Abstract
Diabetes mellitus is a major metabolic disorder affecting a huge population all over the world. The aim of the current study was to validate the folkloric use of Artemisia indica as an antidiabetic plant by using the isolated compound carnosol from the chloroform fraction of Artemisia indica in streptozotocin-induced diabetes mellitus in rats. The antidiabetic activity-guided isolation of the chloroform fraction of Artemisia indica linn (Asteraceae) led to the isolation and characterization of carnosol. Carnosol was tested for its possible antidiabetic potential in streptozotocin [50 mg/kg. intra peritoneal (i.p)]-induced diabetic Sprague Dawley rats. Blood glucose level, body weight, serum lipid profile and activities of liver enzymes and effects on histopathological parameters were determined. A daily oral dose of carnosol (1–100 mg/kg b.w) for 15 days caused a significant reduction in blood glucose level, which was comparable to the standard antidiabetic drug, glibenclamide (0.5 mg/kg, p.o). Carnosol also showed reduction in triglycerides, total cholesterol, and low density lipoproteins (LDL) as well as serum glutamate pyruvate transaminase, serum glutamate oxaloacetate transaminase, alkaline phosphatase and serum creatinine level in diabetic rats. Furthermore, in histopathological studies, carnosol reversed streptozotocin-induced changes in the pancreatic islets of Langerhans and caused regeneration and restored the integrity of pancreatic islets of Langerhans which may be responsible for its antihyperlgycemic effect. In conclusion, carnosol possesses hypoglycemic, antihyperlipidemic and useful protective effects on the liver and renal functions in diabetic rats, which suggests that the antidiabetic activity of Artemisia indica may be due in part to carnosol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is one of the leading metabolic disorders in the world characterized by hyperglycemia due to an absolute or relative secretion of insulin and the failure of insulin to act on its target tissues (Valiathan 1998). Diabetes mellitus causes both microvascular and macrovascular complications. The microvascular complications of diabetes mellitus include neuropathy, retinopathy and nephropathy whereas the macrovascular complications include various cardiovascular disorders, including myocardial infarction, coronary artery disease, diabetic ketoacidosis, nonketotic hyperosmolar coma and diabetic coma (Fowler 2008). The number of people suffering from diabetes mellitus has increased exponentially over the last few years and is on the rise. Currently there are approximately 170 million people suffering from diabetes mellitus globally and this number is projected to increase up to approximately 380 million by 2030 (Wild et al. 2004). The problem is even more alarming in South Asia with India and China representing more than half of the people suffering from diabetes mellitus.
Carnosol is a naturally occurring phytopolyphenol diterpene found mainly in herbs such as rosemary (Rosmarinus officinalis) and sage (Salvia pachyphylla). Carnosol was first isolated from Salvia pachyphylla in 1942 (Johnson et al. 2008) and its chemical structure was confirmed in 1964 by Brieskorn and his coworkers (Brieskorn et al. 1964). Carnosol has been shown to possess various pharmacological activities. The antioxidant activity of rosemary and sage is responsible for their health improving activities (Johnson 2011). More than 90 % of antioxidant activity has been attributed to the presence of carnosol in rosemary (Lo et al. 2002). It has also been reported to possess anti-inflammatory and antitumor activities (Johnson 2011). Topical administration of carnosol has been shown to reduce inflammation and skin cancer via inhibition of TPA-induced ornithine decarboxylase activity, TPA-induced inflammation, arachidonic acid-induced inflammation and TPA-induced hyperplasia in mouse models (Huang et al. 1994) and inhibition of nitric acid production in activated macrophages (Chan et al. 1995). Carnosol has also been shown to be effective in the prevention and treatment of colon and prostate cancers via G2 cell cycle arrest in PC3 cells decreasing cell viability (Johnson 2011; Johnson et al. 2008). Carnosol also exhibits antiangiogenic effects inhibiting proliferation, differentiation, migration and proteolytic capability of endothelial cells (Lopez-Jimenez et al. 2013). Carnosol has also shown antimicrobial activity against yeasts and bacteria and thus could be effective in the retardation of microbial spoilage of foods (Collins and Charles 1987). Furthermore, carnosol has been reported to display antidepressant like activity in the tail suspension test (Machado et al. 2013), hepatoprotective and antiplatelet activities (Lee et al. 2006). Thus, it is clear from the cited literature that carnosol exert a variety of pharmacological effects via various mechanisms. The blood glucose lowering effect of rosemary and sage extracts has been attributed to the presence of carnosol present in these extracts (Rau et al. 2006). However, till date, no study has been done to investigate the hypoglycemic potential of pure carnosol.
Previously we have shown that the chloroform fraction of Artemisia indica exerted significant antidiabetic and anti hyperlipidemic activity in streptozotocin (STZ)-induced diabetic rats (Ahmad et al. 2014). The present study was designed to evaluate the antidiabetic activity of carnosol isolated from the chloroform fraction of Artemesia indica and to establish its therapeutic potential in the treatment of diabetes and related complications.
Materials and methods
Chemicals
Streptozotocin (Sigma Aldrich), glibenclamide (Sanofi Aventis Pharma (Pvt) Ltd., Pakistan) and glucose estimation kits (S.D Chek Gold Germany) were used in this study. The different organic solvents and chemicals used for extraction were purchased from local suppliers of Merck Germany. Other reagents used in this study were tween-80 (Scharlau Chem. Spain), normal saline (Utsoka Pharma (Pvt) Ltd Pakistan), biochemical reagents for lipid profile, liver function tests (LFTs) Kits (Human Germany) and renal function tests (RFTs) kit (Bioneed Germany diagnostic).
Extraction and isolation of carnosol
The aerial parts of the plant were collected from upper Dir (Sherin Gal) Khyber Pakhtunkhwa, Pakistan, in the month of July 2008 and were authenticated by a taxonomist. The plant specimen was deposited in the Department of Pharmacy, University of Malakand, Pakistan. Specimen having voucher number (200300106) was deposited at the same institution. A general extraction procedure (Ahmad et al. 2014) was employed for isolation. The powdered plant material (5 kg) was extracted with methanol at room temperature. The crude methanolic extract (750 g) was fractionated into the n-hexane fraction (80 g), chloroform fraction (250 g), ethyl acetate fraction (70 g), n-butanol fraction (100 g) and finally the aqueous fraction (200 g). The chloroform fraction (250 g) was subjected to column chromatography on silica gel (70–230 mesh size). The column was initially eluted with pure n-hexane and then with gradient polarities of ethyl acetate and n-hexane which afforded six sub fractions (F1–F6). The sub-fraction (F-1, 47 g) was subjected to isolation and purification on flash column chromatography (3 g, Silica gel) and preparative thin layer chromatography by eluting with n-hexane:ethyl acetate (3:7) that yielded the compound which was identified as carnosol (Fig. 1).
Spectroscopic characterization of carnosol
Different spectroscopic methods were used to elucidate the structure of carnosol. 1H NMR, 13C NMR and Electrospray mass spectroscopy (ESMS) were carried out. Mass spectra were recorded on Varian MAT-112 mass spectrometer. 1H NMR, 13C NMR spectra were recorded using (DMSO-d 6 ) as solvent on Bruker 400 NMR spectrometer with tetramethylsilane as internal standard. ESMS spectroscopy showed the molecular ion peak at 331 that corresponds to the molecular formula C20H26O4.
1H NMR (DMSO-d 6 ) δ ppm: 6.68 (H, s, H-14), 5.44 (1H, dd, J = 3.8, 1.4 Hz, H-7), 3.21 (1H, sept, J = 6.8, H-15), 2.64 (1H, dd, J = 14, 1.6 Hz, H-1 α), 2.43 (1H, ddd, J = 14, 4.6 Hz, H-1β), 2.01 (1H, ddd, H-6 α), 1.6 (1H, m, H-6β), 1.59 (1H, dd, J = 10.6, 5.4 Hz, H-5), 1.50 (1H, m, H-2β), 1.42 (1H, dd, J = 13, 1.4 Hz, H-3 α), 1.21 (1H, ddd, J = 13.4, 3.2 Hz, H-3β), 1.10 (6H, d, J = 6.8, H-17, H-16), 0.79 (3H, s, H-18), 0.77 (3H, s, H-19
13C NMR (DMSO- d 6 ) δ ppm: 175.9 (C-20), 143.7 (C-11), 143.5 (C-12), 134.7 (C-13), 132.0 (C-8), 122.3 (C-9), 111.7 (C-14), 77.4 (C-7), 48.3 (C-10), 45.4 (C-5), 41.0 (C-3), 34.6 (C-4), 31.8 (C-18), 29.7 (C-6), 29.2 (C-1), 26.6 (C-15), 23.2 (C-17), 23.1 (C-16), 19.8 (C-19), 19.0 (C-2).
The isolated compound was identified as carnosol by comparison of 1H NMR with literature confirmed with 2D-COSY and HRMS.
Animals
Adult Sprague Dawley rats in the weight range of 150–200 g were purchased from the Department of Pharmacy, University of Peshawar. Animals were housed in the Department’s animal house with fresh water and standard food available ad libitum. The animals were maintained at 12 h light and dark cycle and with room temperature maintained at 22–25 °C in the animal house. All animal procedures were approved by the Departmental Animal Ethical Committee (DAEC/PHARM/2012/11) and were conducted according to the UK animal scientific procedure act, 1986.
Acute toxicity study of carnosol
The acute toxicity of carnosol was determined by using Sprague Dawley rats (1–200 mg/kg), according to the method described by Ahmad and coworkers (Ahmad et al. 2014). The animals were divided into six groups (n = 6). One group served as a control and received tween-80 suspension orally. Carnosol at the dose level of 50,100,150 and 200 mg/kg b.w to each rat orally. Each animal was subjected to various parameters including writhing, convulsions, aggressiveness, hypersensitivity, salivation, lacrimation, spontaneous activity, ataxia and catalepsy 30 min prior to injection (baseline) and then at 0 (straight after injection), 30 and 60 min, 24, 48 and 72 h and 1 week after administration for any kind of behavioral, physical and pharmacological toxic effects.
Induction of hyperglycemia
Hyperglycemia was induced in Sprague Dawley rats by a single i.p injection of 50 mg/kg of STZ reconstituted with normal saline (0.9 %) after overnight fasting. After 72 h of STZ administration, blood glucose levels were measured in blood samples collected from tail vein puncture with one touch Glucometer strips using an SD glucometer (Germany). Rats with fasting blood glucose level more than 300 mg/dl were considered diabetic and selected for the study.
Experimental design
Animals were randomly divided into six groups (8 rats in each group). The first group served as normal control (nondiabetic) and received normal saline whereas the second group served as diabetic control and received 5 % Tween-80 suspension. The third group received standard drug glibenclamide (0.5 mg/kg, p.o). The fourth, fifth, sixth and seventh groups received carnosol 1, 10, 30 and 100 mg/kg b.w. (p.o) respectively. The treatment was continued once daily at 0900 am for 15 days. Body weight and blood glucose levels were estimated on the 0, 4th, 7th, 10th and 15th day of the treatment (Gupta et al. 2004).
Estimation of serum lipid and liver physiological profile
After completion of the antidiabetic assay on the 15th day, all animals were anesthetized with pentobarbital sodium (35 mg/kg) and euthanized by cervical decapitation using the method described in schedule 1 of animal scientific procedure act 1986 and blood samples were collected through cardiac puncture for biochemical parameter studies (Nagappa et al. 2003). Collected blood was centrifuged at 1500×g for 10 min for serum separation. The serum sample was then analyzed by a spectrophotometer (Perkin Elmer Germany) for determination of serum serum glutamate pyruvate transaminase (SGPT), serum serum glutamate oxaloacetate transaminase (SGOT) and serum alkaline phosphatase (ALP) using standard IFCC kinetic Method (Bioneed kit Germany). Total cholesterol (TC), triglycerides (TG), LDL, high density lipoprotein (HDL) and serum creatinine was determined by CHOD-PAP and GPO-PAP methods on UV-Spectrophotometer using the Human kit, Germany.
Histopathological studies
On the 15th day, all animals were sacrificed by cervical dislocation and the pancreases were removed. Pancreatic samples were preserved in 10 % formalin solution for tissue histopathological studies. Tissue samples were processed in tissue processor and pancreatic sections were stained with haematoxylin and eosin Y (H/E) dyes. The sections of the pancreas were observed under a light microscope (Olympus) for histopathological study (Ghazanfar et al. 2014).
Statistical analysis
All the values of blood glucose, body weight, and biochemical parameters were represented as mean ± S.E.M. When two groups were compared, Student’s t-test was used and when more than two groups were compared, one way ANOVA followed by Dunnett’s post hoc multiple comparison test was used. Differences between groups were considered significant at p < 0.05, **p < 0.01 as compared with diabetic control at the same time (One way ANOVA followed by Dunnett’s multiple comparison test).
Results
Acute toxicity tests
Acute toxicity studies revealed that the administration of carnosol (50–200 mg/kg) did not produce significant changes in the behavior of the animals as observed by lack of convulsions, respiratory distress, writhing, changes to reflex activity or mortality. A slight increase in irritability and escape behavior was observed at 200 mg/kg in one out of eight animals. At 24 h–1 week, all animals seemed well with no observable changes in behavior or appearance. No deaths were observed up to 1 week of study.
Antihyperglycemic effect of carnosol in STZ- induced diabetic rats
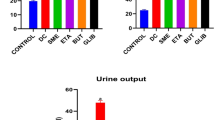
Table 1 shows the effect of carnosol and glibenclamide on serum glucose levels in STZ-induced diabetic rats. The standard reference drug glibenclamide (0.5 mg/kg, p.o.) significantly reduced fasting blood glucose level (**p < 0. 01, n = 8; one way ANOVA with Dunnett’s post hoc test). Glibenclamide showed significant reduction in blood glucose values on day 4, 7, 10 and 15 respectively in comparison to the diabetic control group. Glibenclamide reduced the mean blood glucose level from 440.8 ± 40 to 210.2 ± 40** mg/dl at the end of the treatment (Table 1). Similarly oral administration of carnosol (1, 10, 30 and 100 mg/kg b.w.) caused a significant (**p < 0. 01, n = 8; one way ANOVA with Dunnett’s post hoc test) reduction in blood glucose level compared to diabetic control. Carnosol 1, 10, 30 and 100 mg/kg decreased blood glucose level from 465.7 ± 15, 446.7 ± 53, 443 ± 52 and from 437 ± 30 to 264.3 ± 15**, 260.6 ± 16**, 230.8 ± 13** and 220.3 ± 12** mg/dl respectively. The lowering in blood glucose level at various doses tested was not significant at the end of 15 days treatment. The significant reduction in blood glucose level was evident from the 4th day onwards. The antihyperglycemic effect of carnosol was comparable to glibenclamide (**p < 0. 01, n = 8; one way ANOVA with Dunnett’s post hoc test) (Table 1).
Effects of carnosol on body weight in STZ- induced diabetic rats
The results of changes in body weight of control and experimental rats treated with carnosol and glibenclamide are shown in Table 2. STZ-induced diabetic rats produced significant (***p < 0.001, n = 8; Student’s t test) loss in body weight as compared to normal rats during the study. In the diabetic control rats, continued weight loss was observed till the end of the study (15 days treatment). During this period, 13.8 % reduction in their body weight (Table 2) was recorded. STZ-mediated body weight reduction was reversed by carnosol at the dose level of 1, 10, 30 and 100 mg/kg b.w. At the end of 15 days treatment, carnosol 1, 10, 30 and 100 mg/kg caused increases in body weight by 9.9, 10.5, 11.2 and 12.0 % respectively. This improvement in body weight was comparable to that of glibenclamide (Table 2).
Effects of carnosol on lipid profile in STZ- induced diabetic rats
The lipid profiles in control and experimental rats are depicted in Table 3. In STZ-induced diabetic control rats, there was a significant (**p < 0.01, n = 8; Student’s t test) increase in TC, TG, LDL cholesterol and serum creatinine compared to normal control. In addition, there was a significant decrease (*p < 0.05, n = 8; Student’s t test) in HDL cholesterol in diabetic control rats in comparison to normal control.
Administration of carnosol 1, 10, 30 and 100 mg/kg for 15 days showed a significant (**p < 0.01, ***p < 0.001, n = 8; one way ANOVA with Dunnett’s post hoc test) reduction in TC, TGs and LDL cholesterol in comparison to diabetic control rats. The effect was comparable to the standard drug glibenclamide (0.5 mg/kg). Carnosol (1, 10, 30 and 100 mg/kg) dose dependently increased HDL cholesterol (**p < 0.01 n = 8; one way ANOVA with Dunnett’s post hoc test). Glibenclamide (0.5 mg/kg) also significantly (**p < 0.01 n = 8; one way ANOVA with Dunnett’s post hoc test) increased HDL cholesterol in diabetic rats.
The effect of carnosol on liver function in STZ-induced diabetic rats
Table 4 summarized the effects of STZ on the activity of hepatic marker enzymes in serum. In the present study, the levels of SGPT, SGOT and ALP in STZ-induced diabetic rats were elevated. Administration of carnosol (1, 10, 30 and 100 mg/kg) significantly reduced (**p < 0.01, n = 8; one way ANOVA with Dunnett’s post hoc test) serum SGPT, SGOT and ALP in rats intoxicated with STZ. The restoration of hepatic enzymes observed with carnosol (1, 10, 30 and 100 mg/kg) was comparable to standard drug glibenclamide (**p < 0.01, n = 8; one way ANOVA with Dunnett’s post hoc test). Carnosol (1, 10, 30 and 100 mg/kg) also caused a significant reduction (***p < 0.001 n = 8; one way ANOVA with Dunnett’s post hoc test) in serum creatinine comparable to standard drug glibenclamide (Table 4).
STZ-induced histopathological changes in rat pancreas
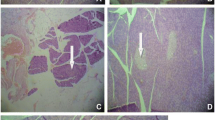
Figure 2a shows the normal acini and normal cellular population in the islets of Langerhans of the pancreas of Sprague Dawley rats. As shown in Fig. 2b, administration of STZ caused significant reduction in the number of pancreatic beta (β) cells in Sprague Dawley rats. The pancreatic sections harvested from the non-treated diabetic group also showed shrunken islets of Langerhans associated with degenerative necrosis. Furthermore, STZ also caused a reduction in the dimension of islets as marked by arrows (Fig. 2b) compared to non diabetic rats (Fig. 2a).
Normal acini and normal cellular population in the islets of Langerhans a. STZ induced extensive damage to the islets of Langerhans and reduced dimension of islets as marked by arrow b. Restoration of normal cellular size of islet with hyperplasia shown by glibenclamide c; partial restoration of normal cellular population and enlarge size of β cells shown by carnosol (10 mg/kg b.w.) d; partial restoration of β cells with hyperplasia and enlarge cells shown by carnosol (100 mg/kg b.w.) e
Figure 2c shows that the administration of glibenclamide (0.5 mg/kg b.w.) caused the restoration of normal cell size of islets of Langerhans accompanied by hyperplasia. Also the islets of Langerhans appeared less shrunken as compared to the untreated diabetic group. Similarly carnosol 10 mg/kg, b.w. also caused partial restoration of normal cellular population and enlarged the size of β cells of islets of Langerhans (Fig. 2d). Furthermore 100 mg/kg of carnosol not only caused the partial restoration and increased in size of β cells of islets of Langerhans but the partial restoration was also accompanied by hyperplasia of β cells of islets of Langerhans (Fig. 2e). This effect was similar and comparable to glibenclamide.
Discussion
Carnosol is a naturally occurring phenolic diterpene found mainly in the rosemary plant. Carnosol is primarily responsible for the high antioxidant activity of Rosmarinus officinalis (Huang et al. 1994; Singletary et al. 1996). Carnosol significantly increased tyrosine hydroxylase activity, suggesting that it may be a potential agent for the treatment for Parkinson’s disease (Kim et al. 2006). Carnosol has been shown to inhibit the binding of [35S]t-butylbicyclophosphorothionate ([35S] (TBPS) to the chloride channel of the GABA/benzodiazepine receptor (Rutherford et al. 1992) and to inhibit GABA-A receptors expressed in Xenopus ooctyes in electrophysiological studies (Abdelhalim et al. 2014). Investigation of the aqueous extract of Rosmarinus officinalis linn (Rosemary) in STZ-induced diabetic rats has shown that the extract exerts significant hypoglycemic and hypolipidemic activities (Al-Jamal and Alqadi 2011). Furthermore, an infusion of Salvia officinalis linn has been shown to exert metformin-like effects in STZ-induced diabetic rats (Lima et al. 2006). The blood glucose lowering effect of rosemary and sage extracts may be attributed to the activation of peroxisome proliferator-activated receptor gamma (PPARgamma) transcription factors, by carnosol and carnosic acid present in these extracts (Rau et al. 2006). Chronic administration of carnosol for 15 days to the STZ-induced diabetic rats produced a significant fall in blood glucose level. Thus carnosol may be considered to have good antihyperglycemic activity and did not cause any hypoglycemic effect unlike insulin and other synthetic drugs (Chaulya et al. 2011). The antihyperglycemic effect was not found to be dose-dependent as there were not significant differences between 1, 10, 30 and 100 mg/kg of the carnosol-treated groups. Elevated plasma TC, TG and LDL cholesterol are the major risk factors of various cardiovascular disorders. Diabetic rats showed elevated plasma TC, TG and LDL cholesterol. Reduction in hyperglycemia also resulted in significant reduction in the level of serum cholesterol and TG. Furthermore, carnosol reversed the significant loss of body weight caused by STZ. This reversal of body weight loss can be attributed to the hypoglycemic effect of carnosol. Studies have shown that liver cells are destroyed irreversibly by STZ that cause increased levels of different enzymes, including SGPT, SGOT and serum ALP in the blood (Daisy et al. 2008). Carnosol was found to have significantly reduced activities of these enzymes, thus indicating the protective effects of carnosol on hepatocytes. Chronic hyperglycemia can also cause nephropathy that increases serum creatinine (Fowler 2008). The reduction of serum creatinine in treated rats may be due to lowering of blood glucose level. STZ usually causes destruction of β-cells of islets of Langerhans after 72 h of STZ administration and the maximum destruction is achieved after 3–4 weeks of STZ administration. This causes an increase in free radical formation. Pancreatic β-cells of islets of Langerhans are highly sensitive to nitric oxide and free radicals due to decreased activity of free radical scavenging enzymes (Spinas 1999). In the current study, carnosol (10 and 50 mg/kg, b.w) caused increased partial restoration of the normal cellular population and hyperplasia of pancreas β-cells. Furthermore an increase in size of the pancreatic β-cells was also observed. This regeneration and hyperplasia of pancreatic β-cells could be due to the prevention of free radical formation by carnosol as it has been shown to reduce the production of reactive oxygen species by increasing the level of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, glutathione-S-transferases and glutathione reductase which may limit the hyperglycemia-induced microvascular and macrovascular complications (Labban et al. 2014). Since some residual β-cells still retains the regenerative capability, the partial restoration of the cells could be due to neogenesis or by replication of the already existing differentiated β-cells (Govan et al. 1986). Although STZ has been reported to cause rapid and irreversible necrosis of pancreatic β-cells, administration of alkaloid extract of Ephedra distachya and L-ephedrine (Xiu et al. 2001) and various extracts of Artemisia amygdalina decne (Ghazanfar et al. 2014) have been reported to regenerate both functioning and atrophic pancreatic islets of Langerhans suggesting differentiation and proliferation of residual pancreatic β-cells. These reports support the histophathological observations wherein both the number and structural integrity of pancreatic β-cells were restored. Thus, this effect of carnosol on pancreas β-cells could lead to increase insulin synthesis and secretion thereby reducing hyperglycemia and correcting diabetic state. Furthermore the ability of carnosol to activate of PPARgamma transcription factors (Rau et al. 2006) could also contribute to its hypoglycemic effect observed in this study.
Conclusion
In conclusion, carnosol decreased blood glucose level significantly in fasting STZ-induced diabetic rats. It also caused a significant reduction in the lipid profile of these animals. Carnosol also significantly lowered various biochemical parameters including SGPT, SGOT, ALP and serum creatinine in diabetic rats. In the histopathological studies, there was a partial restoration of pancreatic β-cells, hyperplasia and normal cellular population of pancreatic islets of Langerhans comparable to the standard oral hypoglycemic drug, glibenclamide. The lowering of blood glucose level and effects on the pancreatic β-cells of islets of Langerhans indicate a strong antidiabetic potential of carnosol. However, further studies are required to elucidate other molecular mechanism/s involved in the antidiabetic activity of carnosol.
References
Abdelhalim A, Chebib M, Aburjai T, Johnston G, Hanrahan J (2014) GABAA receptor modulation by compounds isolated from Salvia triloba L. Adv Biol Chem 4:148–159
Ahmad W, Khan I, Khan M, Ahmad M, Subhan F, Karim N (2014) Evaluation of antidiabetic and antihyperlipidemic activity of Artemisia indica linn (aeriel parts) in Streptozotocin induced diabetic rats. J ethnopharmacol 151:618–623
Al-Jamal A, Alqadi T (2011) Effects of Rosemary (Rosmarinus officinalis) on lipid profile of diabetic rats. Jordan J Biol Sci 4:199–204
Brieskorn C, Fuchs A, Brendenberg J, McChesney J, Wenkert E (1964) The structure of carnosol. J Org Chem 29:2293–2298
Chan M, Ho C, Huang H (1995) Effects of three dietary phytochemicals from tea, rosemary and turmeric on inflammation-induced nitrite production. Cancer Lett 96:23–29
Chaulya NC, Halder PK, Mukherjee A (2011) Antidiabetic activity of methanol extract of rhizomes of Cyperus tegetum roxb (Cyperaceae). Acta Pol Pharm 68:989–992
Collins M, Charles H (1987) Antimicrobial activity of Carnosol and Ursolic acid: two anti-oxidant constituents of Rosmarinus officinalis. L Food Microbiol 4:311–315
Daisy P, Eliza J, Ignacimuthu S (2008) Influence of Costus speciosus (Koen.) Sm. rhizome extracts on biochemical parameters in Streptozotocin induced diabetic rats. J Health Sci 54:675–681
Fowler M (2008) Microvascular and macrovascular complications of diabetes. Clin Diabetes 26:77–82
Ghazanfar K, Ganai B, Akbar S, Mubashir K, Dar S, Dar M, Tantry M (2014) Antidiabetic activity of Artemisia amygdalina Decne in Streptozotocin induced diabetic rats. Biomed Res Int 2014:185676
Govan A, Macfarlane P, Callander R (1986) Pathology Illustrated, vol 2. Churchill Levingstone, New York, NY
Gupta S, Kataria M, Gupta P, Murganandan S, Yashroy R (2004) Protective role of extracts of neem seeds in diabetes caused by Streptozotocin in rats. J ethnopharmacol 90:185–189
Huang M et al. (1994) Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res 54:701–708
Johnson JJ (2011) Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer Lett 305:1–7
Johnson JJ, Syed DN, Heren CR, Suh Y, Adhami VM, Mukhtar H (2008) Carnosol, a dietary diterpene, displays growth inhibitory effects in human prostate cancer PC3 cells leading to G2-phase cell cycle arrest and targets the 5’-AMP-activated protein kinase (AMPK) pathway. Pharma Res 25:2125–2134
Kim SJ et al. (2006) Carnosol, a component of rosemary (Rosmarinus officinalis L.) protects nigral dopaminergic neuronal cells. Neuroreport 17:1729–1733
Labban L, Mustafa US, Ibrahim YM (2014) The Effects of Rosemary (Rosmarinus officinalis) leaves powder on glucose level, lipid profile and lipid perodoxation. Int J Clin Med 5:297–304
Lee JJ, Jin YR, Lim Y, Hong JT, Kim TJ, Chung JH, Yun YP (2006) Antiplatelet activity of carnosol is mediated by the inhibition of TXA2 receptor and cytosolic calcium mobilization. Vasc Pharmacol 45:148–153
Lima CF, Azevedo MF, Araujo R, Fernandes-Ferreira M, Pereira-Wilson C (2006) Metformin-like effect of Salvia officinalis (common sage): is it useful in diabetes prevention? Br J Nutr 96:326–333
Lo AH, Liang YC, Lin-Shiau SY, Ho CT, Lin JK (2002) Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-κB in mouse macrophages. Carcinogenesis 23:983–991
Lopez-Jimenez A, Garcia-Caballero M, Medina MA, Quesada AR (2013) Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur J Nutr 52:85–95
Machado DG et al. (2013) Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem 136:999–1005
Nagappa AN, Thakurdesai AP, Singh N (2003) Antidiabetic activity of Terminlia catappa linn fruits. J ethnopharmacol 88:45–50
Rau O et al. (2006) Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med 72:881–887
Rutherford DM, Nielsen MP, Hansen SK, Witt MR, Bergendorff O, Sterner O (1992) Isolation and identification from Salvia officinalis of two diterpenes which inhibit t butylbicyclophosphoro[35S]thionate binding to chloride channel of rat cerebrocortical membranes in vitro. Neurosci Lett 135:224–226
Singletary K, MacDonald C, Wallig M (1996) Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett 104:43–48
Spinas GA (1999) The dual role of nitric oxide in islet beta-cells. News Physiol Sci 14:49–54
Valiathan MS (1998) Healing plants. Curr Sci 75:1122–1127
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Xiu LM, Miura AB, Yamamoto K, Kobayashi T, Song QH, Kitamura H (2001) Pancreatic islet regeneration by ephedrine in mice with streptozotocin-induced diabetes. Am J Chin Med 29:493–500
Acknowledgments
The authors are thankful to the Higher Education Commission of Pakistan for financing this research from beginning till completion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Khan, I., Ahmad, W., Karim, N. et al. Antidiabetic activity and histopathological analysis of carnosol isolated from Artemisia indica linn in streptozotocin-induced diabetic rats. Med Chem Res 26, 335–343 (2017). https://doi.org/10.1007/s00044-016-1750-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1750-4