Newcastle-Ottawa quality assessment scale was used for quality evaluation of studies to be incorporated in the meta-analysis [21]. Eligibility was assessed based on the following criteria: the selection of the study groups (0–4 points), the comparability of the groups (0–2 points), and the ascertainment of either the exposure or outcome of interest (0–3 points), with a total score of 9. A score ≥ 5 was deemed suitable for inclusion in the meta-analysis.
Furthermore, it was stated that the studies selected for the meta-analysis should have included patients aged over 18 years. In addition, animal experimental studies as well as case reports of HSS administration without a control group should have been eliminated from the meta-analysis. Similarly, all studies not written in English, duplicated studies, review articles, editorials, and expert opinions would have to be excluded.
Eligibility assessment and data extraction were carried out independently by two investigators (RDV and CE), with discrepancies resolved by consensus in consultation with a third author (CA).
Outcomes of interest
Primary outcomes of interest were the pooled relative risk ratio (RR) of mortality and heart failure hospital re-admission in patients. Secondary outcomes were length of hospitalization, weight loss, and elevation in serum creatinine levels.
Statistical analysis
Statistical analyses were performed using Review Manager 5.0.4 software (available from the Cochrane Collaboration at: http//www.cochrane.org) and Stata version 10 (Stata CorpLP, College Station, Tex., USA). As regards dichotomous variables, such as (a) the proportion of patients who experienced hospitalization and (b) mortality from all causes, the effect of therapy with HSS plus i.v. furosemide vs. i.v. furosemide alone was presented as a relative risk (RR) with a 95 % confidence interval (CI), using a random effects model. On the other hand, for the other three outcome measures taken into account (mean length of hospital stay, mean weight loss, and mean variation in serum creatinine), which were computed as continuous variables, we adopted the weighted mean difference (WMD) for estimating the effect size of the combined therapy (HSS plus i.v. furosemide) compared with therapy with i.v. furosemide alone, using a random effects model once again. Heterogeneity was evaluated by Cochran’s Q test, and calculation of the I2 statistic (I2) was assumed to represent the percentage of variability due to between-study variability. We rated I2 of less than 25, 25–50 %, and more than 50 % as low, moderate, and high amounts of heterogeneity, respectively. Publication bias was assessed using Begg’s funnel plot. Results were regarded as statistically significant if p was less than 0.05.
Results
Search results
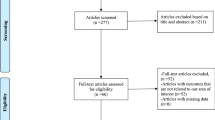
In total, 99 studies were collated. Among them, 75 were excluded because of the ascertained inconsistency with our inclusion criteria, as inferred on the basis of the abstracts. Of the remaining 24 articles, 15 were eliminated after reading the article in full and detecting unavailable or inappropriate data, i.e., the lack of proper and well-planned comparison between a group of patients with ADHF treated by combined therapy with HSS plus furosemide and another ADHF group treated with furosemide alone (Fig. 1).
Overall, nine studies were eligible for inclusion in the meta-analysis. Among these studies, five were used for building the meta-analysis estimating the RR of death from all causes in patients treated with HSS plus i.v. furosemide compared with those treated with furosemide alone. Similarly, four studies were used for the meta-analysis evaluating the RR for heart failure-related re-hospitalization in HSS-treated vs. untreated patients. Furthermore, seven studies served as a source of data for the estimation of WMD for length of hospital stay, eight studies served as a source of data for the estimation of WMD for weight loss, and eight studies were judged suitable for the estimation of WMD for change in serum creatinine. Some aspects of these studies are briefly outlined in the next section focusing on the qualitative findings.
Qualitative analysis
The qualitative findings of these studies are briefly summarized and reported in the online Appendix.
General characteristics of studies included in the meta-analysis
General aspects of the selected studies (number of patients, outcomes of interest, duration and dosing of HSS therapy, etc.) are summarized in Tab. 1 and Tab. 2. The first feature to be emphasized is that the majority of studies included in the quantitative analysis provided a sodium chloride concentration in aqueous solution suitable for the serum sodium level detected at the time of admission. In all studies, the loop diuretic used was furosemide. The design of the meta-analysis did not provide for special criteria to be fulfilled for diuretic dose adjusting, except the fact that the furosemide had to be administered intravenously (irrespective of whether it was administered as a rapid bolus or as a slow i.v. infusion). The diuretic dosage should have been left to the discretional judgment of the treating physician and it might have ranged from 40 to 2,000 mg/day. Also, the duration of treatment might have varied (in fact, treatment with i.v. diuretic administration was applied across the various studies for a minimum of 3 days to a maximum of 12 days). Similarly, no parameter [such as a given volume of urinary output or a given estimated glomerular filtration rate (eGFR)] was preventively suggested as an indication for suspending or re-modulating drug dosage. In particular, six out of a total of nine studies used a diversified sodium chloride concentration based on the individuals’ serum sodium values at admission, in order to prevent possible electrolyte problems. As regards the dietary intake of sodium advised in the studies, in four studies it was planned that the group of patients receiving HSS were to follow a diet with 120 mEq daily of Na+, while a reduced sodium intake (not more than 80 mEq of Na+ per day) was scheduled for the group treated with i.v. diuretic alone. On the other hand, in another study by Paterna et al. [2]—also included in our meta-analysis—all patients, i.e., both those receiving HSS and those exempted from it, were asked to comply with a diet entailing a daily sodium intake of 120 mEq. On the contrary, in the study of Tuttolomondo et al. [29], the dietary regimen prescribed to all patients included a restricted sodium intake that did not exceed 70 mEq/day. In the remaining studies, the sodium content of the diet was not disclosed.
Regarding the adopted therapeutic regimen, in addition to the i.v. furosemide with or without HSS supplementation, some data concerning the medical treatment during hospital stay for ADHF have been collected from the studies admitted to meta-analysis. They are represented in Tab. 3. Briefly, ACE-inhibitors (ACE-i), digoxin, and nitrates were present as background therapy (i.e., the therapeutic regimen maintained during in-hospital administration of i.v. furosemide with or without HSS) in a substantial percentage of cases in each of the evaluated studies. By contrast, the administration of beta-blockers and aldosterone receptor antagonists (ARAs) was scheduled only in the most recent studies. Intravenous administration of inotropic drugs was not reported except for the study by Issa et al. [25].
We evaluated the quality of included studies using the Newcastle-Ottawa scale (Tab. 1). A score of ≥ 5 was deemed proper quality for inclusion. On the whole, the quality of studies was high. Six studies [4, 22, 24, 25, 28, 29] scored 8 points, two studies [2, 27] received 7 points, and one study [26] was given 6 points. There was no publication bias based on the symmetry of the funnel plots.
Quantitative analysis (meta-analysis)
Mortality
Exhaustive data on mortality were reported by five studies. The forest plot displayed in Fig. 2 summarizes the effects of therapy with HSS plus i.v. furosemide as regards death from all causes. All-cause mortality was observed in 154 (14.9 %) of the 1,032 patients belonging to the HSS plus i.v. furosemide group vs. 277 (26.8 %) of the 1,032 controls, i.e., the patients who had been assigned to i.v. furosemide alone. A significant RR of 0.57 (95 % CI: 0.44–0.74; p = 0.0003) was found, so that a reduction in all-cause mortality in patients treated with HSS plus furosemide was demonstrated compared with patients receiving i.v. furosemide alone.
Forest plot for relative risks of death from all causes for chronic heart failure (CHF) patients treated with HSS plus i.v. furosemide vs. CHF patients treated with i.v. furosemide alone assumed as controls. Test for heterogeneity: Q = 11.715 on 4 degrees of freedom (p = 0.0195); I2 (percentage of variability due to inter-study variability) = 65.86 %. HSS hypertonic saline solution, fur furosemide, CI confidence interval
We performed sensitivity analysis to determine the effect of plausible changes in assumptions on the association between HSS–furosemide combined therapy and all-cause mortality (Fig. 3).
The forest plot for all-cause mortality is compared with the corresponding exclusion sensitivity plot. In the latter, there are the hypothetical values that the pooled relative risk ratio (RR) would assume after removal in turn of each of the five studies previously incorporated in the meta-analysis. In the exclusion sensitivity plot (right panel), each of the five small diamonds represents the value of the overall RR arising from a pooled analysis of only four of the five studies originally included in the meta-analysis. Note that the exclusion of the study by Licata et al. [4] as well as the exclusion of the one by Paterna et al. [24] causes loss of statistical significance of the association between HSS use and decreased risk of death from all causes
When the study by Paterna et al. [24] was excluded, the point estimate changed to become nonsignificant (RR = 0.64; 95 % CI: 0.36–1.13; p = 0.1248). Likewise, when the study by Licata et al. [4] was excluded, a loss of statistical significance was detected (RR = 0.65; 95 % CI: 0.38–1.1; p = 0.1075). The significant difference in all-cause mortality was not affected by the exclusion of the other individual trials. Thus, these two studies, especially the study by Paterna et al. [24], had a major impact on the point estimate of the pooled data.
ADHF-related re-hospitalization
By pooling and evaluating the four RCTs analyzing the respective proportions of patients experiencing ADHF-related hospital re-admission, combined therapy with HSS plus i.v. furosemide was shown to be associated with a reduced risk of ADHF-related re-hospitalization (RR = 0.51; 95 % CI: 0.35–0.75; p = 0.001; see Fig. 4). In particular, ADHF-related hospital re-admission was observed in 188 (18.6 %) of the 1,012 patients in the HSS group vs. 372 of the 1,020 (36.5 %) controls. Based on a sensitivity analysis (Fig. 5), the removal of the study by Licata et al. [4] was shown to induce a loss of statistical significance, by reducing the RR to 0.13 (95 % CI: 0.016–1.13; p = 0.065). Likewise, the removal of the study by Paterna et al. [24] significantly weakened the role of HSS as a predictor of reduced risk of re-hospitalization (RR = 0.14; 95 % CI: 0.015–1.237; p = 0.076).
Forest plot of relative risks for experiencing heart failure hospital readmission for patients treated with HSS plus i.v. furosemide vs. patients treated with i.v. furosemide alone assumed as controls. Test for heterogeneity: Q =7.11 on 3 degrees of freedom (p=0.068); I2=57.85%. pts patients, HSS hypertonic saline solution, fur furosemide, CI confidence interval
Forest plot for the frequency of re-hospitalizations related to heart failure is compared with the corresponding exclusion sensitivity plot. In the latter, there are the hypothetical values that the pooled relative risk ratio (RR) would assume after removal in turn of each of the four studies previously incorporated in the meta-analysis. In the exclusion sensitivity plot (right panel), each of the four small diamonds represents the value of the overall RR arising from a pooled analysis of only three of the four studies originally included in the meta-analysis. Note that the exclusion of the study by Licata et al. [4] as well as the exclusion of the one by Paterna et al. [24] causes loss of statistical significance of the association between HSS use and decreased risk of re-hospitalization
Length of hospital stay
Seven studies complying with the inclusion criteria were analyzed to compare the mean length of hospital stay in patients treated with HSS plus i.v. furosemide (n = 1,392) vs. those treated with i.v. furosemide only (n = 1,327; Fig. 6). Based on the estimate of weighted mean difference (WMD), combined therapy with HSS plus furosemide was found to be associated with reduced length of hospital stay (WMD = − 3.13 days; 95 % CI: − 4.23, − 2.03; p < 0.00001). Sensitivity analysis demonstrated similar results when each individual study was removed.
Forest plot of studies estimating the mean length of hospital stay in CHF patients undergoing therapy with HSS plus i.v. furosemide compared with those treated with i.v. furosemide alone. Test for heterogeneity: Q =107.25 on 6 degrees of freedom (p < 0.001); I2 (percentage of variability due to between-study variability) =94.41%. SD standard deviation, CI confidence interval, CHF chronic heart failure
Weight loss
Based on eight RCTs making a comparison between the average weight loss achieved among patients (n = 1,319) treated with HSS plus furosemide and that achieved among the controls (n = 1,334), combined therapy with HSS plus furosemide was found to be associated with greater weight loss from initial admission to discharge (WMD = 2.22 kg; 95 % CI: 1.03–3.40; p = 0.0002; Fig. 7) Sensitivity analysis demonstrated similar results when each individual study was removed.
Meta-analysis of studies estimating the weight loss (kg) induced by HSS plus i.v. furosemide compared with the one caused by i.v. furosemide alone in CHF patients undergoing a single course (some days) of i.v. diuretic therapy. Test for heterogeneity: Q =440.65 on 7 degrees of freedom (p < 0.0001); I2 (percentage of variability due to between-study variability) =98.41%. CI confidence interval, i.v. intravenous, SD standard deviation, HSS hypertonic saline solution, pts patients
Variation in serum creatinine
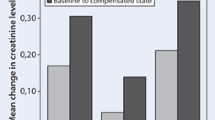
Eight RCTs were included in our meta-analysis aimed at comparing the change in serum creatinine in patients treated with HSS plus i.v. furosemide (n = 1,319) vs. controls (n = 1,334), i.e., the patients receiving i.v. furosemide only (Fig. 8). The use of combined therapy was proven to be associated with better preservation of renal filtration function, judging by a significantly smaller increase in serum creatinine from admission to discharge found in patients receiving HSS (WMD = − 0.42 mg/dl; 95 % CI: − 0.52, − 0.33; p < 0.00001). Most patients had renal dysfunction at study entry. Sensitivity analysis demonstrated similar results when each individual study was removed.
Meta-analysis of studies estimating the renal safety profile, expressed as increase in serum creatinine exhibited by HSS plus i.v. furosemide vs. i.v. furosemide alone (mg/dl) in CHF patients undergoing a single course (some days) of i.v. diuretic therapy. Test for heterogeneity: Q =3719.83 on 7 degrees of freedom (p < 0.0001); I2 (percentage of variability due to between-study variability) =99.81%. i.v. intravenous, CI confidence interval, SD standard deviation, HSS hypertonic saline solution, pts patients
Discussion
It is noteworthy that in decompensated heart failure a condition of reduced effective circulating volume (apparently conflicting with the clinical picture of marked peripheral edema) may frequently occur, so as to entail a relative intravascular depletion, which in turn may be aggravated by i.v. loop diuretics at high doses. The relative hypoperfusion of the proximal renal tubules carries a condition of tubular ischemia, leading to partial loss of the ability to adequately re-absorb the intraluminal sodium. This increased load of excreted sodium inside the tubules, due both to possible ischemic problems at the level of hypoperfused proximal tubules and inherent pharmacodynamic characteristics of furosemide, is able to elicit a mechanism of defensive response against the volume depletion, consisting of intense hyperactivation of the macula densa and generation of increased vasoconstrictor drive at the level of the afferent arteriolar bed of the nephrons. This reflex mechanism, known as“tubuloglomerular feedback,” is instantaneous and occurs irrespective of renin-angiotensin-aldosterone system (RAAS) stimulation. It could be defined as a vasomotor adjustment that attenuates natriuresis when an increase in tubular sodium load is detected by the macula densa. Sodium clearance is thus reduced back to baseline levels, and the person is thereby kept in sodium balance. Indeed, a diuretic-related increase in tubular sodium delivery elicits the same counterbalancing autoregulatory response as seen associated with hypoperfusion of the proximal renal tubules during hypovolemic shock. However, tubuloglomerular feedback is usually interpreted as a maladaptive response in the case of HF. Therefore, there has been much effort to weaken or suppress it by clinicians and researchers. In particular, to effectively antagonize the reduction in effective circulating volume and renal blood supply which, in turn, propitiates the tubuloglomerular feedback, some compounds capable of exerting an osmotic attraction on the plasmatic water from extravascular to intravascular fluid compartments have been proposed, such as mannitol [30], dextran, and other plasma expanders [15, 16, 17], albumin [31, 32], as well as HSS [1, 33]. In particular, HSS may be able to preserve renal flow and glomerular filtration rate (GFR) during intensive i.v. furosemide therapy maintained for several days [23], thereby impeding the feared massive release of renin and antidiuretic hormone, which usually occurs after only a few days of i.v. loop diuretic administration. Thus, the addition of small volumes of HSS during loop diuretic unloading treatment may be a very valuable therapeutic option capable of attenuating the possible harmful effects of neuro-hormonal excitation as well as the i.v. diuretic-related reduction in GFR and subsequent rise in serum creatinine [34].
Moreover, it is well known that after a few days of once-daily dosing with a high-potency diuretic formulation, the increase in 24-h natriuresis that occurs after the first dose disappears or becomes markedly attenuated, a process usually referred to as the braking phenomenon. According to some authors [22, 25], it could be effectively prevented by simultaneous administration of appropriate HSS doses during i.v. diuretic therapy. In fact, HSS possesses the remarkable property of strengthening the effect of furosemide by transiently increasing the serum Na+ concentration, so that an adequate delivery of this ion at the level of the tubular lumen of Henle’s loop [22] is maintained simultaneously with the period of the pharmacodynamic action of furosemide. Notably, it is recommended that HSS is given in conjunction with loop diuretics. Actually, in the hypothetical case of HSS being given without concomitant i.v. diuretic administration, the increased concentrations of NaCl (caused by HSS infusion) can be sensed by the macula densa in the distal tubule, and can enhance the conversion of adenosine triphosphate to adenosine and vasoconstriction through tubuloglomerular feedback. The concurrent administration of furosemide inhibits this tubuloglomerular feedback response and prevents the reflex increase in renal vascular resistances caused by the increased filtered sodium load [35, 36]. The result is a significant net increase in salt and water excretion.
On this basis, several studies have been carried out to explore the potential of HSS to protect renal function during i.v. diuretic therapy in patients with HF, and also to exert a favorable preventive effect against the so-called diuretic resistance [3, 5]. Subsequently, the interest of researchers focused also on the issue of whether integration with HSS was able to achieve a more rapid and complete regression of the signs and symptoms of congestion. Thus, some studies addressed the hypothesis of whether a possible improvement in hydrosaline retention might have been achieved by combined therapy (HSS plus i.v. diuretic), resulting in increased urine output and enhanced weight loss [2, 4, 22]. Finally, some studies aimed to explore whether combination therapy with HSS plus furosemide is able to shorten hospital stay, reduce the frequency of re-hospitalizations, and improve survival in patients with advanced cardiac failure and prone to relapses of clinical decompensation [24].
On the whole, the results of our meta-analysis are encouraging because the combined approach of HSS plus i.v. furosemide has been proved to impact favorably on each of the five examined endpoints, including mortality. However, for some of these endpoints, sensitivity analyses showed that the benefit of HSS ascertained by analysis of the overall pooled data was sometimes lost when a single study was removed from the analysis, a sign of relatively poor robustness of the underlying assumption in favor of HSS. In particular, the exclusion of some studies in the sensitivity analysis led to a loss of statistical significance for both mortality and frequency of re-hospitalizations due to heart failure (see“Results,” sections“Mortality” and“ADHF-related re-hospitalization”). Moreover, whether the marked basal renal insufficiency of patients enrolled in the study by Engelmeier [26]and the very high Na+ concentration (7.5 %) used in the study by Issa et al. [25]might have caused the relatively disappointing outcomes registered in these two studies is still a matter of debate. In fact, the small study of Engelmeier (only 50 patients of whom 25 were assigned to combination therapy with i.v. furosemide plus HSS and 25 to i.v. furosemide alone) substantially differs from the other trials because in this study all enrolled patients were affected by advanced renal insufficiency (eGFR < 40 ml/min/1.73 m2). Therefore, the fact that no significant advantage with the use of HSS was achieved in this study as regards the prevention of the worsening renal function (WRF), diuretic-related, might depend on the already markedly impaired renal conditions at enrollment. Furthermore, in the very small trial of Issa et al. (20 patients assigned to combination therapy with HSS plus furosemide compared with 12 controls receiving i.v. furosemide alone) all-cause mortality was higher in the HSS arm compared with that of controls receiving furosemide alone, although a statistical significance was not attained (50 % vs. 33.3 %, respectively; odds ratio = 2, p = 0.3607). However, just in the study by Issa et al., the planned therapeutic protocol included the HSS use at a concentration much higher than that adopted in other studies (100 ml of 7.5 % sodium chloride administered twice a day for 3 days), with no indication to appropriately modulate the HSS dose according to the serum sodium level. Therefore, the approximate care of dosing as well as inadequate adjustment to the patients’ electrolyte levels may have contributed to generate the ascertained lack of statistical significance with respect to the endpoint“death from all causes.” However, as regards the primary endpoint investigated by Issa and colleagues, i.e., WRF resulting from i.v. diuretic therapy, a significantly lower frequency of this complication was detected in the HSS–furosemide group compared with the control group receiving only furosemide (Fig. 8).
Thus, based on the findings of the meta-analysis, a protective effect on renal filtration function appears to be certainly obtained by small volumes of HSS added to i.v. diuretic therapy. Indeed, this effect is noticeable in seven of the eight studies analyzing the change in serum creatinine in patients who received HSS infusion as a supplement compared with those who did not.
Moreover, the association of HSS with a reduced risk of WRF remained unchanged when each of the included studies was eliminated within the sensitivity analysis, which validates the assumption that the association detected by the meta-analysis of the pooled data was sufficiently robust. Therefore, based on the present meta-analysis as well as on previous specific studies that were not included [3, 5], a protective action against iatrogenic increase in serum creatinine, related to high doses of i.v. diuretics, could be recognized as a sufficiently documented therapeutic property of HSS (when given in conjunction with i.v. loop diuretics). Thus, there are sufficient grounds for introducing the use of HSS in the near future as a supplement to be routinely added to i.v. loop diuretics, in order to protect renal function in patients with acute cardiac decompensation both in the case of hyponatremia and in the presence of normal serum sodium levels.
Study limitations
There are also some limitations and weaknesses in the various study designs that must be considered. These are briefly outlined here.
For two of the investigated outcomes, namely, mortality and rate of ADHF-related re-hospitalizations, only five and four studies were considered, respectively, thereby reducing the study’s power and the potential for identifying statistically significant results.
Some studies included in this meta-analysis recruited patients in the 1990s, and patients were less likely to receive beta-blockers, ACE inhibitors, and aldosterone receptor blockers. This may result in the overestimation of the current therapeutic benefit of HSS.
Several studies excluded patients with renal dysfunction. Therefore, although the HSS group was shown to have improved serum creatinine, this analysis did not take into account patients with baseline renal dysfunction, particularly patients with serum creatinine levels > 3 mg/dl (265.2 μmol/l). Hence, the results of this meta-analysis and of the studies that have been incorporated in it should not be extended to patients with heart failure and cardiorenal syndrome.
In addition, to our knowledge, no study to date has shown any possible major adverse effects in the HSS group or has advised reducing its dosing or stopping its administration under certain circumstances.
Perhaps owing to the fact that the HSS concentration was adjusted according to the serum sodium level of the individual CHF patient, electrolyte problems were consistently avoided. Alternatively, this may depend on the close therapeutic monitoring of a clinical trial setting, while HSS safety may be less robust in the case of routine hospital wards not belonging to a tertiary care center.
Another aspect to be emphasized is that the majority of the nine studies used for building the five meta-analyses of our study are attributable to the same research group, which may, although not necessarily, create a bias since researchers usually tend to underscore the results and possible favorable prospects of their research work, if they believe to have the exclusive or prevalent merit for a given advancement of the knowledge or a given therapeutic progress.
Conclusion
In the era of the emergence of novel therapies for advanced CHF, the use of HSS as a therapeutic adjunct to i.v. loop diuretics still needs to be explored on a larger scale. Indeed, compared with inotropic agents, the supplementation of diuretics with HSS seems to be characterized by greater cost-effectiveness with a reduced side-effect profile. Furthermore, owing to its renoprotective effects, this measure may be able to prevent or retard the need for invasive procedures, such as isolated ultrafiltration, continuous veno-venous hemofiltration, or hemodialysis in advanced stages of CHF.
The use of HSS does not require a higher intensity of patient monitoring in a critical care setting, leaving this a feasible option for physicians and patients who are not in a tertiary care center.
In future, large RCTs are needed to assess the benefit of HSS in diverse patient populations, as well as using a patient population on optimal current HF treatment, i.e., comprising beta-blockers and aldosterone receptor antagonists, which had not yet been implemented in medical practice at the time that some of the trials on HSS were performed. Thus, further trials are warranted, especially in the setting of CHF complicated by marked renal dysfunction (serum creatinine of > 2.2 mg/dl). Furthermore, particular attention should be paid in order to plan a trial aimed at evaluating the impact of HSS on all-cause mortality and on the rate of ADHF-related re-hospitalizations, two endpoints that require further research to corroborate the assumption that they may be favorably influenced by the addition of HSS to the diuretics. Meanwhile, the role of small volumes of HSS added to i.v. loop diuretics as a renoprotective measure, able to prevent WRF, may now be deemed sufficiently documented in the clinical setting of CHF patients with seemingly normal renal function or with only mild–moderate renal insufficiency (serum creatinine of ≤ 2.2 mg/dl), who require i.v. diuretics for acute exacerbation of cardiac failure.
References
Liszkowski M, Nohria A (2010) Rubbing salt into wounds: hypertonic saline to assist with volume removal in heart failure. Curr Heart Fail Rep 7(3):134–139
Paterna S, Di Pasquale P, Parrinello G et al (2000) Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as a bolus, in refractory congestive heart failure. Eur J Heart Fail 2(3):305–313
Issa VS, Bacal F, Mangini S et al (2007) Hypertonic saline solution for renal failure prevention in patients with decompensated heart failure. Arq Bras Cardiol 89(4):251–255 (Article in English, Portuguese)
Licata G, Di Pasquale P, Parrinello G et al (2003) Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J 145(3):459–466
De Vecchis R, Ciccarelli A, Ariano C et al (2011) Renoprotective effect of small volumes of hypertonic saline solution in chronic heart failure patients with marked fluid retention: results of a case-control study. Herz 36(1):12–17
Butler J, Forman DE, Abraham WT et al (2004) Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 147(2):331–338
Cioffi G, Tarantini L, Pulignano G et al (2007) Prevalence, predictors and prognostic value of acute impairment in renal function during intensive unloading therapy in a community population hospitalized for decompensated heart failure. J Cardiovasc Med (Hagerstown) 8(6):419–427
Brandimarte F, Mureddu GF, Boccanelli A et al (2010) Diuretic therapy in heart failure: current controversies and new approaches for fluid removal. J Cardiovasc Med (Hagerstown) 11(8):563–570
De Vecchis R, Ciccarelli A, Pucciarelli A (2010) Unloading therapy by intravenous diuretic in chronic heart failure: a double-edged weapon? J Cardiovasc Med (Hagerstown) 11(8):571–574
Wittner M, Di Stefano A, Wangemann P, Greger R (1991) How do loop diuretics act? Drugs 41(Suppl 3):1–13
Penfield WG (1919) The treatment of severe and progressive hemorrhage by intravenous injections. Am J Physiol 48:121–128
Crystal GJ, Gurevicius J, Kim SJ et al (1994) Effects of hypertonic saline solutions in the coronary circulation. Circ Shock 42:27–38
Maningas P (1987) Resuscitation with 7.5 % NaCl in 6 % dextran-70 during hemorrhagic shock in swine: effects on organ blood flow. Crit Care Med 15:1121–1126
Rocha-e-Silva M, Negraes GA, Soares AM et al (1986) Hypertonic resuscitation from severe hemorrhagic shock: patterns of regional circulation. Circ Shock 19:165–175
Mazzoni MC, Borgström P, Arfors KE, Intaglietta M (1988) Dynamic fluid redistribution in hyperosmotic resuscitation of hypovolemic hemorrhage. Am J Physiol 255(3 Pt 2):H629–H637
Maningas PA, Mattox KL, Pepe PE et al (1989) Hypertonic saline-dextran solutions for the prehospital management of traumatic hypotension. Am J Surg 157:528–534
Armistead CW, Vincent JL, Preiser JC et al (1989) Hypertonic saline solution-hetastarch for fluid resuscitation in experimental septic shock. Anesth Analg 69:714–720
Ing RD, Nazeeri MN, Zeldes S et al (1994) Hypertonic saline/dextran improves septic myocardial performance. Am Surg 60:507–508
Kien ND, Kramer GC (1989) Cardiac performance following hypertonic saline. Braz J Med Biol Res 22:2245–2248
Kazory A, Ross EA (2008) Contemporary trends in the pharmacological and extracorporeal management of heart failure: a nephrologic perspective. Circulation 117(7):975–983
Detsky AS, Naylor CD, O’Rourke K et al (1992) Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol 45(3):255–265
Paterna S, Di Pasquale P, Parrinello G et al (2005) Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high-dose furosemide and hypertonic saline solution versus high-dose furosemide alone in refractory congestive heart failure: a double-blind study. J Am Coll Cardiol 45(12):1997–2003
Paterna S, Fasullo S, Di Pasquale P (2005) High-dose torasemide is equivalent to high-dose furosemide with hypertonic saline in the treatment of refractory congestive heart failure. Clin Drug Investig 25(3):165–173
Paterna S, Fasullo S, Parrinello G et al (2011) Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study). Am J Med Sci 342(1):27–37
Issa VS, Andrade L, Ayub-Ferreira SM et al (2013) Hypertonic saline solution for prevention of renal dysfunction in patients with decompensated heart failure. Int J Cardiol 167(1):34–40
Engelmeier RS, Le TT, Kamalay SE et al (2012) Randomized trial of high dose furosemide-hypertonic saline in acute decompensated heart failure with advanced renal disease. JACC 59(13):E958
Parrinello G, Di Pasquale P, Torres D et al (2012) Troponin I release after intravenous treatment with high furosemide doses plus hypertonic saline solution in decompensated heart failure trial (Tra-HSS-Fur). Am Heart J 164(3):351–357
Parrinello G, Paterna S, Di Pasquale P et al (2011) Changes in estimating echocardiography pulmonary capillary wedge pressure after hypersaline plus furosemide pert furosemide alone in decompensated heart failure. J Card Fail 17(4):331–339
Tuttolomondo A, Pinto A, Di Raimondo D et al (2011) Changes in natriuretic peptide and cytokine plasma levels in patients with heart failure, after treatment with high dose of furosemide plus hypertonic saline solution (HSS) and after a saline loading. Nutr Metab Cardiovasc Dis 21(5):372–379
Redfors B, Swärd K, Sellgren J, Ricksten SE (2009) Effects of mannitol alone and mannitol plus furosemide on renal oxygen consumption, blood flow and glomerular filtration after cardiac surgery. Intensive Care Med 35(1):115–122
Filippatos GS, Desai RV, Ahmed MI et al (2011) Hypoalbuminaemia and incident heart failure in older adults. Eur J Heart Fail 13(10):1078–1086
Clarke MM, Dorsch MP, Kim S et al (2013) Baseline albumin is associated with worsening renal function in patients with acute decompensated heart failure receiving continuous infusion loop diuretics. Pharmacotherapy 33(6):583–588
Drazner MH, Palmer BF (2003) Hypertonic saline: a novel therapy for advanced heart failure? Am Heart J 145(3):377–379
Schrier RW (1990) Effects of furosemide treatment in patients with congestive heart failure. In: Lasix 25th Anniversary Symposium. Proceedings of the International Symposium, pp 83–94, Frankfurt am Main 1990. Media Medica, Frankfurt am Main
Tucker BJ, Blantz RC (1984) Effect of furosemide administration on glomerular and tubular dynamics in the rat. Kidney Int 26:112–121
He XR, Greenberg SG, Briggs JP, Schnermann J (1995) Effects of furosemide and verapamil on the NaCl dependency of macula densa-mediated renin secretion. Hypertension 26:137–142
Compliance with ethical guidelines
Conflict of interest. R. De Vecchis, C. Esposito, C. Ariano, and S. Cantatrione declare that there are no conflicts of interest. The accompanying manuscript does not include studies on humans or animals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Additional material onlineThis article includes additional material online. You will find this supplemental at dx.doi.org/10.1007/s00059-013-4041-6.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
De Vecchis, R., Esposito, C., Ariano, C. et al. Hypertonic saline plus i.v. furosemide improve renal safety profile and clinical outcomes in acute decompensated heart failure. Herz 40, 423–435 (2015). https://doi.org/10.1007/s00059-013-4041-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-013-4041-6