Abstract
Paysandisia archon was accidentally introduced into Europe where it damages endemic and ornamental palms, also threatening the date palm from North Africa. Little was known about sex pheromones in the Castniidae day-flying moth before a recent paper that concluded on the absence of female sex pheromone in P. archon. A putative identification of a short-range male pheromone, present on fore- and hindwings, was reported. In this paper, we describe the original structure of the male androconia located on the tarsi of the mid-legs. The extracts of mid-legs were analysed by GC/MS and the chemical structure of the male androconia component was identified as E2,Z13-18:OH. After extraction in solvent, biological activity of the extracts was assessed by EAG. The chemistry and the morphology of the androconia reinforce the current classification of the Castniidae in the Cossoid/Sesioid assemblage and provide new information on the chemical ecology in day-flying Lepidoptera and suit the recent paper describing the courtship behaviour
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paysandisia archon (Burmeister 1879) was introduced into southern Europe with new palm species originating from South America, probably from Argentina or Uruguay, where damage by this insect has never been reported. In Europe, the population spread rapidly and destroyed ornamental and patrimonial palms. The spread of this alien insect is threatening the date palms in North Africa and thus an important human food resource. The larvae develop as a stem borer and are not controlled by insecticide. Females lay eggs on the palm crown and the young larvae, after feeding on the young palm, bore into the apex of the palm stem. In this context of alien insect invasion, it was interesting to decipher the mating behaviour (Delle-Vedove et al. 2012, 2013) and identify the putative sex pheromone in P. archon to provide new tool to survey population expansion and/or monitor the insect.
In the Castniidae family, male moths have long been known to exhibit diverse and often spectacular androconia (Le Cerf 1936). Most of them are located on the abdomen and hindlegs. Jordan (1923) described in Gazera heliconioides Herrich-Schäffer, under the name of Castnia linus Cramer in the original article, male androconia on paronychia of the mid-tarsus and mentioned similar structures on the tarsi of Haemonides cronida Herrich-Schäffer, Geyeria hubneri Latreille and Frostetola gramivora Schaus, which are all species belonging to the subfamily Castniinae, as does P. archon (Lamas 1995). Such androconia were found and studied in P. archon and in this paper we present a description and the identification the chemical content.
Little is known about the Castniidae moth sex pheromone, with one species investigated (Rebouças et al. 1999), leading to the identification of hydrocarbons and unsaturated fatty acids from female ovipositor extracts. A recent paper also dealing with sex pheromone in P. archon was published while this paper was being drafted. The authors identified a putative sex pheromone from male wing extracts. No female sex pheromone was identified (Sarto i Monteys et al. 2012). Observations of the courtship behaviour evidence that males scratch the substratum with the mid-legs, inducing take off and hovering flight in the female (Delle-Vedove et al. 2013). The results we present in this paper demonstrate that the male androconia produce large amounts of a compound already known as a Cossidae female pheromone component. The identification of pheromones is of course of interest for insect monitoring when they can be used as long-range attractants, but it also contributes to the understanding of insect phylogeny and taxonomy. The question is even more interesting as the taxonomic position of the Castniidae has changed over time. After being included within the Cossoidea superfamily in the past, Castniid moths are now filiated with the Sesiidae and classified as a family in the Sesioidea superfamily.
Identification of the component from P. archon male androconia is reported in this paper and discussed in accordance with taxonomic considerations and with the recent papers.
Methods
Insects
The insects used in the experiments were collected as last instar larvae or pupae in infested palms cut down in the Montpellier area between January and July from 2007 to 2010. After collection, the larvae were placed in individual plastic boxes with palm tissue and kept in a glasshouse under natural conditions (temperature ranging from 10 to 25 °C) until pupation. Pupae obtained from April to August were kept in the laboratory under a natural photoperiod at 25 °C. Each day, emerging moths were sexed and kept separately in a fine-mesh cage (30.5 cm; http://www.livemonarch.com) under laboratory conditions.
Adult moth extracts and pheromone collection
The male forewings, mid-legs and genitalia of dissected males were extracted in hexane. The extractions were carried out between 2 and 3 h after emergence as this period was previously determined as the period of sexual activity under our experimental conditions (Beaudoin-Ollivier and Frérot 2006; Delle-Vedove et al. 2012). Some of the extracts were pooled and condensed under a nitrogen flow before analysis when no candidate pheromone component was detected, i.e. 5 male wings or genitalia in 100 or 10 μL.
Chemical analysis
Gas chromatography (GC) analyses were conducted using a Varian 3400 CX with a split spitless injector, a FID detector heated to 260 °C fitted with an Rtx®-Wax column, 30 m × 0.32 mm i.d. (Restek®, France). The oven temperature was ramped from 50 to 100 °C at 15 °C/min, then to 245 °C at 5 °C/min. Helium was the carrier gas (15 psi pressure). GC and mass spectrometer (GC/MS) analyses were carried out on a Saturn QISMS ion trap coupled to a 3400 CX GC, in electron impact mode (EI), 70 eV, 40–400 amu. GC conditions were as follows: the injector was set to 250 °C, the oven temperature was ramped from 50 to 300 °C at 8 °C/min, kept for 19 min at 300 °C, equipped with an Rxi-5 Sil-MS (Restek®, France), 30 m × 0.32 mm i.d.
Compounds were identified according to their mass spectra and retention indexes (RI). The RI were computed on AMDIS 32 using n-alkanes from C10 to C24, eluted under the same conditions as the samples on a non-polar column. All compound spectra and RI were compared with the RI and spectra of the laboratory libraries using AMDIS32 software.
Assertion of the chemical structure was thereafter achieved for the male pheromone through proton NMR spectroscopy in d6 acetone (Varian 300 MHz Mercury+) as the quantity collected, about 500 μg, allowed such assessment by NMR analyses.
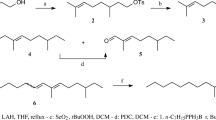
Chemical synthesis of (2E,13Z)-octadecadien-1-ol (E2,Z13-18:OH)
A solution of (2E,13Z)-octadien-1-yl acetate (846 mg, 3 mmol) in THF (5 mL) was added to a solution of NaOH (600 mg, 15 mmol) in MeOH (10 mL). The reaction mixture was stirred for 2 h at 40 °C. It was then concentrated under reduce pressure, diluted with water, and extracted with hexane. The organic layer was washed with water (5 mL) and aq. NaCl sat. (5 mL). It was dried over magnesium sulfate and filtered. Evaporation under reduce pressure afforded the expected product, (2E,13Z)-octadien-1-ol, as colourless oil (708 mg, 100 %). IR spectra were recorded on a PerkinElmer Spectrum 100 FT-IR spectrometer, in “ATR mode”. NMR spectra were recorded on Bruker AC 300 spectrometer [300.13 MHz (1H) and 75.47 MHz (13C)] using tetramethylsilane as the internal standard and CDCl3 as the solvent. Chemical shifts are reported in δ ppm values in Hertz.
IR ν max (cm−1) 3,570–3,110 (OH), 1610 (C=C).
1 H NMR δ H: 0.88–0.93 (3H, m); 1.29–1.41 (20H, m); 2.02–2.08 (6H, m); 4.09 (2H, d, 3 J H1-H2 = 6 Hz; 5.34-5.38 (2H, m); 5.60-5.73 (2H, m).
13 C NMR δ C: 13.98; 22.34; 26.91; 27.19; 29.14; 29.19; 29.29; 29.49; 29.53 (2C); 29.76; 31.96; 32.21; 63.78; 128.81; 129.85 (2C); 133.51.
This detailed NMR study prompted us to develop an efficient synthesis of (2E,13Z)-octadien-1-ol. Commercially available (2E, 13Z)-octadien-1-yl acetate served as the starting material. The acetate was diluted in THF and treated with sodium hydroxide in aqueous methanol at 40 °C. Saponification was completed after 2 h of stirring, as deduced from the IR data of the crude product and the absence of the absorption band at 1,740 cm−1 for the C=O bond of the acetate group. The purity of the crude product was confirmed by GC/MS analysis and NMR experiments. Assignments have been made using 1H and 13C 1D experiments, 1H–1H 2D COSY, 1H–13C HSQC and HMBC experiments, and spin decoupling. 1H NMR clearly showed the presence of a doublet at 4.09 ppm assigned to H-1 coupled with the vinyl proton H-2. 1H–1H 2D COSY and HMBC experiments allowed the assignments of H-3 at the same chemical shifts of H-2. The identification of H-13 and H-14 between 5.34 and 5.38 could be easily confirmed from the 1-H NMR and HSQC data. The small difference of the chemical shifts of the H-1–H-2 and H-13–H-14 vinylic systems has constituted a real difficulty to establish the configuration of the double bond C=C. Therefore, spin decoupling experiments were performed to remove the signal splitting and to simplify the vinylic systems. The irradiation of the signals at 2.04 ppm corresponding to H-4, H-12 and H-15, allowed the determination of the 3 J H-13–H-14 coupling constant. The value of 12 Hz indicated that the H-13 proton was placed in the cis position of H-14. The irradiation of the H-1 signal at 4.09 ppm allowed the determination of the 3 J H-2–H-3 coupling constant. The value of 17 Hz was in agreement with the 2-E configuration.
Synthetic compounds
The synthetic compounds belonged to the INRA, PISC laboratory collection; isomeric purity was about 90 %.
Electroantennography
The EAG technique was used to assess the biological activity of crude extracts of male tarsus. Electrical depolarisations were recorded with a Syntech EAG Device (Hilversum, Netherlands). The proximal and tip parts of the antenna were cut and connected to the glass electrodes filled with a KCl solution. Electrical contact between the preparation and the amplifier was made with AgCl-coated silver electrodes. Charcoal-filtered and humidified air passed continuously at 15 mL/s over the preparation through a metal tube outlet 5 mm away from the antennae. The stimulation was deposited on a filter paper and placed in a Pasteur pipette. At the beginning of each series of stimulations, air and filter paper with 10 μL of hexane were applied as control stimulations. Before any stimulation, the filter papers were left 10 min to allow solvent evaporation and renewed each hour. The pulse flow lasted 0.5 s and was delivered at 13 mL/s.
For male tarsus pheromone extracts quantification was possible and carried out by GC according to the calibration curves of the dienic compound (8E,10E)-dodecadien-1-ol. We applied a dose of around 5 μg (10 μL of crude extract). Each dose of male extracts was tested on 12 female and 15 male antennae, respectively. Synthetic compounds, E2,Z13-18:OH and E2,Z13-18:Ac, were tested at a dose of 5 μg (n = 12 males and 14 females).
Statistics
Mean EAG responses to crude extracts or synthetic stimulations were compared with the corresponding hexane response, for males and females, respectively, using a Wilcoxon test for paired samples. Statistical tests were performed using R 2.12.0 (R Development Core Team 2010).
Results
Description of the male androconia
In P. archon, the mid-legs of males are modified. The first segment of the tarsus is larger and broader than in females (Fig. 1a), and the last segment of the tarsus bears distally a pair of brush-like structures, just below the claws (Fig. 1b), which correspond to the paronychia (Séguy 1967; Gordh and Headrick 2001). These hairbrush organs of males are assumed to release sex pheromones into the air, as previously described in almost all families and body parts of Lepidoptera. Authors agree on the generic term androconia (Birch et al. 1990).
Male pheromone component identification
The mid-leg extracts showed a huge quantity (μg) of a component at the retention time of an octadecadienol. The mass spectrum showed the following ions: 41, 55, 81, 95, 109, 248 (M+ −18) characteristic of a long-chain dienic compound. On an apolar column and on a polar column, the retention times matched that of E2,Z13-18:OH. As this compound was the only one available among the four 2,13 and four 3,13-octadecadienols, and as the quantity of extract was enough, we used NMR for identification purposes.
The NMR spectrum exhibited three characteristic multiplets at 4.10, 5.35 and 5.6 ppm, each integrating for 2 protons, in agreement with the presence of two unsaturations and a primary alcohol. It was deduced from the coupling pattern of these three multiplets that one of the unsaturations was at the C-2 position, but the stereochemistry of the double bonds remained unclear. A comparison with NMR data obtained under the same conditions from a synthetic sample of E2,Z13-18:OH unambiguously elucidated the chemical structure.
GC/MS reconstruction of the ion currents of diagnostic ions for putative long-chain alcohols or acetates, known to be pheromone components, did not reveal any other components in the mid-leg extracts.
Electroantennography
The overall intensities of EAG responses were weak in both male and female antennae, at less than 0.7 mV under our experimental conditions (CEFE Montpellier and PISC Versailles), but responses were always significantly different from the air control (Wilcoxon rank test, p < 0.01) and from the respective hexane control (Fig. 2 Male and female EAG responses to crude extracts of male mid-legs and to synthetic compounds are reported in Fig. 2 and compared to the hexane control response.
a Mean EAG responses (µV) of male (grey) and female (white) antennae to crude extracts of male mid-legs. b Mean EAG responses (µV) of male (grey) and female (white) antennae to synthetic compounds. Asterisks represent significant differences in responses from the corresponding control (hexane) for males or females, according to Wilcoxon test (*p < 0.05; **p < 0.01; ***p < 0.001). Extr. crude extract
Male mid-leg extract elicited significant responses on both male and female antennae, as well as the identified E2,Z13-18:OH, male pheromone component.
Discussion
Moths of the Lepidoptera belonging to the Castniidae family are known to exhibit diverse and often spectacular androconia (Le Cerf 1936) and we found that P. archon also bears such a structure with hairbrushes and claws characterizing paronychia. As shown in mid-leg extracts, the paronychia of P. archon contain a large quantity of a single pheromone component and the enlarged first article of the mid-tarsus is probably the site of male sex pheromone emission, but further investigations are needed to locate the site of pheromone biosynthesis, which may be different from the emission site in male moths (Birch et al. 1990). The male pheromone might be produced either in the paronychia, as seems to be a common feature in all Castniidae males (Jordan 1923), or in a remote part of the leg or body. Jordan 1923, postulated that the large tarsi contain strong muscles that are used to grasp the female firmly during mating. This behaviour was not observed in P. archon (Delle-Vedove et al. 2013). Only histological examination would answer that question.
The existence of androconia in P. archon shown in this study reinforces the already proposed uniformity of the Castniidae family. The chemical structure of the identified component in male mid-leg extract was surprisingly chemically similar to that of female moth sex pheromone included in the Cossoidea superfamily, and in several species of clearwing moths classed in the Sesioidea, Sesidae. Most of the known components identified from male moth androconia originate from food intake and are related either to amino acids or specific chemicals from plants (Blum 1987). A chemical analogy between male and female sex pheromone has rarely been described, but was found recently in another moth (Lassance and Löfstedt 2009).
Our results corroborate the monophyly of the Cossoidea and Sesioidea demonstrated by Minet (1991). To our knowledge, the components 2,13- and 3,13-octadecadienols have only been identified in species classed in these superfamilies (see Pherobase for details), confirming what has been called the Cossoid/Sesioid assemblage (Edwards et al. 1999).
EAG demonstrated that the scent released from the male androconia was perceived by the conspecific antennae. The intensity of the EAG responses was weak, as already mentioned by Sarto i Monteys et al. (2012). This point and the finding of E2,Z13-18:OH in male extract were the only similar results, although the extract in the Sarto i Monteys et al. (2012) paper was wing extract. The presence of this compound on wings could originate from the adsorption on the cuticle of the huge amount of pheromone component deposited by males during pre-courtship behaviour. In the first behavioural study on mating behaviour (Delle-Vedove et al. 2013), the male did appear to be the primary attractive sex and first released the androconia pheromone by scratching the substratum with the mid-legs. This behavioural event also contradicts the hypothesis put forward by Sarto i Monteys et al. (2012) of a release during flight.
GC/MS analyses did not reveal any other component from male androconia, even from wing extracts, and in particular no farnesal-like compounds. However, our findings showed that day-flying Lepidoptera possess pheromone-like secretions, as already found in other species (Boppré 1978; Zagatti and Renou 1984; Lassance and Löfstedt 2009; Nieberding et al. 2008 and literature cited therein). Further behavioural studies with synthetic chemicals will be undertaken to further investigate the phenomenon of long and short-range attraction in this day-flying Lepidoptera.
References
Beaudoin-Ollivier L, Frérot B (2006) Paysandisia archon: vous avez dit phéromone? Phytoma 594:30–32
Birch MC, Poppy GM, Baker TC (1990) Scents and eversible scent structures of male moths. Ann Rev Entomol 35:25–58
Blum MS (1987) Biosynthesis of arthropod exocrine compounds. Annu Rev Entomol 32:381–413
Boppré M (1978) Chemical communication, plant relationships, and mimicry in the evolution of Danaid butterflies. Entomol Exp Appl 24:264–277
Delle-Vedove R, Beaudoin-Ollivier L, Hossaert-McKey M, Frérot B (2012) Reproductive biology of the palm borer, Paysandisia archon (Lepidoptera: Castniidae). Eur. J. Entomol. 109:289–292
Delle-Vedove R, Frérot B, Hossaert-McKey M, Beaudoin-Ollivier L (2013) Courtship behavior of the Castniid palm borer Paysandisia archon: potential roles of male scents and visual cues in a day-flying moth. J Insect Sci (in press)
Edwards ED, Gentili P, Horak M, Kristensen NP, Nielsen ES (1999) The cossoid/sesioid assemblage, chap 11, pp 181–195. In: Kristensen NP (ed) Lepidoptera. (Encyclopedia of entomology J. Capinera; B. Heppner)
Gordh G, Headrick DH (2001) A dictionary of entomology. Cabi, Wallingford
Jordan K (1923) On the scent organs in the males of certain American Castniidae. Novit Zool 30:159–162
Lamas G (1995) A critical review of J. Y. Miller’s Checklist of the Neotropical Castniidae (Lepidoptera). Rev Peru Entomol 37:73–87
Lassance JM, Löfstedt C (2009) Concerted evolution of male and female display traits in the European corn borer, Ostrinia nubilalis. BMC Biol 7:10
Le Cerf F (1936) Un type remarquable d’androconie chez certaines Gazera Boisduval (Lep. Castniidae). Bul Soc Entomol Fr 41:191–195
Minet J (1991) Tentative reconstruction of the ditrysian phylogeny (Lepidoptera/Glossata). Entomol Scand 22:69–95
Nieberding CM, de Voss H, Schneider MV, Lassance JM, Estramil N, Andersson J, Bång J, Hedenström E, Löfstedt C, Brakefield PM (2008) The male sex pheromone of the butterfly Bicyclus anynana: towards an evolutionary analysis. PLoS ONE 3(e2751):1–12 (Public Library of Science)
Rebouças LMC, Do Caraciolo MSB, Sant’Ana AEG, Pickett JA, Wadhams LJ, Pow EM (1999) Composição química da glândula abdominal da fêmea da mariposa Castnia licus (Drury) (Lepidoptera:Castniidae): possíveis feromônios e precursores. Quím Nova 22:645–648
Sarto i Monteys V, Acin P, Rosell G, Quero C, Jiménez MA, Guerrero A (2012) Moths behaving like butterflies. Evolutionary loss of long range attractant pheromones in Castniid moths: a Paysandisia archon model PLoS ONE 7(1):1–11
Séguy E (1967) Dictionnaire des termes techniques d’entomologie élémentaire. Lechevalier, Paris
Zagatti P, Renou M (1984) Sex pheromones of Zygaenid moths. Mating behavior of the 6-spotted burnet moth Zygaena filipendulae (Lepidoptera Zygenidae). Ann Soc Entomol Fr 20:439–454
Acknowledgments
The project was funded by the “Région Languedoc-Roussillon and CIRAD” grant. We would like to thank the arborist, city of Montpellier, for providing infested palms.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frérot, B., Delle-Vedove, R., Beaudoin-Ollivier, L. et al. Fragrant legs in Paysandisia archon males (Lepidoptera, Castniidae). Chemoecology 23, 137–142 (2013). https://doi.org/10.1007/s00049-013-0128-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-013-0128-z