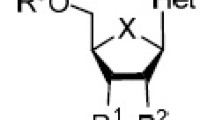Abstract
Ribavirin is a broad antiviral compound with demonstrated activity against herpes simplex virus (HSV), human immunodeficiency virus HIV-1, influenza virus, respiratory syncytial virus, and hepatitis C virus, among other viruses. However, routine clinical use of ribavirin is limited because this compound is considerably cytotoxic. Herein, we describe the design, synthesis, and antiviral activity of new nucleoside ribavirin analogs based on the following: (1) ring bioisosterism of a 1,2,4-triazole for a 1,2,3-triazole; (2) amide group exchange for other substituents, such as c-propyl, methyl carboxylate, or trifluoromethyl groups; and (3) the ribofuranose remained linked to the triazole ring. Compounds 5a–c were obtained with yields of 65–36 % and tested against Influenza A and HSV-1 replication as well as reverse transcriptase (RT) from human immunodeficiency virus type 1 (HIV-1 RT). Compound 5b (R = CO2CH3) was the most effective analog, with IC50 values 14 and 3.8 μM for Influenza A and HIV-1 RT, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ribavirin is a broad antiviral compound that targets viral DNA/RNA polymerases and cellular enzymes (De Clercq, 2004; Magden et al., 2005). Its activity has been demonstrated for herpes simplex virus (HSV) (De Clercq, 2004), human immunodeficiency virus (HIV-1) (De Clercq, 2004), influenza virus (Saladino et al., 2010), respiratory syncytial virus (RSV) (Krilov, 2011), and hepatitis C virus (HCV) (Sarrazin et al., 2012), among other viruses. However, routine clinical use of ribavirin is limited because it is considerably cytotoxic (De Clercq, 2004; Magden et al., 2005). This drug is primarily used in a combinatory therapy for treating HCV infection with α-interferon (Sarrazin et al., 2012) and in severe cases of acute respiratory infection from RSV (Krilov, 2011). More recently, given the emergence of multidrug-resistant influenza strains since the 2009 pandemics, clinical trials have evaluated the potency of ribavirin and neuraminidase inhibitor (NAIs) combinations (Kim et al., 2011). Therefore, the ribavirin chemical structure can be an interesting prototype for developing novel antiviral compounds.
A series of 4H-1,2,4-triazole 3,4,5-trisubstituted derivatives was investigated for antiretroviral activity against HIV-1 reverse transcriptase enzyme (HIV-1 RT). The key interactions between the triazoles and HIV-1 RT were identified by X-ray crystallography (Kirschberg et al., 2008). Girardet and co-workers described synthesis and the antiviral activities of 1,2,4-triazoles derivatives against several non-nucleoside reverse transcriptase inhibitor (NNRTI)-resistant strains of HIV-1. Several such compounds exhibited potent antiviral activities against efavirenz- and nevirapine-resistant viruses with K103N and/or Y181C mutations or a Y188L mutation (Rosa et al., 2006).
The 1,2,4-triazolacetamide derivatives were evaluated for inhibitory effect on HIV-1 RT. This study identified a series of triazoles with inhibitory activity against wild-type HIV-1 and the viral strains with a K103N mutation (Zhan et al., 2011). Chrysina and co-workers confirmed the bioisosteric relationship between the amide moiety and the 1,2,3-triazole ring (Chrysina et al., 2009).
The 1,2,3-triazole and its derivatives can be synthesized using “click chemistry” and have been widely used in medicinal chemistry, such as in the pathogenesis of Alzheimer’s disease (as IDO inhibitors) (Huang et al., 2011) as well as for antitubercular (Boechat et al., 2011a; Jordão et al., 2011a), antifungal (Aher et al., 2009), antibacterial (Wang et al., 2010), antileishmanial (Ferreira et al., 2007; Coghi et al., 2008), trypanocidal (Júnior et al., 2009; Silva et al., 2008), and anti-cancer (Yan et al., 2010) activities.
Triazole scaffolds show significant biological potential for developing novel antiviral agents to treat various viral diseases (Kharb et al., 2011; Jordão et al., 2009, 2011b; Pérez-Castro et al., 2007) in the future, including through in vitro inhibitory profiles against HIV-1 RT (Piotrowska et al., 2012; Silva et al., 2009).
Synthetic methodologies for preparing triazole and nucleoside derivatives have been described in the literature (Kharb et al., 2011; Amblard et al., 2009; Shawali, 2010). Preparing 1,2,3-triazoles through 1,3-dipolar cycloaddition using various acetylenes and an azido group connected to a sugar unit is a widely used method for synthesizing triazole nucleosides and nucleotides with biological activity (Chrysina et al., 2009; Pérez-Castro et al., 2007; El Akri et al., 2007; Elayadi et al., 2012; Rocha et al., 2012; Ferreira et al., 2010a, b; Hou et al., 2011).
We have prepared bioactive triazoles using the standard medicinal chemistry such as isosteric replacement of functional groups and ring bioisosterism (Boechat et al., 2011a, b; Ferreira et al., 2007) (Fig. 1). Herein, we have designed new nucleosides 5a–c, which are ribavirin analogs with potential antiviral activity. Such derivatives were based on ring bioisosterism wherein a 1,2,3-triazole substitutes for the 1,2,4-triazole. We also investigated the different groups bound to a 1,2,3-triazole ring instead of the ribavirin amide group. The selected substituents were c-propyl, methyl carboxylate, and trifluoromethyl.
To synthesize nucleosides 5a–c, the respective triazoles were first synthesized and then coupled to the ribose sugar moiety. For the last step, we used the methodology described by de Souza and co-workers wherein the nitrogen base is coupled to the sugar group (Matta et al., 1996, 1999).
Importantly, the ribofuranose moiety remains protected based on previous docking studies that described optimal intermolecular (docking) energies. Moreover, in a previous work we have identified a better performance of protected nucleoside when assayed against virus (Silva et al., 2009).
Results and discussion
Chemistry
To prepare the target compounds 5a–c using the synthetic route described herein (Scheme 1), we began with (azidomethyl)benzene (2) that had previously been generated by nucleophilic substitution of (chloromethyl)benzene (1) with sodium azide and then reacted (2) with terminal alkynes to generate the 1-benzyl-1H-1,2,3-triazoles (3a–c) (Pagliai et al., 2006). Click chemistry reaction conditions involved using CuSO4·5H2O as a catalyst that is fully controlled for reaction regioselectivity, and only the 1,4-regioisomers were generated (Lu and Gervay-Hague, 2007; Kolb et al., 2001).
To synthesize the 1H-1,2,3-triazoles (4a–c), the respective benzyl-1H-1,2,3-triazoles (3a–c) were treated with palladium hydroxide on carbon under hydrogen flow, which generated the products (Estermam and Seebach, 1988; Kacprzak, 2005). The structure of compounds 3b and 4a were characterized using X-ray crystallography by our group (Boechat et al., 2010).
Compounds 5a–c were prepared in accordance with the methodology described in the literature (Matta et al., 1996, 1999). The 1H-1,2,3-triazole (4a–c) derivatives were silylated with N,O-bis-(trimethylsilyl)trifluoroacetamide (BSTFA) containing trimethylchlorosilane (TMS-Cl). The corresponding trimethylsilyl derivatives formed were immediately condensed with 1-O-acetyl-2,3,5-tri-O-benzoyl-β-d-ribofuranose in a one-pot reaction under trimethylsilyl trifluoromethanesulfonate (TMSOTf). Good yields of the compounds were obtained and fully characterized by 1H, 13C, and 19F nuclear magnetic resonance (NMR), IR, HRMS as well as elemental analysis (CHN).
The synthetic route was initiated by preparing a key intermediate via a reaction between (chloromethyl)benzene (1) and sodium azide to produce the (azidomethyl)benzene (2), as a yellow oil with a 99 % yield. Fourier transform infrared spectroscopy (FTIR) analysis showed a strong absorption band at 2113 cm−1, which is characteristic of the stretching vibrations for the N3 bond. The IR and the 1H NMR confirm the chemical structure of the intermediate 2 as described in literature (Ankati and Biehl, 2009), (Campbell-Verduyn et al., 2009). The azide was used in the next step without purification to prevent degradation.
Synthesis of 1-benzyl-1H-1,2,3-triazoles (3a–c) through a 1,3-dipolar cycloaddition reaction between the appropriate acetylenes, benzyl azide (2), and sodium ascorbate and catalyzed by Cu(I) was performed at 25 °C and the compounds protected from light because the aromatic azides are photosensitive. After purification in a flash-type column, compounds 3a–c were produced as white or yellow crystals with yields from 86 to 31 %. FTIR of 3a–c demonstrated the absence of stretching vibrations from an azide group. In the 1H NMR spectra, signals from the respective protons were observed as a singlet at 8.99–7.86 ppm, which corresponds to the triazole ring, whereas signals that correspond to protons in the phenyl moiety appear as a multiplet at 7.41–7.35 ppm, and the benzylic CH2 group was observed at 5.69–5.50 ppm. The 3b derivative was described by our group previously and showed characteristic 1H and 13C NMR signals at 3.82 and 160.50 ppm due to the methyl group and ester carbonyl, respectively, and the melting points (m.p.) value is in accordance with the literature (Boechat et al., 2010).
The 19F NMR spectrum for compound 3c showed a characteristic signal at −59.5 ppm that confirms the trifluoromethyl group.
The 1-benzyl-1H-1,2,3-triazole (3a–c) derivatives were reduced to the respective 1H-1,2,3-triazoles (4a–c) with palladium hydroxide on carbon under hydrogen flow in PARR Reactor hydrogenator for 72 h at 25 °C. The compounds were purified by silica gel flash-type column chromatography, and the triazoles (4a–c) were produced as white or light-yellow crystals at yields from 76 to 40 %.
The structures were confirmed by 1H, and 13C NMR, which did not exhibit signals related to a benzyl group. The 4a derivative was described by our group previously and the m.p. value is in accordance with the literature (Boechat et al., 2010). Compound 4c was directly used in the next step without purification to prevent degradation and was characterized by 1H NMR and mass spectroscopy (MS).
To prepare the nucleosides (5a–c), a modified procedure was used that included the 1H-1,2,3-triazoles (4a–c) with BSTFA/TMS-Cl under an argon atmosphere at 70 °C for 6 h. This procedure yielded the corresponding trimethylsilyl derivatives, which were immediately condensed with ribofuranose in a one-pot reaction under TMSOTf. This procedure generates acceptable yields (65–35 %) of the corresponding protected nucleosides. Spectroscopic analysis confirmed the formation of such products.
The anomeric protons of nucleosides 5a–b were identified by the peaks at 6.65 and 6.79 ppm, respectively (d, J = 3.1 Hz), and for 5c a double duplet at 5.76 ppm (t, J = 5.9 Hz) was observed because of H–F couples. The 19F NMR spectrum for compound 5c showed a characteristic signal at −74.6 ppm that confirmed the trifluoromethyl group.
Molecular modeling
The target compounds 5a–c were modeled in silico, and energy minimization was performed over 1,000 steps using the steepest descent method, Gasteiger–Hückel charges, a dielectric constant 80, and the Tripos force field. The structures were further optimized using the conjugated gradient method.
The crystal structure of HIV-1 RT (PDB entry: 3V4I) (Das et al., 2012) was used as the molecular target for ligand–enzyme docking simulations (Dias and Azebedo, 2008) by applying the MolDock algorithm (Thomsen and Christensen, 2006) implemented in Molegro Virtual Docker software (Molegro, 2009).
The optimum docking protocol was constructed by adding flexibility to the enzyme-binding pocket (“induced fit”). Each compound was docked with softened potentials (steric, hydrogen bonding, and electrostatic forces); the enzyme residues remained rigid in the default conformation. Next, the residue side chains that were sufficiently close to the compound to interact were energy minimized. The final step was energy minimization of the compounds.
Importantly, the docking simulations were performed with the protected form of the compounds i.e., the ribofuranose ring remained bound to the triazole ring through C–N coupling (Fig. 1); this was the form with the best intermolecular (docking) energies.
Antiviral activity of novel ribavirin analogs
Considering the broad range of viruses targeted by ribavirin (De Clercq, 2004), we tested the effects of the novel analogs of this compound against Influenza A and HSV-1 replication as well as the activity of HIV-1 RT. Shown in Table 1, our analogs inhibited Influenza A replication and RT activity with different potencies; compound 5b (R = CO2CH3) was the most effective analog, with IC50 values 14 and 3.8 μM for the two targets, respectively.
When we compared the antiviral data for ribavirin from the literature using assay conditions similar to ours (Markland et al., 2000; Kornev et al., 2011; Fernandez-Larsson and Patterson, 1990), we found that our molecules are generally less cytotoxic and more potent against influenza A and RT. Ribavirin inhibits influenza A replication with an IC50 at approximately 30 μM (Markland et al., 2000; Kornev et al., 2011), which is comparable to our observations for compound 5a but 2.0- and 1.5-fold higher than the IC50 values for compounds 5b and 5c, respectively.
In regard to HIV-1, in its unphosphorylated, diphosphorylated, and triphosphorylated forms, ribavirin inhibits the enzyme RT with IC50 of 615, 81, and 112 μM (Fernandez-Larsson and Patterson, 1990), respectively. Thus, compound 5b is ~20-fold more potent against HIV-1 RT than the most active form of ribavirin (Fernandez-Larsson and Patterson, 1990) (Table 1).
It is described in the literature that ribavirin cytotoxicity varies for different cell types from 70 to ~500 μM (Kornev et al., 2011), whereas the CC50 values for our compounds were in the 550 μM range. Thus, our compounds tend to be in the low cytotoxicity range compared with their counterpart, ribavirin (Kornev et al., 2011).
Although the ribavirin analogs developed herein are superior to ribavirin in antiviral activity against influenza A and HIV-1 RT, other compounds with routine clinical use for such viruses, including oseltamivir carboxylate for influenza A and AZT-TP for HIV-1 RT, are more potent than our ribavirin analogs. Nevertheless, our molecules are less cytotoxic than AZT. As shown, HSV-1 replication was not affected by our ribavirin analogs, which may suggest that our compounds are more specific (Table 1).
The development of novel strategies to control HIV-1 and influenza A replication is an important task given the large economic and public health burden from such agents (Pasman, 2012). In particular, the emergence of multidrug-resistant strains of such agents further motivates the search for novel compounds (Pasman, 2012). The ribavirin mechanism of action may vary with inhibition of different viral agents, including inhibition of inosine monophosphate dehydrogenase (which would reduce GTP levels in the cells) and viral RNA polymerase and/or generation of an error catastrophe through its incorporation by viral RNA polymerase (De Clercq, 2004; Magden et al., 2005). Thus, our analogs are interesting for the development of novel anti-influenza and antiretroviral agents, which may have a mechanism of action mentioned above or another one. Considering the fact that the tested compounds were potent against HIV-1 RT, we performed in silico studies for further characterize the docking sites for our compound on HIV-1 RT.
For the interactions between HIV-1 RT and compounds 5a–c, docking scores, hydrogen bonds, and steric and van der Waals energy contributions are shown in Table 2. Three additional compounds were included: AZT, ribavirin, and zalcitabine. The first was used as the standard molecule in the experimental assays. Ribavirin was included because the work herein includes design of its analogs. Consequently, ribavirin is the primary reference compound. Zalcitabine is also an example of a known reverse transcriptase inhibitor. According to the relevant values, both compounds 5a and 5b interact better with the enzyme than compound 5c. Moreover, the correlation between the docking score, hydrogen bonds, and van der Waals energies is clear. This correlation was confirmed by the calculated cross-correlation matrix of the energy values. Figure 2 shows a detailed depiction of the interactions between the compounds and HIV-1 RT generated from docking calculations. Figure 3 shows a 2D-representation of the interactions between the compounds and enzyme (Stierand and Rarey, 2010). Notably, the intermolecular hydrogen bonds, which are shown as black dashed lines, are important and operate as “molecular anchors” to bind the compounds to the enzyme active site.
Conclusions
Three novel nucleosides of 1,2,3-triazole analogs were successfully synthesized via cyclization of azides and terminal alkyne using “click chemistry” with a 65–35 % yield. The nucleoside structures are supported by IR; 1H, 19F, and 13C NMR [DEPT, 1H-1H-COSY, HETCOR] spectroscopy; CHN and MS data.
Compound 5b was superior to ribavirin in terms of antiviral activity against influenza A replication and HIV-1 RT activity; however, it was less potent than other drugs, such as oseltamivir carboxylate and AZT-TP. Remarkably, the most effective compound tested against HIV-1 RT, molecule 5b, required Tyr115 in this enzyme structure for docking. This characteristic is consistent with another potent antiretroviral and anti-HSV-1 molecule which we previously described (Souza et al., 2009).
Finally, our results indicate that the chemical structure of our synthesized 1,2,3-triazole analogs can be interesting prototypes for development of novel antiretroviral and anti-influenza drugs.
Experimental section
The 1H, 13C, and 19F nuclear magnetic resonance (NMR) spectra were obtained generated at 400.00, 100.00, and 376.00 MHz, respectively, on a BRUKER Avance instrument equipped with a 5-mm probe and using tetramethylsilane as an internal standard.
The chemical shifts (δ) are reported in ppm and the coupling constants (J) in Hertz. The FTIR absorption spectra were recorded on a Shimadzu mode IR Prestige-21 spectrophotometer through KBr reflectance (cm−1). The electron ionization mass spectrometry (EI-MS, scan ES + Capilar (3.0 kV)/cone (30 V)/extractor (1 V)/RF lens (1.0 V)/source temperature (150 °C)/desolvation temperature (300 °C)) were recorded using a Micromass/Waters Spectrometer (model: ZQ-4000). The HRMS data were obtained using LC–MS—Bruker Daltonics MicroTOF (analyzer time of flight). The CHN data were obtained using a Perkin-Elmer 2400 CHN Analyzer. The (m.p.) were determined using a Büchi model B-545 apparatus. The Mini Bench Top Reactor PARR Model 4842 hydrogenator was used for the reactions for obtaining the compounds (4a–c). Thin layer chromatography (TLC) was performed using a silica gel F-254 Glass Plate (20 × 20 cm2). Column chromatography was performed using a silica gel 60 (0.040–0.063 mm). The remaining reagents and solvents used were analytical grade.
Procedure for preparing (azidomethyl)benzene (2)
These compounds were prepared by the reaction of (chloromethyl)benzene (1) (7.9 mmol) and NaN3 (11.8 mmol) in CH3CN (50 mL) and DMF (3 mL). The reaction mixture was kept under reflux with stirring for 24 h. The development of the reaction was followed by TLC. The mixture was diluted with 50 mL of H2O and extracted with CH2Cl2 (3 × 30 mL), and the combined organic extracts were washed with water (3 × 30 mL), dried over anhydrous MgSO4, and filtered; the solvent was removed by vacuum. The product was obtained as yellow oil with 99 % yield.
IR (KBr): 2113 (N3). 1H NMR (DMSO-d6, 400 MHz, δ in ppm): 7.31–7.41 (m, 5H), 4.34 (s, 2H). [lit. IR (KBr): 2098.3 (N3) (Ankati and Biehl, 2009). 1H NMR 7.25–7.43 (m, 5H), 4.35 (s, 2H) (Campbell-Verduyn et al., 2009)].
General procedure for preparing the compounds 3a–c
These compounds were prepared by the reaction of (azidomethyl)benzene (2) (22.5 mmol), with the appropriate acetylene (33.7 mmol) and l-ascorbic acid sodium salt (2.25 mmol), and of CuSO4·5H2O (0.225) mmol in a mixture of H2O/t-BuOH (1:1) (40 mL). The reaction mixture was kept under vigorous stirring at 25 °C for 24 h. The development of the reaction was followed by TLC. After cooling, the reaction mixture was poured into ice-cold water (50 mL). The precipitate was filtered and dried. The residual crude product was purified via silica gel column chromatography using a gradient mixture of hexane/ethyl acetate. The compounds 3a–c were obtained as a white solid with 86–31 % yield.
1-Benzyl-4-cyclopropyl-1H-1,2,3-triazole (3a)
Yield: 84 %. m.p. 61.2–62.4 °C. IR (KBr): 3462; 3076; 1635; 1215; 1047; 1028; 1012; 719. 1H NMR (DMSO-d6, 400 MHz): 0.67–0.71 (m, 2H, CH 2 cyclopropyl); 0.89–0.85 (m, 2H, CH 2 cyclopropyl); 1.88–1.95 (m, 1H, CH cyclopropyl); 5.50 (s, 2H, CH 2); 7.28–7.29 (m, 2H, H-Ph); 7.28–7.38 (m, 3H, H-Ph); 7.86 (s, 1H, CH triazole). 13C NMR (DMSO-d6, 100 MHz): 6.48 (CH cyclopropyl); 7.58 (2C, CH2 cyclopropyl); 52.6 (CH2); 120.8; 127.7; 127.9; 128.6; (all C-Ph); 136.1 (CH triazole); 149.2 (Cq triazole).
Methyl 1-benzyl-1H-1,2,3-triazole-4-carboxylate (3b)
Yield: 86 %. m.p. 104.1–106.6 °C (Lit. m.p. 104.0–107.0 °C). IR (KBr): 3431; 3111; 1724; 1541; 1230; 1047; 1020; 713. 1H NMR (DMSO-d6, 400 MHz): 3.82 (s, 3H, CO2CH 3); 5.66 (s, 2H, CH 2); 7.33–7.38 (m, 5H, H-Ph); 8.89 (s, 1H, CH triazole). 13C NMR (DMSO-d6, 100 MHz): 51.6 (CO2 CH3); 53.0 (CH2); 127.9; 128.2; 128.7; 129.1; (all C-Ph); 135.3 (CH triazole); 138.6 (Cq triazole); 160.5 (CO2CH3).
1-Benzyl-4-(trifluoromethyl)-1H-1,2,3-triazole (3c)
Yield: 31 %. m.p. 73.0–75.0 °C. IR (KBr): 3458; 3089; 1570; 1382; 1217; 1151; 1051; 997; 709. 1H NMR (DMSO-d6, 400 MHz): 5.69 (s, 2H, CH 2); 7.35–7.41 (m, 5H, H-Ph); 8.99 (s, 1H, CH triazole). 13C NMR (DMSO-d6, 100 MHz): 120.75 (q, J = 265 Hz, CF3); 125.6 (d, J = 2.5 Hz, CH triazole); 128.0; 128.3; 128.8; 135.0 (all C-Ph); 136.7 (CH triazole); 137.0 (Cq triazole). 19F NMR (DMSO-d6, 376 MHz): −59.5 (s, 3F, CF 3).
General procedure for preparing the compounds 4a–c
These compounds were prepared by the mixture of appropriate 1-benzyl-1H-1,2,3-triazole (3a–c) (18.6 mmol) and palladium hydroxide on carbon (20 wt.% loading, dry basis, matrix carbon, and wet support) (14.2 mmol) in a mixture of H2O/MeOH (1:1; 100 mL). The reaction remained under hydrogen flow for 72 h at 25 °C. The reaction mixture was filtered on Celite and extracted with CHCl3 (3 × 50 mL); the combined organic extracts were washed with water (3 × 30 mL), dried over anhydrous MgSO4, and filtered; the solvent was removed by vacuum. The residual crude product was purified via silica gel column chromatography using a gradient mixture of hexane/ethyl acetate. The compounds 4a–c were obtained as a white solid with 76–40 % yield.
4-Cyclopropyl-1H-1,2,3-triazole (4a)
Yield: 76 %. m.p. 54.8–55.2 °C, (Lit. m.p. 54.8–55.2 °C). IR (KBr): 3446; 2983; 1637; 1230; 1006; 995. 1H NMR (DMSO-d6, 400 MHz): 0.67–0.74 (m, 2H, CH 2 cyclopropyl); 0.90–0.91 (m, 2H, CH 2 cyclopropyl); 1.92–1.98 (m, 1H, CH cyclopropyl); 7.55 (s, 1H, CH triazole); 14.63 (s, 1H, NH). 13C NMR (DMSO-d6, 100 MHz): 6.31 (CH cyclopropyl); 7.79 (2C, CH2 cyclopropyl); 130.3 (CH triazole); 149.1 (Cq triazole).
Methyl 1H-1,2,3-triazole-4-carboxylate (4b)
Yield: 40 %. m.p. 125.7–127.1 °C. IR (KBr): 3446; 3136; 1714; 1354; 1240; 1211; 779. 1H NMR (DMSO-d6, 400 MHz): 3.82 (s, 3H, CO2CH 3); 8.52 (s, 1H, CH triazole); 15.7 (s, 1H, NH). 13C NMR (DMSO-d6, 100 MHz): 53.0 (CO2 CH3); 138.6(Cq triazole); 160.5 (CO2CH3).
4-(Trifluoromethyl)-1H-1,2,3-triazole (4c)
Yield: 48 %. IR (KBr): 3444; 3091; 1635; 1217; 1153; 709. 1H NMR (DMSO-d6, 400 MHz): 8.50 (s, 1H, CH triazole). 19F NMR (DMSO-d6, 376 MHz): −59.1 (s, 3F, CF 3). MS m/z [M + 1]+: 138.0.
General procedure for preparing the compounds 5a–c
These compounds were prepared by the reaction of appropriate 1H-1,2,3-triazoles (4a–c) (1.83 mmol), N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) (6.86 mmol), and 4.6 mmol of chlorotrimethylsilane (TMS-Cl) in CH3CN (4 mL). The reaction mixture was kept under argon atmosphere at 70 °C for 6 h. Thereafter, the reaction mixture was cooled to 25 °C, and a solution of 1-O-acetyl-2,3,5-tri-O-benzoyl-β-d-ribofuranose (1.83 mmol) and trimethylsilyl trifluoromethanesulfonate (TMSOTf) (0.915 mmol) in CH3CN (5 mL) was slowly added and remained under vigorous stirring for 24 h. The development of the reaction was followed by TLC. The resulting mixture was poured into ice-cold water (10 g) and neutralized with a saturated aqueous NaHCO3 solution. The solution was extracted with CH2Cl2 (3 × 50 mL), the combined organic layers were washed with water (3 × 20 mL), and then dried over anhydrous MgSO4. The solvent was removed under reduced pressure. The residual crude product was purified via silica gel column chromatography using a gradient mixture of hexane/ethyl acetate. The compounds 5a–c were obtained as a white solid with 65–35 % yield.
(2R,3R,4R)-2-((benzoyloxy)methyl)-5-(4-cyclopropyl-1H-1,2,3-triazol-1-yl)tetrahydrofuran-3,4-diyl dibenzoate (5a)
Yield: 65 %. m.p. 142.7–143.5 °C. IR (KBr): 3446; 2981; 1637; 1724; 1274; 1126; 1093; 709. 1H NMR (DMSO-d6, 400 MHz): 0.68–0.72 (m, 2H, CH 2 cyclopropyl); 0.88–0.92 (m, 2H, CH 2 cyclopropyl); 1.92–1.99 (m, 1H, CH cyclopropyl); 4.55 (dd, J = 4.6, 12.2 Hz, 1H, CH 2); 4.66 (dd, J = 4.6, 12.2 Hz, 1H, CH 2); 4.91–4.95 (m, 1H, H-4′); 6.10 (t, J = 6.0 Hz, H-3′); 6.22 (dd, J = 3.1, 5.2 Hz, 1H, H-2′); 6.65 (d, J = 3.1 Hz, H-1′); 7.44–7.52 (m, 2H, H-3″, H-5″); 7.63–7.69 (m, 1H, H-4′); 7.90–7.95 (m, 2H, H-2″, H-6″); 8.12 (s, 1H, CH triazole). 13C NMR (DMSO-d6, 100 MHz): 6.27 (CH); 7.52 (CH2); 7.57 (CH2); 63.3 (2C, CH2); 71.0 (C-3′); 74.2 (C-2′); 79.5 (C-4′); 88.9 (C-1′); 120.7 (CH triazole); 128.2, 128.4, 129.0 (C-1″); 128.6, 128.6, 128.7 (C-3″, C-5″); 129.2, 129.2, 129.3 (C-2″, C-6″); 133.4, 133.8, 133.9 (C-4″); 149.4 (Cq triazole); 164.3 (C=O); 164.5 (C=O); 165.3 (C=O). Anal. Calcd. for C31H27N3O7: C, 67.26; H, 4.92; N, 7.59; Found: C, 67.52; H, 5.20; N, 7.64. HRMS (ESI) m/z [M + Na] Calcd.: 576.1747; Found: 576.1745
(2R,3R,4R)-2-((benzoyloxy)methyl)-5-(4-(methoxycarbonyl)-1H-1,2,3-triazol-1-yl)tetrahydrofuran-3,4-diyl dibenzoate (5b)
Yield: 35 %. m.p. 190.0–192.0 °C. IR (KBr): 3429; 3124; 1720, 1600; 1274; 1126; 1103; 711. 1H NMR (DMSO-d6, 400 MHz): 3.85 (s, 3H, CO2CH 3); 4.60 (dd, J = 4.7, 12.3 Hz, 1H, CH 2); 4.72 (dd, J = 4.7, 12.3 Hz, 1H, CH 2); 4.98–5.01 (m, 1H, H-4′); 6.15 (t, J = 5.8 Hz, H-3′); 6.31 (dd, J = 3.1, 5.2 Hz, 1H, H-2′); 6.79 (d, J = 3.1 Hz, H-1′); 7.46–7.52 (m, 1H, H-4″, H-5″); 7.65–7.70 (m, 1H, H-3″); 7.91–7.95 (m, 2H, H-2″, H-6″); 9.11 (s, 1H, CH triazole). 13C NMR (DMSO-d6, 100 MHz): 51.9 (CO2 CH3); 63.2 (2C, CH2); 70.9 (C-3′); 74.5 (C-2′); 80.1 (C-4′); 89.6 (C-1′); 128.7 (CH triazole); 129.2, 129.3, 129.4 (C-1″); 128.6, 128.8, 129.0 (C-2″); 128.4, 128.2, (C-4″); 133.5, 133.9, 134.1 (C-3″); 139.0 (Cq triazole); 160.3 (CO2CH3), 164.3 (C=O); 164.6 (C=O); 165.3 (C=O). Anal. Calcd. for C30H25N3O9: C, 63.04; H, 4.41; N, 7.35; Found: C, 62.80; H, 4.58; N, 7.54. HRMS (ESI) m/z [M + Na] Calcd.: 594.1489; Found: 594.1483.
(2R,3R,4R)-2-((benzoyloxy)methyl)-5-(4-(trifluoromethyl)-1H-1,2,3-triazol-1-yl)tetrahydrofuran-3,4-diyl dibenzoate (5c)
Yield: 35 %. m.p. decomp. IR (KBr): 3435; 3109; 1680; 1123; 1093; 1130; 709. 1H NMR (DMSO-d6, 400 MHz): 4.51–4.63 (m, 2H, CH 2), 4.66–4.69 (m, 1H, H-2′or H-3′ or H-4′), 5.75–5.77 (m, 1H, H-2′or H-3′ or H-4′), 5.82–5.84 (m, 1H, H-2′or H-3′ or H-4′), 5.89 (t, J = 5.9 Hz, 1H, H-1′), 7.40–7.53 (m, 6H, OBz), 7.61–7.68 (m, 3H, OBz), 7.85 (d, J = 8.0 Hz, 2H, OBz), 7.93 (d, J = 4.0 Hz, 2H, OBz), 8.02 (d, J = 8.0 Hz, 2H, OBz), 10.62 (d, J = 6.1 Hz, 1H, CH triazole). 13C NMR (DMSO-d6, 100 MHz): 63.47 (2C, CH2), 70.50 (C-1′), 73.69 (C-2′), 78.03 (C-3′), 82.60 (C-4′), 115.42 (q, J = 286.4 Hz, CF3), 128.41 (d, J = 5.8 Hz, CH triazole), 128.59, 128.74, (C-4″), 129.26, 129.19, 129.16 (C-1″), 133.72, 133.44 (C-3″), 133.85 (Cq triazole), 156.79 (C=O), 164.54 (C=O), 165.37 (C=O). 19F NMR (DMSO-d6, 376 MHz): −74.6 (s, 3F, CF 3).
Compounds
Compounds 5a–c were diluted in 100 % dimethylsulfoxide (DMSO) and stored at −20 °C. The concentration used for the assays was below 0.01 %
Cells and virus
Vero (African green monkey kidney cells) and MDCK (Mardin–Darby canine kidney cells) cells were cultured with Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Grand Island, NY, USA), and a T-lymphoid cell line (Supt1) was cultured in RPMI1640 (GIBICO). The cultures were supplemented with 10 % fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 U/mL penicillin as well as 100 μg/mL streptomycin and incubated at 37 °C in 5 % CO2.
The viral stocks were prepared as we have described previously (Souza et al., 2010; Denizot and Lang, 1986). The Vero cells were infected with HSV-1 (AR-29 strain) in FBS-free DMEM, and the MDCKs were infected with influenza A (A/England/42/1972 strain) in DMEM with 0.2 % serum albumin and trypsin at 4 μg/mL (inoculation medium). After a 1-h inoculation period, the cells were washed using PBS, and DMEM with 2 % FBS or inoculation medium was added to HSV-1- or influenza A-infected cells, respectively. At 48-h post-infection (p.i.), the cells were lysed using three cycles of freezing and thawing then centrifuged at 1.500×g and 4 °C for 20 min; the supernatant was collected and stored at −70 °C for further studies.
Cytotoxicity assay
In 96-multiwell plates (1 × 104/well), Vero, MDCK, or Supt1 cells were treated with different concentrations of compounds 5a–c for 72 h; then 50 μL of a 1 mg/mL solution comprising 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma) was added to cells diluted in DMEM without serum. MTT was removed after 3 h, 50 μL of acid–isopropanol (0.04 N HCl in isopropanol) was added and the optical density (OD) was determined using an automatic plate reader with a 570 nm test wavelength and 690 nm reference wavelength (Kuo et al., 2001). The cytotoxic concentration in 50 % (CC50) was calculated by linear regression analysis of the dose–response curves generated from the data.
Anti-HSV-1 assays
Yield reduction assay
In 24-well plates (1 × 105/well), Vero cell monolayers were infected with HSV-1 at a MOI 5 for 1 h at 37 °C. The cells were washed and treated with different concentrations of compounds 5a–c in DMEM with 2 % FBS. After 20 h, the cells were lysed, the cellular debris was cleared by centrifugation, and virus titers at the supernatant were determined using a plaque-forming assay and the Vero cells, which is described in the following subsection. The HSV-1 yield was also generated at different concentrations of acyclovir (ACV).
Plaque-forming assay
In 6-well plates (3 × 105/well), Vero cell monolayers were exposed to the supernatant from the yield reduction assay for 1 h at 37 °C. Next, the residual viruses were washed out, and DMEM with 5 % FBS and 1 % methylcellulose (Fluka) (overlay medium) was added to cells. After 72 h at 37 °C, the monolayers were fixed using 10 % formaldehyde in PBS and stained with a 0.1 % crystal violet solution in 70 % methanol, and the virus titers were calculated by scoring the plaque-forming units (PFU) (Souza et al., 2009). The inhibitory concentration in 50 % (CC50) was calculated by linear regression analysis of the dose–response curves generated from the data.
Anti-HIV-1 RT inhibitory activity
The inhibitory effect of compounds 5a–c on the RTHXB2 RNA-dependent DNA polymerase (RDDP) activity was evaluated using purified recombinant HIV-1 enzyme as reported (Souza et al., 2009) with minor modifications. RDDP activity was assayed in 50 mM Tris HCl (pH 7.8), 6 mM MgCl2, 1 mM dithiothreitol, 50 mM KCl, 5 μM dTTP, 80 μg/mL poly(rA) oligo(dT)12–18 template primer (Pharmacia, Piscataway, NJ, USA), and 3 U of enzyme (one unit is the enzyme concentration that incorporates 1 pmol of dTTP per minute per mg of enzyme at 37 °C under the standard assay conditions). The isotopic dilutions for the reactions were prepared at the ratio 2 μCi [³H]dTTP (49 Ci/mmol)/2.7 μM dTTP. The reactions were initiated at 37 °C, incubated for 30 min, and arrested with 0.5 M EDTA. The precipitate was collected on a Whatman DE 81 filter and washed with 0.1 M sodium phosphate; the incorporated nucleotides were measured by liquid scintillation (Packard tri carb 2100). The inhibitory concentration in 50 % (CC50) was calculated by linear regression analysis of the dose–response curves generated from the data. The polymerization reactions were performed with and without as well as various concentrations of the tested compounds and with and without AZT-TP.
Anti-influenza assay
To evaluate the effect of compounds 5a–c on Influenza A replication, MDCK cells were seeded in a 6-well plate and grown to 80 % confluence in DMEM with 10 % FBS. Prior to infection, the cells were washed two times with inoculation medium. Then, Influenza A was added in inoculation medium at an MOI 5 for 1 h at 35 °C and 5 % CO2. Thereafter, the cells were washed to remove unbound virus and treated with different concentrations of the compounds or with NAI oseltamivir carboxylate as a reference compound. Influenza A-infected cells were incubated with the compounds for 3 days in inoculation medium; the culture supernatant was harvested and RNA was extracted using the QIAmp Viral RNA mini kit (Qiagen, CA) in accordance with the manufacturer’s instructions. The purified RNA was subjected to a one-step real-time RT-PCR to detect a viral matrix gene in accordance with the CDC–WHO protocol for detecting this pandemic virus (WHO, 2009).
References
Aher NG, Pore VS, Mishra NN, Kumar A, Shukla PK, Sharma A, Bhat MK (2009) Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues. Bioorg Med Chem Lett 19(3):759–763
Amblard F, Cho JH, Schinazi RF (2009) Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem Rev 109(9):4207–4220
Ankati H, Biehl E (2009) Microwave-assisted benzyne-click chemistry: preparation of 1H-benzo[d][1,2,3]triazoles. Tetrahedron Lett 50:4677–4682
Boechat N, Ferreira MLG, Bastos MM, Camilo ALS, Wardell SMSV, Wardell JL, Tiekink ERT (2010) Crystal and molecular structures of two triazole derivatives: 4-cyclopropyl-4,5-dihydro-1H-1,2,3-triazole and methyl 1-benzyl-1H-1,2,3-triazole-4-carboxylate. J Chem Crystallogr 40(12):1137–1141
Boechat N, Ferreira VF, Ferreira SB, Ferreira MLG, Silva FC, Bastos MM, Costa MS, Lourenco MCS, Pinto AC, Krettli AU, Aguiar AC, Teixeira BM, Silva NV, Martins PRC, Bezerra FAFM, Camilo ALS, Silva GP, Costa CCP (2011a) Novel 1,2,3-triazole derivatives for use against Mycobacterium tuberculosis H37Rv (ATCC 27294) strainz. J Med Chem 54(17):5988–5999
Boechat N, Pinheiro LCS, Santos-Filho OA, Silva IC (2011b) Design and synthesis of new N-(5-trifluoromethyl)-1H-1,2,4-triazol-3-yl benzenesulfonamides as possible antimalarial prototypes. Molecules 16(9):8083–8097
Campbell-Verduyn LS, Mirfeizi L, Dierckx RA, Elsinga PH, Feringa BL (2009) Phosphoramidite accelerated copper(I)-catalyzed [3 + 2] cycloadditions of azides and alkynes. Chem Commun 16:2139–2141
Chrysina ED, Bokor E, Alexacou KM, Charavgi MD, Oikonomakos GN, Zographos SE, Leonidas DD, Oikonomakos NG, Laszlo S (2009) Amide-1,2,3-triazole bioisosterism: the glycogen phosphorylase case. Tetrahedron 20(6-8):733–740
Coghi P, Vaiana N, Pezzano MG, Rizzi L, Kaiser M, Brun R, Romeo S (2008) Parallel synthesis and antileishmanial activity of ether-linked phospholipids. Bioorg Med Chem Lett 18(16):4658–4660
Das K, Martinez SE, Bauman JD, Arnold E (2012) HIV-1 reverse transcriptase complex with DNA and nevirapine reveals non-nucleoside inhibition mechanism. Nat Struct Mol Biol 19(2):253–259
De Clercq E (2004) Antiviral drugs in current clinical use. J Clin Virol 30(2):115–133
Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89(2):271–277
Dias R, Azebedo WF Jr (2008) Molecular docking algorithms. Curr Drug Targets 9(12):1040–1047
El Akri K, Bougrin K, Balzarini J, Faraj A, Benhida R (2007) Efficient synthesis and in vitro cytostatic activity of 4-substituted triazolyl-nucleosides. Bioorg Med Chem Lett 17(23):6656–6659
Elayadi H, Mesnaoui M, Korba BE, Smietana M, Vasseur JJ, Secrist JA, Lazrek HB (2012) Preparation of 1,4-disubstituted-1,2,3-triazolo ribonucleosides by Na2CuP2O7 catalyzed azide-alkyne 1,3-dipolar cycloaddition. ARKIVOC viii:76–89
Estermam H, Seebach D (1988) Diastereoselektive alkylierung von 3-aminobutansäure in der 2-stellung. Helv Chim Acta 71(7):1824–1839
Fernandez-Larsson R, Patterson JL (1990) Ribavirin is an inhibitor of human immunodeficiency virus reverse transcriptase. Mol Pharmacol 38(6):766–770
Ferreira SB, Costa MS, Boechat N, Bezerra SRJ, Genestra MS, Canto-Cavalheiro MM, Kover WB, Ferreira VF (2007) Synthesis and evaluation of new difluoromethyl azoles as antileishmanial agents. Eur J Med Chem 42(11–12):1388–1395
Ferreira ML, Souza MVN, Wardell SMSV, Wardell JL, Vasconcelos TRA, Ferreira VF, Lourenço MCS (2010a) Synthesis and antitubercular evaluation of new bis-1,2,3-triazoles derived from d-mannitol. J Carbohydr Chem 29(6):265–274
Ferreira SB, Sodero ACR, Cardoso MFC, Lima ES, Kaiser CR, Silva FP Jr, Ferreira VF (2010b) Synthesis, biological activity, and molecular modeling studies of 1H-1,2,3-triazole derivatives of carbohydrates as α-glucosidases inhibitors. J Med Chem 53(6):2364–2375
Hou S, Liu W, Ji D, Zhao Z (2011) Efficient synthesis of triazole moiety-containing nucleotide analogs and their inhibitory effects on a malic enzyme. Bioorg Med Chem Lett 21(6):1667–1669
Huang Q, Zheng M, Yang S, Kuang C, Yu C, Yang Q (2011) Structure–activity relationship and enzyme kinetic studies on 4-aryl-1H-1,2,3-triazoles as indoleamine 2,3-dioxygenase (IDO) inhibitors. Eur J Med Chem 46(11):5680–5687
Jordão AK, Afonso PP, Ferreira VF, Souza MCBV, Almeida MCB, Beltrame CO, Paiva DP, Wardell SMSV, Wardell JL, Tiekink ERT, Damaso CR, Cunha AC (2009) Antiviral evaluation of N-amino-1,2,3-triazoles against Cantagalo virus replication in cell culture. Eur J Med Chem 44(9):3777–3783
Jordão AK, Ferreira VF, Souza TML, Faria GGS, Machado V, Abrantes JL, Souza MCBV, Cunha AC (2011a) Synthesis and anti-HSV-1 activity of new 1,2,3-triazole derivatives. Bioorg Med Chem 19(6):1860–1865
Jordão AK, Sathler PC, Ferreira VF, Campos VR, Souza MCBV, Castro HC, Lannes A, Lourenco A, Rodrigues CR, Bello ML, Lourenco MCS, Carvalho GSL, Almeida MCB, Cunha AC (2011b) Synthesis, antitubercular activity, and SAR study of N-substituted-phenylamino-5-methyl-1H-1,2,3-triazole-4-carbohydrazides. Bioorg Med Chem 19(18):5605–5611
Júnior ENS, Moura MABF, Pinto AV, Pinto MCFR, Souza MCBV, Araújo AJ (2009) Cytotoxic, trypanocidal activities and physicochemical parameters of nor-β-lapachone-based 1,2,3-triazoles. J Braz Chem Soc 20(4):635–643
Kacprzak K (2005) Efficient one-pot synthesis of 1,2,3-triazoles from benzyl and alkyl halides. Synlett 6:943–946
Kharb R, Yar MS, Sharma PC (2011) Recent advances and future perspectives of triazole analogs as promising antiviral agents. Mini Rev Med Chem 11(1):84–96
Kim WY, Young Suh G, Huh JW, Kim SH, Kim MJ, Kim YS, Kim HR, Ryu YJ, Han MS, Ko YG, Chon GR, Lee KH, Choi SH, Hong SB, Korean Society of Critical Care Medicine H1N1 Collaborative (2011) Triple-combination antiviral drug for pandemic H1N1 influenza virus infection in critically ill patients on mechanical ventilation. Antimicrob Agents Chemother 55(12):5703–5709
Kirschberg TA, Balakrishnan M, Huang W, Hluhanich R, Kutty N, Liclican AC, McColl DJ, Squires NH, Lansdon EB (2008) Triazole derivatives as non-nucleoside inhibitors of HIV-1 reverse transcriptase-structure-activity relationships and crystallographic analysis. Bioorg Med Chem Lett 18(3):1131–1134
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl 40(11):2004–2021
Kornev AB, Peregudov AS, Martynenko VM, Balzarini J, Hoorelbeke B, Troshin PA (2011) Synthesis and antiviral activity of highly water-soluble polycarboxylic derivatives of [70]fullerene. Chem Commun 47(29):8298–8300
Krilov LR (2011) Respiratory syncytial virus disease: update on treatment and prevention. Expert Rev Anti Infect Ther 9(1):27–32
Kuo YC, Chen CC, Tsai WJ, Ho YH (2001) Regulation of herpes simplex virus type 1 replication in Vero cells by Psychotria serpens: relationship to gene expression, DNA replication, and protein synthesis. Antiviral Res 51(2):95–109
Lu Y, Gervay-Hague J (2007) Synthesis of C-4 and C-7 triazole analogs of zanamivir as multivalent sialic acid containing scaffolds. Carbohydr Res 342(12–13):1636–1650
Magden J, Kääriäinen L, Ahola T (2005) Inhibitors of virus replication: recent developments and prospects. Appl Microbiol Biotechnol 66(6):612–621
Markland W, McQuaid TJ, Jain J, Kwong AD (2000) Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother 44(4):859–866
Matta AD, Bernardino AMR, Romeiro GA, Oliveira MRP, Souza MCBV, Ferreira VF (1996) Nucleosides having quinolones derivatives as nitrogenated base: regiospecific and stereospecific ribosylation of 3-carbethoxy-1,4-dihydro-4-oxoquinoline. Nucleosides Nucleotides 15(4):889–898
Matta AD, Santos CVB, Pereira HS, Frugulhetti ICPP, Oliveira MRP, Souza MCBV, Moussatché N, Ferreira VF (1999) Synthesis of novel nucleosides of 4-oxoquinoline-3-carboxylic acid analogues. Heteroatom Chem 10(3):197–202
Molegro Virtual Docker/4.0 Molegro ApS: Aarhus, Denmark, 2009
Pagliai F, Pirali T, Gross ED, Brisco RD, Tron GC, Sorba G, Genazzani AA (2006) Rapid synthesis of triazole-modified resveratrol analogues via click chemistry. J Med Chem 49(2):467–470
Pasman L (2012) The complication of coinfection. Yale J Biol Med 85(1):127–132
Pérez-Castro I, Caamano O, Fernández F, García MD, López C, De Clercq E (2007) Synthesis of 4-substituted-1,2,3-triazole carbanucleoside analogues of ribavirin via click chemistry. Org Biomol Chem 5:3805–3813
Piotrowska DG, Balzarini J, Glowacka IE (2012) Design, synthesis, antiviral and cytostatic evaluation of novel isoxazolidine nucleotide analogues with a 1,2,3-triazole linker. Eur J Med Chem 47:501–509
Rocha DR, Santos WC, Lima ES, Ferreira VF (2012) Synthesis of 1,2,3-triazole glycoconjugates as inhibitors of α-glucosidases. Carbohydr Res 350:14–19
Rosa M, Kim HW, Gunic E, Jenket C, Boyle U, Koh Y, Korboukh I, Allan M, Zhang W, Chen H, Xu W, Nilar S, Yao N, Hamatake R, Lang SA, Hong Z, Zhang Z, Girardet J-L (2006) Tri-substituted triazoles as potent non-nucleoside inhibitors of the HIV-1 reverse transcriptase. Bioorg Med Chem Lett 16(17):4444–4449
Saladino R, Barontini M, Crucianelli M, Nencioni L, Sgarbanti R, Palamara AT (2010) Current advances in anti-influenza therapy. Curr Med Chem 17(20):2101–2140
Sarrazin C, Hézode C, Zeuzem S, Pawlotsky JM (2012) Antiviral strategies in hepatitis C virus infection. J Hepatol 56(1):S88–S100
Shawali AS (2010) Tandem in situ generation and 1,5-electrocyclization of N-hetaryl nitrilimines. A facile methodology for synthesis of annulated 1,2,4-triazoles and their acyclo C-nucleosides. ARKIVOC i:33–97
Silva EN Jr, Menna-Barreto RFS, Pinto MCFR, Silva RFS, Teixeira DV, Souza MCBV, Simone CA, Castro SL, Ferreira VF, Pinto AV (2008) Naphthoquinoidal [1,2,3]-triazole, a new structural moiety active against Trypanosoma cruzi. Eur J Med Chem 43(8):1774–1780
Silva FC, Souza MCBV, Frugulhetti ICPP, Castro HC, Souza SLO, Souza TML, Rodriques DQ, Souza AMT, Abreu PA, Passamani F, Rodrigues CR, Ferreira VF (2009) Synthesis, HIV-RT inhibitory activity and SAR of 1-benzyl-1H-1,2,3-triazole derivatives of carbohydrates. Eur J Med Chem 44(1):373–383
Souza TM, Rodrigues DQ, Ferreira VF, Marques IP, Santos FC, Cunha AC, Souza MC, Frugulhetti ICPP, Bou-Habib DC, Fontes CF (2009) Characterization of HIV-1 enzyme reverse transcriptase inhibition by the compound 6-chloro-1,4-dihydro-4-oxo-1-(beta-d-ribofuranosyl) quinoline-3-carboxylic acid through kinetic and in silico studies. Curr HIV Res 7(3):327–335
Souza TM, Salluh JI, Bozza FA, Mesquita M, Soares M, Motta FC, Pitrowsky MT, Oliveira ML, Mishin VP, Gubareva LV, Whitney A, Rocco SA, Gonçalves VM, Marques VP, Velasco E, Siqueira MM (2010) H1N1pdm influenza infection in hospitalized cancer patients: clinical evolution and viral analysis. PLoS One 5(11):e14158
Stierand K, Rarey M (2010) Drawing the PDB: protein-ligand complexes in two dimensions. ACS Med Chem Lett 1(9):540–545
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49(11):3315–3321
Wang X-L, Wan K, Zhou C-H (2010) Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activities. Eur J Med Chem 45(10):4631–4639
World Health Organization (2009) CDC protocol of realtime RTPCR for influenza A(H1N1) http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf. Accessed 30 Apr 2009
Yan S-J, Liu Y-J, Chen Y-L, Liu L, Lin J (2010) An efficient one-pot synthesis of heterocycle-fused 1,2,3-triazole derivatives as anti-cancer agents. Bioorg Med Chem Lett 20(17):5225–5228
Zhan P, Chen X, Li X, Li D, Tian Y, Chen W, Pannecouque C, De Clercq E, Liu X (2011) Arylazolylthioacetanilide. Part 8: Design, synthesis and biological evaluation of novel 2-(2-(2,4-dichlorophenyl)-2H-1,2,4-triazol-3-ylthio)-N-arylacetamides as potent HIV-1 inhibitors. Eur J Med Chem 46(10):5039–5045
Acknowledgments
The authors thank the Coordination of Improvement of Higher Education (CAPES), the National Council of R&D of Brazil (CNPq), and Carlos Chagas Filho Foundation for Research of the State of Rio de Janeiro (FAPERJ) for fellowships granted. We also thank the financial support of FAPERJ and Technological Development Program on Products for Health (PDTIS/FIOCRUZ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Lourdes G. Ferreira, M., Pinheiro, L.C.S., Santos-Filho, O.A. et al. Design, synthesis, and antiviral activity of new 1H-1,2,3-triazole nucleoside ribavirin analogs. Med Chem Res 23, 1501–1511 (2014). https://doi.org/10.1007/s00044-013-0762-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0762-6