Abstract
Solanum macrocarpon Linn. (SM) is a well eaten vegetable and prominent for treatment of diseases in folk medicine. This study evaluated the antidiabetic potentials of fractions of methanol extract of Solanum macrocarpon in streptozotocin – induced diabetic male Wistar rats.
Diabetes mellitus (DM) was induced in Wistar rats by single (i.p.) dose of 50 mg/kg streptozotocin (STZ) and diabetic rats were administered the extract(s) once daily for four weeks. The effect was determined on body weight (BW), blood glucose concentration (BGC), oral glucose tolerance test (OGGT), heamatological indices and lipid profiles. Also, the pancreatic level of oxidative stress and histopathology were determined. Treatment with extracts significantly decreased the elevated BGC, and improved OGTT and BW of diabetic rats. Heamatological indices, lipid profiles and pancreatic level of oxidative stress were augmented to near normalcy in diabetic rats administered the extract. Histopathology slides showed improvement in structural integrity of diabetic rats upon treatment with the extracts. The findings of this study have clearly shown that Solanum macrocarpon possess antidiabetic ability and could be used in the management of diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is an endocrine disorder, persisted with increase in blood glucose as a result of decrease in insulin secretion or inability to utilize the secreted insulin (Karigidi et al. 2020). It is presently a global menace threatening around 500 million people and the number has been predicted to rise to 650 million in 2040 (IDF 2021). This increase may be ascribed to enhanced life expectancy, consumption of energy rich diet, and physical inactivity of many people, nowadays (Ibrahim et al. 2018). The high blood glucose concentration of diabetic patient always cause disturbance in lipid metabolism and enhanced free radicals production which is the hallmark of hypertension in diabetic patients (Vellapandian and Ramachandram 2022). Certainly, Pancreas being an organ responsible for secretion, storage and utilization of insulin in the body is central to development of DM. One of the prominent causes of pancreas’ loss of function is its vulnerability to free radicals attack due to its low antioxidant defense status (Lenzen 2017). Presently, many hypoglycemic drugs with different mechanisms of action have been developed for treatment of DM but there have been many reported shortcomings on them. Because of this, many people still rely on plant-based therapy to treat and manage DM. One of the vegetables used for management/treatment of DM in folk medicine is Solanum macrocarpon.
Vegetables are prominent part of human diet because of their richness in protein, micronutrients and vitamins. Solanum macrocarpon (SM) belongs to Solanaceae family and popularly referred to as African eggplants. It is well cultivated in the tropics because of its numerous uses; the leaves are used as vegetable for soup or herb for treatment of ailments. Its use in preparing soup is due to the fact that it is considered very rich in fibre, zinc, protein, fat and calcium (Komlaga et al. 2014). The leaves are reservoirs of phytochemicals of biological significance (Oyesola et al. 2022). The aqueous extract of the vegetable has been shown to possess antihyperglycemic potential in alloxan-induced DM (Ajiboye et al. 2021).
More than 80% of diabetes burden are carried by low and middle income countries that do not have sufficient financial means to obtain medical care (IDF 2021; Karigidi and Olaiya 2020b). Therefore, they depend on plant-based drugs for treatment and management of DM. Due to this, there is call to scientifically validate some of the plant-based drugs use for treatment of DM. This study is designed to investigate the antidiabetic effects of methanol extract of Solanum macrocarpon and its fractions in streptozotocin-induced diabetic male Wistar rats.
Materials and methods
The Solanum macrocarpon Linn explored in this study was bought at Bodija market, Ibadan, Nigeria. It was identified and authenticated at Department of Botany, University of Ibadan (UIH-22,543). The leaves were sorted to remove shriveled leaves and washed and dried in the laboratory at ambient temperature for three weeks and milled with electric blender.
Preparation of extract and fractions
The procedure of Karigidi and Olaiya (2020a) was adopted for preparation of extract and fractions.
Animal
Healthy male Wistar rats of 115–120 g weight were bought from the University of Ibadan animal house. They were fed standard pellet diet with and water ad libitum. The rats were kept individually in metabolic cage and acclimatized under 12-h light and dark cycle for 14 days prior the induction of experimental DM. The protocol for this study was approved by University of Ibadan, animal care use and research ethics committee for care and use of laboratory animals with reference number (UI-ACUREC/02-0219/21).
Induction of DM
Induction of DM was done as reported by Karigidi and Olaiya (2020a). Rats with fasting blood glucose ≥ 250 mg/dL were used for the study.
Experimental design
After confirmation of DM, the rats were categorized into groups (6) of six rats.
Control: Control rats.
Dcontrol: Diabetic control rats.
SME: Diabetic rats administered SME (300 mg/kg).
ETA: Diabetic rats administered ETA (300 mg/kg).
BUT: Diabetic rats administered BUT (300 mg/kg).
GLIB: Diabetic rats administered Glibenclamide (1 mg/kg).
These extract(s) were administered orally for four weeks with oral gavage. Body weight and blood glucose level were measured every week during the experiment. After four weeks, the rats were euthanized.
Oral glucose tolerance test (OGTT)
On the 27th day of treatment, OGTT was measured as described by Karigidi and Olaiya (2020a).
Heamatological indices determination
Whole blood samples were used for the determination of the heamatological indices using automated hematology auto machine -Mindray BC 5300 (UK).
Estimation of lipid profile
Serum cholesterol and high density lipoprotein was determined using the procedure of Allain et al. (1974). Total triglycerides were determined by the method of Jacobs and Denmark (1960). LDL was calculated by dividing triglycerides by 5.
Preparation of homogenate
Excised pancreas tissues were homogenized in phosphate buffer (0.1 M, pH 7.4), and centrifuged at 10,000 g for 10 min at 4 °C. The protein content was assayed using protocol of Bradford (1976).
Markers of oxidative stress and antioxidant status determination
Lipid peroxidation was evaluated using the method of Farombi et al. (2000). Nitric oxide was assayed based on the method of (Green et al. 1982). Reduced glutathione (GSH) was measured using the procedure of (Jollow et al. 1974). Glutathione peroxidase (GPx) was measured by the protocol of (Rotruck et al. 1973). Superoxide dismutase (SOD) was evaluated by the procedure of (Misra and Fridovich 1972). Catalase (CAT) was measured using the method of (Clairborne 1995).
Histopathology
Pancreas were fixed in 10% formaldehyde for 24 h, and processed using the method of Avwioro (2010).
Statistical analysis
Data are presented as the mean ± SD. The significance of the differences between means was established by ANOVA, p < 0.05 using SPSS and charts were drawn with graph pad prism 9.
Results
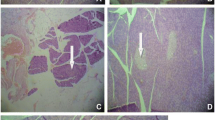
As shown in Table 1, induction of DM led to elevation in blood glucose level of diabetic rats. However, treatment with the extract(s) lowered the level of FBG significantly (p < 0.05) in the diabetic rats. Table 2 presents the effect of the treatment on the body weight of diabetic rats. There was reduction in the body weight of the diabetic rats when compared with the control rats. Administration of the extract(s) was able to increase the weight of diabetic rats significantly (p < 0.05) in relation to untreated diabetic rats. The impact of the extract(s) on metabolic output of diabetic rats is shown is Fig. 1. The increased food intake, water consumption and urine output were significantly (p < 0.05) reduced. Figure 2 presents the effect of treatment on OGTT. After 30 min of glucose load, diabetic rats treated with the extract(s) have started showing decline in their blood glucose levels unlike diabetic control rats. The role of treatment on heamatological indices of the diabetic rats is shown in Table 3. Packed cell volume (PCV), platelets (PLT), red blood cell (RBC) and heamoglobin (Hg) levels were declined while white blood cell (WBC) was increased in diabetic rats. Treatment of diabetic rats with the vegetable extract(s) significantly normalized these parameters (p < 0.05) against the control rats. Figure 3 shows the effect of the treatment on the lipid profile; total cholesterol, triglycerides, and LDL-cholesterol were significantly increased while HDL-cholesterol was decreased in diabetic rats. Upon administration of the extract(s) for 28 days, the parameters were significantly (p < 0.05) reversed in diabetic rats. The effect of the treatment with extract(s) on lipid peroxidation (LPO), nitric oxide (NO) and H2O2 is shown in Fig. 4. The formation of lipid peroxide, nitric oxide and hydrogen peroxide were significantly (p < 0.05) increased in diabetic rats. Treatment with extract(s) was able to decrease the generation of these free radicals significantly (p < 0.05). The role of treatment on GSH, GPX, GST, SOD and CAT is shown in Fig. 5. There was significant (p < 0.05) reduction in GSH level and activities of GPX, GST, SOD and CAT of diabetic rats which was significantly modulated by administration of the extract(s) for 28 days. The effect of the administration of the extract(s) on the structural integrity (Pancreas) of the diabetic rats is presented in Fig. 6. The control rats revealed normal architecture while the diabetic control rats showed poor architecture with severe fibrosis and diffused islet of Langerhans. The treated groups showed moderate architecture with mild infiltration of inflammatory cells.
Discussion
Numerous studies have reported positive results on the utilization of vegetable plants in the treatment of streptozotocin - induced diabetes and its associated ailments in experimental animals (Omoboyowa et al. 2020; Boye et al. 2020; Ajiboye et al. 2021). Apart from hyperglycemia, the induction of DM is usually accompanied by enhanced food intake, weight loss, urination and thirst (Ibrahim et al. 2018). The positive responses on these metabolic parameters upon treatment might be attributed to the anti-hyperglycemic ability of the vegetable extract(s) which may be linked to their capacity to regenerate of pancreas β-cells (Karigidi and Olaiya 2020a). An OGTT is a more dependable index of untimely irregularity in glucose metabolism than fasting blood glucose (Jagannathan et al. 2020). The improvement in the OGTT of diabetic rats further confirms the antihyperglycemic potential of the extract(s). Enhanced blood glucose concentration promotes oxidative attachment of glucose to heamoglobin leading to its loss of structure and function (Dordevic et al. 2017). Anaemia in DM is due to the elevated non-enzymatic glycosylation of membrane proteins in RBC. Also, white blood cells are activated by advanced glycation products. In the present study, the vegetable extract(s) modulate the anaemia and abnormalities of immune functions. Induced DM lead to a persistent increase in cholesterol, triglycerides and decrease in HDL concentrations as reported by similar studies (Karigidi et al. 2020). In DM, hormone-sensitive lipase is activated leading to excessive liberation of free fatty acids (FFAs) from adipose tissue. Therefore, the marked hyperlipidemia that is usually associated with DM may be considered a result of the uncontrolled activities of lipolytic hormones on fat deposits (Cyriac and Eswaran 2016). In this study, these parameters were mitigated indicating that treatment with the vegetable can reduce the risk of cardiovascular diseases in DM.
These overproduced free radicals in DM attack biological membranes and different biomolecules leading to membrane damage and loss of functions of many organs. Treatment with the SM extract(s) was able to modulate the elevated free radical production. The reduced free radicals production might be attributed to the normalization of glucose homeostasis in treated diabetic rats. In response to increased ROS production, antioxidants are activated to mop up ROS in order to maintain REDOX homeostasis in the body (Abdulwahab et al. 2021). The enhanced antioxidant status of the treated diabetic rats might be attributed to presence of antioxidant molecules in SM (Idowu et al. 2021). Antidiabetic property of the SM was further confirmed on its ability to promote regeneration of pancreas’ cell after induction of DM. In the present study, histopathology plates revealed improved structural integrity in pancreas of treated diabetic rats.
Conclusion
Data obtained in the present study have shown that Solanum macrocarpon can lower blood glucose level, improved glucose tolerance, modulate aberrant lipid and heamatological indices and mitigate hyperglycemia - mediated oxidative stress in pancreas; thereby justifying the use of this vegetable for treatment of diabetes mellitus in folk medicine.
References
Abdulwahab DA et al (2021) Melatonin protects the heart and pancreas by improving glucose homeostasis, oxidative stress, inflammation and apoptosis in T2DM-induced rats. Heliyon 7:e06474. https://doi.org/10.1016/j.heliyon.2021.e06474
Ajiboye BO et al (2021) Aqueous extract of Solanum macrocarpon Linn leaves abates hyperglycaemia and expression of glucose transporters gene in alloxan-induced diabetic rats. J Endocrinol Invest 44(2):265–276
Allain GC, Poon LS, Chan CS, Richmond W (1974) Quantitative determination of cholesterol using enzymatic colorimetric method. Clin Chem 20:470–475
Avwioro OG (2010) Histochemistry and tissue pathology, principle and techniques. Claverianum press, Nigeria
Boye A et al (2020) Glucose lowering and pancreato-protective effects of Abrus precatorius (L.) leaf extract in normoglycemic and STZ/ nicotinamide-induced diabetic rats. J Ethnopharmacol 258:112918. https://doi.org/10.1016/j.jep.2020.112918
Bradford MM (ed) (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254.
Clairborne A. (1995) Catalase activity. In: AR Greewald, editor. Handbook of methods for oxygen radical research. Boca Raton, FL: CRC Press; p. 237–42
Cyriac B, Eswaran K (2016) Anti- hyperglycemic effect of aqueous extract of Kappaphycus alvarezii (Doty) Doty ex. P. Silva in alloxan-induced diabetic rats. J Appl Phycol 28:2507–2513
Đorđević M, Mihailović M, Arambašić et al (2017) Methanol extract protects red blood cells from oxidative damage in streptozotocin-induced diabetic rats. J Ethnopharmacol 202:172–183
Farombi EO et al (2000) Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-a Garcinia kola seed extract. Food Chem Toxicol 38:535–541
Green LC et al (1982) Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal Biochem 126:131–138
Ibrahim HO et al (2018) Antidiabetic and haematological effects of Chrysophyllum albidum supplemented diet on streptozotocin induced diabetic rats. J Appl Life Sci Int 20(2):1–17
Idowu GP, Obuotor EM, Onajobi FD (2021) In vitro and in silico investigation of cholinesterase inhibition and anti-radical properties of Solanum macrocarpon leaf extracts: a preliminary anti-alzheimer’s study. Alzheimer’s Dement 17(Suppl 9). https://doi.org/10.1002/alz.049605
International Diabetes Federation (2021) The IDF Diabetes Atlas, 10th edn. Belgium
Jacobs NJ, Van Denmark PJ (1960) Determination of triglycerides. Arch Biochem Biophys 88:250–255
Jagannathan R, Neves JS, Dorcely B et al (2020) The oral glucose tolerance test: 100 years later. Diabetes Metab Syndr Obes 3787–3805
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Karigidi KO, Olaiya CO (2020a) Antidiabetic activity of corn steep liquor extract of Curculigo pilosa and its solvent fractions in streptozotocin-induced diabetic rats. J Tradit and Complement Med 10:555–564
Karigidi KO, Olaiya CO (2020b) Curculigo pilosa mitigates against oxidative stress and structural derangements in pancreas and kidney of streptozotocin-induced diabetic rats J Complement Integrat Med; 20190217
Karigidi KO, Akintimehin ES, Omoboyowa DA, Adetuyi FO, Olaiya CO (2020) Effect of Curculigo pilosa supplemented diet on blood sugar, lipid metabolism, hepatic oxidative stress and carbohydrate metabolism enzymes in streptozotocin-induced diabetic rats. J Diabetes Metab Disord 19:1173–1184
Komlaga G, Sam GH, Dickson RA, Mensah MLK, Fleischer TC (2014) Pharmacognostic studies and antioxidant properties of the leaves of Solanum macrocarpon. J Pharm Sci Res 6:1–4
Lenzen S (2017) Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic beta-cells. Biochim Biophys Acta Gen Subj 1861:1929–1942
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Omoboyowa DA, Karigidi KO, Aribigbola TC (2020) Bridelia ferruginea Benth leaves attenuates diabetes nephropathy in STZ-induced rats via targeting NGAL/KIM-1/cystatin c gene. Clin Phytosci 6, 63 (2020)
Oyesola OA, Sampson II, Augustine AA, Adejoke OB, Taiwo GE (2022) Comparison of phytochemical constituents of ethanol leaf extracts of Solanum macrocarpon and Vernonia amygdalina. Asian J Trop Biotechnol 19:6–10
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Vellapandian CRR, Ramachandram DS (2022) Renoprotective activity of anethole- rich fraction from aromatic herbs on junk food induced diabetic nephropathy in rats. J Diabetes Metab Disord. https://doi.org/10.1007/s40200-022-01101-4
Funding
None.
Author information
Authors and Affiliations
Contributions
OJA designed, performed experiment and wrote the first draft of manuscript, KOK performed experiment and statistical analysis of data, MOA performed experiment and statistical analysis of data COO designed, supervised and reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anjorin, O.J., Karigidi, K.O., Agunloye, M.O. et al. Antidiabetic effects of fractions of methanol extract of Solanum macrocarpon Linn. On streptozotocin – induced diabetic male Wistar rats. Vegetos (2023). https://doi.org/10.1007/s42535-023-00751-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42535-023-00751-w