Abstract
The edible pseudo bulbs of Malaxis acuminata D. Don are used as a constituent of an Indian drug ‘Astavarga’ and in several marketed Ayurvedic formulations. The aim of the present work was to investigate the in vitro antiproliferative activity of the ethanolic extract and its fractions of the pseudo bulbs of Malaxis acuminata and to develop the HPLC-ESI-QTOF-MS/MS method for rapid de-replication of the phytoconstituents present in the bioactive fraction. The antiproliferative activity was evaluated against four human cancer cell lines, such as A549 (non-small cell lung cancer cells), DU145 (human prostate carcinoma), DLD1 (human colorectal adenocarcinoma), and MCF-7 (human breast adenocarcinoma) using the sulphorhodamine B assay. Eleven compounds including two flavonoids, three bisphenanthrene compounds, three stillbenoid compounds, two phenanthrene derivatives, and one prenylated benzoic acid were identified and characterized by HPLC-ESI-QTOF-MS/MS analysis along with the isolation of three steroidal compounds by column chromatography. The present investigation indicated that pseudo bulbs of Malaxis acuminata possessed a potent antiproliferative activity. LC–MS/MS analysis provided rapid dereplication of compounds, which might be responsible for its antiproliferative activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaxis acuminata D. Don, a synonym for Microstylis wallichii Lindl. (Orchidaceae) is an endangered, terrestrial orchid of tropical Himalaya, distributed in the pine forests at an altitude of 1800–2300 m (Chinmay et al. 2011). M. acuminata is a perennial medium-sized orchid up to 30 cm in height with pseudo bulbs at the base and fibrous roots. The leaves are simple, 3–4 in number, alternate, ovate-lanceolate, membranous, 5–15-cm long, and acute apex with a sheathing leaf base. The flowers are terminal racemes, yellowish green in color with a purple tinge and 3 mm in diameter (Cheruvathur et al. 2010; Chinmay et al. 2011; Uma et al. 2015). M. acuminata is reported as Rasayana and is an important constituent of an Ayurvedic formulation ‘Ashtavarga’ (group of eight medicinal plants) used in the preparation of ‘Chyavanprash’, which is a rejuvenating agent (Govindarajan et al. 2007). The plant is used to treat tuberculosis and is also used as an aphrodisiac (Sharma et al. 2014). The bulbs of this plant are well known for its medicinal value in the Indian System of Medicine, traded as Jeevak and are used as edible bulbs in the north-eastern region of India (Sharma et al. 2014; Rai et al. 2001). Fresh bulbs are used to treat bleeding diathesis, burning sensation, fever, and phthisis (lung diseases) and are externally used as a paste in the treatment of insect bites and rheumatism along with other plants (Cheruvathur et al. 2010; Chinmay et al. 2011). The ethanolic extract of pseudo bulbs is reported for in vivo analgesic and anti-inflammatory activity (Chinmay et al. 2011). In the local area, the pseudo bulbs are used in bronchitis and as a tonic. Similarly, it is used as an ingredient in several formulations such as chavanprash, Bramha Rasayana, and Haritak-Kyadiyoga (Govindarajan et al. 2007; Narayana et al. 2016). The decoction of pseudo bulbs of M. acuminata is used to cure fever, burning sensation, bleeding diathesis, phthisis, cooling, febrifuge, and spermopiotic by the people of Nagaland, India (Rajurkar and Gaikwad 2014; Uma et al. 2015).
The medicinal properties of the family Orchidaceae are due to the presence of different classes of compounds, including alkaloids, flavanoids, stilbenoids, phenanthrenes, terpenoids, bibenzyl derivatives, and so on (Cheruvathur et al. 2010; Rajurkar and Gaikwad 2014; Teoh 2016). The genus Malaxis is known for its nutritive values and is used in the form of a tonic, lactagouage, and rejuvenating drugs which are reported to treat insect bites, rheumatism, burning sensation, fever, general body weakness, and phthisis (Szlachetko and Kolanowska 2014; Hossain 2011). Malaxis species are reported to contain alkaloids, glycosides, and flavonoids (Teoh 2016). The gas chromatography–mass spectrometry analysis of pseudo bulbs of M. acuminata has been reported to possess long-chain saturated and unsaturated fatty acids (Rajurkar and Gaikwad 2014). The chemical analysis of pseudo bulbs of M. acuminata showed to possess a variety of metals, fatty acids, terpenoids and vitamins, α-tocopherol, and γ-tocopherol (Rajurkar and Gaikwad 2014; Chinmay et al. 2011; Teoh 2016).
Literature survey revealed that the phytochemistry of M. acuminata is still unexplored, whereas its formulations are marketed and are having commercial, as well as health importance. Therefore, the development of a selective and efficient analytical method for rapid dereplication of the phytoconstituents of M. acuminata is required. High-performance liquid chromatography/electrospray ionization tandem mass spectrometry (HPLC-ESI-QTOF-MS/MS) is one of the most selective and efficient technique for dereplication and identification of minor constituents (Rubert et al. 2015; Seger and Griesmacher 2007). The present investigation on the pseudo bulbs of M. acuminata showed to possess an antiproliferative activity in ethyl acetate fraction (MAETOAC) which prompted for the dereplication study of the phytoconstituents present in the bioactive fraction using HPLC-ESI-QTOF-MS/MS. To the best of our knowledge and according to literature survey, this is the first report of the fractionation and bioguided identification of compounds by HPLC-ESI-QTOF-MS in the genus Malaxis.
Materials and methods
Reagents and materials
Methanol (LC–MS grade) and formic acid (analytical grade) were purchased from Sigma-Aldrich (St. Louis, USA) and ultrapure water was produced by a Milli-Q system (Millipore, Milford, MA, USA). The pseudo bulbs of M. acuminata were purchased from a plant supplier, Dehradun, India in October 2013 and were identified by Dr. K. R. Arya, Botany Division at CDRI-CSIR, Lucknow and a voucher specimen number (M. acuminate-4781) has been deposited in the medicinal plant herbarium of the same institute.
Extraction and fractionation
The dried and powdered bulbs (4.8 kg) of M. acuminata were extracted with ethanol by percolation. The concentrated ethanol extract (MAETOH; 130.0 g) was treated with 5% HCl solution to obtain pH values of 2–3 and filtered through a filter paper. The precipitate (acid-insoluble part) was removed and the filtrate (acid-soluble part) was successively partitioned by hexane (12.0 mg) and ethyl acetate (MAEPH3; 4.0 g). The aqueous part (pH 2–3) was basified by ammonia (pH 10) and extracted sequentially to get EtOAc (35.0 mg) and n-BuOH (MABPH10, 1.3 g) soluble fractions and the remaining water part was concentrated to yield a water fraction (MAWPH10; 23.0 g). The acid-insoluble precipitate was dissolved in water–methanol (3:1) and partitioned to give hexane (MAHEX, 10.0 g), EtOAc (MAETOAC, 21.0 g), and n-BuOH (MABUOH, 7.0 g) fractions. The ethanol extract of M. acuminata pseudo bulbs and its fractions with appreciable yields were screened for in vitro antiproliferative activity using the sulphorhodamine B (SRB assay. The extract and its fractions were coded as ethanol extract, MAETOH; ethyl acetate fraction at pH-3, MAEPH-3; butanol fraction at pH-10, MABPH-10; and water fraction at pH-10, MAWPH-10; acid-insoluble hexane, ethyl acetate, and n-butanol fractions were coded as MAHEX, MAETOAC, and MABUOH, respectively.
Cell culture
Human cancer cell lines such as A549 (non-small cell lung cancer cells), DU145 (human prostate carcinoma), DLD1 (human colorectal adenocarcinoma), and MCF-7 (human breast adenocarcinoma) were procured from American Type Culture Collection, (Manassas, VA, USA). The cells were maintained in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% antibiotic–antimycotic solution (Life Technology) at 37 °C in 5% CO2.
Cytotoxicity assay
The cytotoxic effect of crude extracts and fractions on different cancer cells was assessed by Sulphorhodamine B dye-based plate assay. The cells (10,000/well in a 96-well plate) were grown overnight at 37 °C in 5% CO2 and incubated with test samples (100 µg/ml) for an additional 48 h. Untreated cells served as controls. After 48 h, the cells were fixed and stained with the SRB dye, as described earlier (Adaramoye et al. 2011) and the plates were read at 540 nm on a plate reader. The cytotoxic effect of the compound was calculated as % inhibition in cell growth as per the formula: [1−(Absorbance of drug-treated cells/Absorbance of untreated cells) × 100].
HPLC analysis
Analyses were carried out using an Agilent 1200 HPLC system (Agilent technologies, USA) interfaced with Agilent 6520 hybrid quadrupole time of a flight mass spectrometer (Agilent technologies, USA) equipped with an electrospray ion source. Agilent 1200 HPLC system was equipped with a quaternary pump (G1311A), an online vacuum degasser (G1322A), an autosampler (G1329A), and a diode-array detector (G1315D) (Kumar et al. 2015). The separation of the compounds from the EtOAc fraction (MAETOAC) of M. acuminata was carried out on a Supelco, C18 column (10 cm × 2.1 mm, 2.7 μm) operated at 25 °C. Analysis was done with a gradient elution program of 0.1% formic acid in water (A) and methanol (B) as a mobile phase at a flow rate of 0.3 mL/min. The following 50 min gradient system was applied: 0–10 min, 40% B; 10–40 min, 40–90% B; 40–42 min, 90–90% B; and 42–50 min, 90–40% B. The sample injection volume was 3 μL. UV detection was performed by scanning the samples at 190–400 nm.
Mass-spectrometric analysis
A mass spectrometer was operated in a negative electrospray ionization (ESI) mode and the spectra were recorded by scanning the mass range of m/z 50–1500 in both MS and MS/MS analyses. In QTOF-MS, nitrogen was used for drying, nebulizing, and collision gas with a drying gas flow rate of 12 L/min. The heated capillary temperature was set to 350 °C and the nebulizer was set to 40 psi. In scan-source parameters, VCap, fragmentor, skimmer, and octapole RF peak voltages were set to 3500, 175, 65, and 750 V, respectively. For the MS/MS analysis, collision energies were used at 25, 30, and 40 eV. The accurate mass data of the molecular ions were processed through the software Mass Hunter Workstation (version B 04.00), which provided a list of possible elemental formulas that are used to generate a molecular formula from the peak (Kumar et al. 2015).
Results and discussion
Fractions and antiproliferative activity
The ethanol extract and its fractions were examined for antiproliferative activity by the SRB assay against four human cancer cell lines such as A549, DU145, DLD1, and MCF-7. The ethanol extract and n-BuOH (MABUOH) fraction showed a moderate antiproliferative activity, whereas acid-soluble fractions were found to be inactive. The EtOAc fraction (MAETOAC) showed a potent antiproliferative activity in comparison with standard doxorubicin against cancer cell lines, such as A549 (70.29 ± 7.22), DLD1 (73.12 ± 8.58), MCF-7 (79.10 ± 9.62), and DU145 (68.65 ± 8.10) (Table 1).
Identification of compounds
The bioactive ethyl acetate fraction (MAETOAC, 1.5 g) that was purified over Si gel column chromatography afforded three compounds such as β-sitosterol, stigmasterol, and stigmasterol-3-O-glucoside which were identified by Nuclear magnetic resonance (NMR), MS, and co-thin layer chromatography with authentic samples available in our laboratory. The base peak chromatogram of the antiproliferative active MAETOAC fraction of M. acuminata is depicted in Fig. 1 in which the peaks are numbered according to their elution order. Eleven compounds were identified and characterized based on their accurate mass, fragmentation behavior, and/or literature data. The MS data of the identified compounds, including observed and calculated mass, molecular formula, ppm error, and MS/MS data are given in Table 2. The HPLC-ESI-QTOF-MS analysis showed that flavonoids, bisphenanthrene, stillbenoids, phenanthrene derivatives, and prenylated benzoic acid were present in the bioactive MAETOAC fraction of M. acuminata.
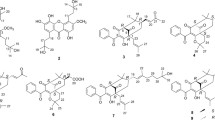
Peaks 1 and 2 (m/z 477.1038 [M – H]−) were identified as isomers of isorhamnetin glycosides eluting at two different values of t R 27.518 and 27.792 min (Schieber et al. 2002). The MS/MS fragmentation pattern of these two flavonoid isomers was found to be similar to that of isorhamnetin O-glycoside identified earlier from the extract of apple fruit (Schieber et al. 2002) and Ginkgo biloba (Tang et al. 2001). Both the compounds yielded common fragments at m/z 314 due to the loss of a sugar moiety, and a fragment ion at m/z 285 was obtained due to the loss of CHO and a fragment ion at m/z 271 corresponding to the loss of CO followed by CH3 from aglycone isorhamnetin at m/z 314, respectively. The peak at m/z 299 was obtained due to the loss of a methyl radical from m/z 314.
Peaks 3, 4, and 7 (m/z 481.1657 [M – H]−) were identified as isomers of bulbophythrin A eluting at three different values of t R 32.557, 33.716, and 38.047 min. These three compounds yielded two common fragments at m/z 465 and 450 due to the consecutive losses of CH4 and CH3 (Scheme 1). Fragment ions at m/z 240 and 225 were obtained by the cleavage of the C–C bond between the bisphenanthrene moiety followed by the loss of CH3. A fragment ion at m/z 433 was obtained due to the simultaneous loss of two methyl groups and one water molecule corresponding to the molecular formula C28H17O5. Earlier, bulbophythrin A and its isomer were isolated from Bulbophyllum odoratissimum and Bletilla striata (Xu et al. 2009).
Three classes of stilbenoid compounds and peaks 5, 6, and 11 were identified as gigantol, batatasin III, and 3′-O-methylbatatasin at t R values of 34.505, 35.230, and 42.265 min, respectively. These three compounds yielded two common fragment ions at m/z 136 and 121. A fragment ion at m/z 136 was obtained due to the cleavage of the C–C bond from the stilbene ring, which further loses the CH3 group-yielded fragment ion at m/z 121. Peak 5 was identified as gigantol at m/z 273.1147 [M – H]−; a yielded fragment ion at m/z 258 was formed due to the loss of CH3 (Scheme 2). Peak 6 was identified as batatasin III at m/z 243.1027 [M – H]−; a yielded fragment ion at m/z 227 was formed due to the loss of CH4 (Scheme 3). Stilbenoids, gigantol, and batatasin III were reported earlier for the antiproliferative activity against HL-60 cell line (Chen et al. 2017; Simoni et al. 2003; Teoh 2016).
Peak 11 at m/z 257.1178 [M – H]−, was identified as 3′-O-methylbatatasin in its MS/MS spectrum. The loss of CH4 from m/z 257 yielded a fragment ion at m/z 241, which further loses CH3 that led to a fragment ion at m/z 226 (Scheme 4). Stilbenoid and 3’-O-methylbatatasin was previously reported (Yamaki et al. 1989).
Peak 8 (m/z 239.0706 [M – H]−) and peak 9 (m/z 271.0976 [M – H]−) were identified as phenanthrene class of compounds; lusianthrin and 2,3-dimethoxy-9,10-dihydrophenanthrene-4,7-diol eluted at t R values of 39.657 and 39.657 min, respectively. Peak 8, at m/z 239.0706 [M – H]−, yielded a fragment ion at m/z 224 due to the loss of CH3, which further loses C2H4-yielded fragment ion at m/z 196 (Scheme 5). Peak 9, at m/z 271.0976 [M – H]−, yielded a fragment ion at m/z 255 due to the loss of CH4 which further loses the CH3 group that led to the fragment ion at m/z 240. A fragment ion at m/z 93 was obtained due to the phenoxide moiety (Schemes 6). 2,3-dimethoxy-9,10-dihydrophenanthrene-4,7-diol was previously isolated from Epidendrum rigidum (Orchidaceae) (Hernandez–Romeo et al. 2005).
Peak 10 was identified as liparacid C, and a prenylated benzoic acid class of a compound at m/z 309.1725 [M – H]−, eluted at t R 41.412 min yielded fragment ions at m/z 265 and 247 due to the successive losses of CO2 followed by H2O. A fragment ion at m/z 291 was obtained due to the loss of H2O from m/z 309 (Scheme 7). Liparacid C was previously isolated from the rhizoma of Liparis nakaharai (Orchidaceae) (Teoh 2016).
Conclusion
This is the first report of the qualitative identification of compounds by the HPLC-ESI-QTOF-MS technique from the antiproliferative fraction of M. acuminata. The analysis of the ethyl acetate fraction of pseudo bulbs of M. acuminata resulted in a different class of compounds, including flavonoids, prenylated acid, stilbenoid, and phenanthrene. Compounds such as gigantol, batatasin III, bulbophythrin A isomers, 2,3-dimethoxy-9,10-dihydrophenanthrene-4,7-diol, liparacid C, and isorhamnetin O-glucoside were identified for the first time from the genus Malaxis, whereas other compounds were reported to be present in the Orchidaceae family. Three steroidal compounds such as β-sitosterol, stigmasterol, and stigmasterol-3-O-glycoside were isolated and identified by NMR and MS analyses. All the compounds were characterized on the basis of their exact mass, molecular formula, and MS/MS fragmentation pattern and might be responsible for the antiproliferative activity in the MAETOAC fraction of M. acuminata.
References
Adaramoye OA, Sarkar J, Singh N, Meena S, Changkija B, Yadav PP, Kanojiya S, Sinha S (2011) Antiproliferative action of Xylopia aethiopica fruit extract on human cervical cancer cells. Phytother Res 25:1558–1563
Bai L, Kato T, Inoue K, Yamaki M, Takagi S (1991) Blestrianol A, B and C, biphenanthrenes from Bletilla striata. Phytochemistry 30:2733–2735
Chen H, Huang Y, Huang J, Lin L, Wei G (2017) Gigantol attenuates the proliferation of human liver cancer HepG2 cells through the PI3K/Akt/NF-κB signaling pathway. Oncol Rep 37:865–870
Cheruvathur MK, Abraham J, Mani B, Thomas TD (2010) Adventitious shoot induction from cultured intermodal explants of Malaxis acuminata D. Don, a valuable terrestrial medicinal orchid. Plant Cell Tiss Organ Cult 101:163–170
Chinmay R, Suman K, Bishnupriya D, Mohanty RC, Dixit R, Padhi MM, Babu R (2011) Phyto-Pharmacognostical studies of two endangered species of Malaxis (Jeevak and rishibhak). Phcog J 3:77–85
Govindarajan R, Singh DP, Rawat AKS (2007) High-performance liquid chromatographic method for the quantification of phenolics in ‘Chyavanprash’a potent ayurvedic drug. J Pharm Biomed Anal 43:527–532
Hernandez-Romeo Y, Acevedo L, Sanchez MDeLA, Shier WT, Abbas HK, Mata R (2005) Phytotoxic activity of bibenzyl derivatives from the orchid Epidendrum rigidum. J Agric Food Chem 53:6276–6280
Hossain MM (2011) Therapeutic orchids: traditional uses and recent advances-an overview. Fitoterapia 82:102–140
Kumar S, Chandra P, Bajpai V, Singh A, Srivastava M, Mishra DK, Kumar B (2015) Rapid qualitative and quantitative analysis of bioactive compounds from Phyllanthus amarus using LC/MS/MS techniques. Ind Crops Prod 69:143–152
Narayana DA, Durg S, Manohar PR, Mahapatra A, Aramya AR (2016) Chyawanprash: A review of therapeutic benefits as in authoritative texts and documented clinical literature. J Ethnopharmacol 197:52–60
Rajurkar NS, Gaikwad KN (2014) Identification and quantification of amino acids from medicinally important plants by using high-performance thin-layer chromatography. J Liq Chromatogr Relat Technol 37:2197–2205
Rai V, Kakkar P, Khatoon S, Rawat AKS, Mehrotra S (2001) Heavy metal accumulation in some herbal drugs. Pharm Biol 39:384–387
Rubert J, Zachariasova M, Hajslova J (2015) Advances in high-resolution mass spectrometry based on metabolomics studies for food–a review. Food Addit Contam Part A 32:1685–1708
Schieber A, Keller P, Streker P, Klaiber I, Carle R (2002) Detection of isorrhamnetin glycoside in extracts of apples (Malus domestica cv. “Brettacher”) by HPLC-PDA and HPLC-APCIMS/MS. Phytochem Anal 13:87–94
Seger C, Griesmacher A (2007) Some important aspects of implementing tandem mass spectrometry in a routine clinical laboratory environment. Biochem Med 17:29–51
Sharma YP, Rani J, Raina R, Bandana K (2014) New insights into the morphology of Malaxis acuminata D Don. Int J Farm Sci 4:136–146
Simoni D, Giannini G, Baraldi PG, Romagnoli R, Roberti M, Rondanin R, Baruchello R, Grisolia G, Rossi M, Mirizzi D, Invidiata FP (2003) A convenient synthesis of unsymmetrically substituted terphenyls of biologically active stilbenes via a double Suzuki cross-coupling protocol. Tetrahedron Lett 44:3005–3008
Szlachetko DL, Kolanowska M (2014) New Malaxis species (Orchidaceae, Epidendroideae) from Colombia. Plant Syst Evol 300:239–244
Tang Y, Lou F, Wang J, Li Y, Zhuang S (2001) Coumaroyl flavonol glycosides from the leaves of Ginkgo biloba. Phytochemistry 58:1251–1256
Teoh ES (2016) Genus: macodes to mycaranthes. In medicinal orchids of Asia. Springer International Publishing, New York
Uma E, Rajendran R, Muthukumar T (2015) Morphology, anatomy and mycotrophy of pseudobulb and subterranean organs in Eulophia epidendraea and Malaxis acuminata (Epidendroideae, Orchidaceae). Flora 217:14–23
Xu J, Yu H, Qing C, Zhang Y, Liu Y, Chen Y (2009) Two new biphenanthrenes with cytotoxic activity from Bulbophyllum odoratissimum. Fitoterapia 80:381–384
Yamaki M, Bai L, Inoue K, Takagi S (1989) Biphenanthrenes from Bletilla striata. Phytochemistry 28:3503–3505
Acknowledgements
The authors are thankful to the director, Central Drug Research Institute (CSIR-CDRI), Lucknow and grateful to SAIF, CSIR-CDRI, Lucknow, India for providing instrumentation facility. This work was financially supported by the project BSC0106i. The authors acknowledge Dr. K. R. Arya, Botany Division, CDRI, Lucknow for authentication of the plant material and Ms. Pooja Soni for technical assistance. CDRI communication number is 9551.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Singh, D., Kumar, S., Pandey, R. et al. Bioguided chemical characterization of the antiproliferative fraction of edible pseudo bulbs of Malaxis acuminata D. Don by HPLC-ESI-QTOF-MS. Med Chem Res 26, 3307–3314 (2017). https://doi.org/10.1007/s00044-017-2023-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-2023-6