Abstract
A series of new pyrazoline compounds bearing a pyridyl moiety (4a–i) were synthesized by condensing appropriate chalcones with hydrazine hydrate and tested for antimicrobial and antioxidant activities. According to in vitro antimicrobial activity against Bacillus subtilis, Staphylococcus epidermidis, Proteus vulgaris, Pseudomonas aeruginosa, Aspergillus niger and Penicillium chrysogenum and antioxidant activity by DPPH method, the compounds 4a, 4d, 4i and 4e, 4f, 4h showed maximum antimicrobial and antioxidant activities, respectively. Physiochemical properties and Lipinski’s ‘Rule of Five’ analysis predicted higher intrinsic quality of the synthesized compounds and revealed that these compounds have good bioavailability and druglikeness properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pyrazoline compounds are not known to occur in nature. They are usually prepared by the reaction of hydrazines with 1,3-diketones. Many synthetic pyrazoline compounds have medicinal importance. Owing to the interesting activity of variously substituted pyrazolines as biological agents, considerable attention has been focused on this class (Mohamad and Payal, 2011). Amongst various pyrazoline derivatives, 2-pyrazoline seems to be the most frequently studied pyrazoline-type compounds. The pharmaceutical importance of these compounds lies in the fact that they can be effectively utilized as antibacterial, antidepressant, anticonvulsant, antihypotensive, psychoanaleptic, antioxidant and tranquillizing agents (Sivakumar et al., 2010; Ozdemir et al., 2007; Turan et al., 2000; Kumar et al., 2009; Ji-Tai et al., 2007). In the development of drugs intended for oral use, good drug absorption and appropriate drug delivery are very important (Hou and Xu, 2004). About 30 % of oral drugs fail in development because of poor pharmacokinetics (Waterbeemed and Gifford, 2003). Among the pharmacokinetic properties, a low and highly variable bioavailability is indeed the main reason for stopping further development of the drug (Hou et al., 2007). Moreover, the knowledge of ionization constants is an important challenge for understanding various phenomenons like biological uptake, pharmacological activity or in vivo studies. Hence, the discovery of new molecules requires accurate determination of molecular properties.
The vital role played by small heterocyclic molecules in drug design cannot be denied. These molecules act as highly functionalized scaffolds. In this context, pyridyl ring, a prominent scaffold present in numerous bioactive molecules, has played a vital role in the development of different medicinal agents (Jyh-Haur et al., 2004). On the other hand, the interesting and versatile biological activities of pyrazolines established them as important pharmacophore. A variety of methods have been reported for the preparation of this class of compounds. In the present article, we report the reaction of 2-acetyl pyridine with different aromatic aldehydes to form pyrazolines bearing a pyridyl moiety and studied their antimicrobial, antioxidant activities and pharmacological properties.
Results and discussion
Chemistry
The present study was undertaken to synthesize some novel pyrazoline derivatives according to steps shown in Scheme 1. In the initial step, chalcones (3a–i) were synthesized by reacting 2-acetylpyridine with appropriate aldehydes in the presence of base by conventional Claisen–Schmidt condensation. The chalcones of the acetylpyridine were reported earlier; however, their preliminary characterizations like IR and NMR were carried out which confirmed them as the desired compounds (Prasad et al., 2008). Reaction between the synthesized chalcones and hydrazine hydrate in acetic acid led to the synthesis of novel pyrazolines (4a–i). Most of the products were found to be homogeneous, checked on pre-coated silica gel TLC plates which were visualized by exposing them to iodine vapours or UV light. Heterogeneous products were readily purified by silica gel column chromatography using a methanol/chloroform eluent.
Structures of the synthesized compounds (4a–i) were established on the basis of elemental analysis and spectral data (IR, 1H NMR, 13C NMR and MS). IR spectra of compounds (4a–i) showed absorption bands in the region of 1,600–1,700 cm−1 corresponding to C=N stretching. Also, infrared spectra revealed N–H and C–H peaks at 2,900–3,250 cm−1.
In the 1H NMR of pyrazoline, the hydrogen atom attached to N of pyrazoline ring appears at 5–6.5 ppm, and the hydrogen atoms attached to C of pyrazoline ring appear at 2–3.5 ppm. Aromatic and other protons were observed at expected ppm values. In the 13C NMR spectra of these compounds (4a–i), the chemical shift values of carbon atoms appeared: 147.8–151.2 (C-3), 33.5–47.6 (C-4) and 48.2–53.3 (C-5).
Physiochemical properties
Physiochemical properties, mainly molecular size, flexibility, hydrophobicity and the presence of various pharmacophoric features, influence the behaviours of molecules in a living organism, including bioavailability. A good bioavailability can be achieved with an appropriate balance between solubility and portioning properties. Thus, in order to achieve good oral drugs, we have investigated a series of pyrazoline derivatives bearing a pyridyl moiety (4a–i) for the prediction of their molecular properties and Lipinski’s ‘Rule of Five’ (Lipinski et al., 1997).
C log of P
Poor solubility and permeability are amongst the main causes for failure during drug development (Avdeef, 2001; Oprea, 2002; Norinder and Bergstorm, 2006). The octanol/water partition coefficient (P) (also referred as Kow) is a measure of the propensity of a neutral compound to differentiate dissolution in these immiscible phases. It is usually referred to as the logarithmic ratio, log P, and serves as a quantitative descriptor of lipophilicity. The octanol/water partition coefficient C log P being a measure of hydrophobicity/lipophilicity was calculated using Marvin Sketch tool (Balogh et al., 2009). Compounds with log P values < 5 (Lipinski’s Rule of Five) mean that they can readily get past ester/phosphate groups in skin membranes. The results obtained are given in Table 1. The calculated values of log P for the derivatives ranged from 2.556 to 4.223. The lipophilic aptitude of a compound increases with the increasing log P. The C log P values of all the compounds show variation which can be attributed to the presence and position of different substituents on the phenyl ring. The molar refractivity (MR—which represents size and polarizability of a fragment of molecule) describing steric effects was calculated using Marvin Sketch Software (Table 1).
‘Rule of Five’ properties
High oral bioavailability is an important factor for the development of bioactive molecules as therapeutic agents. Good intestinal absorption, molecular flexibility (measured by the number of rotatable bonds), low polar surface area or total hydrogen bond count (sum of donors and acceptors), are important predictors of good oral bioavailability (Veber et al., 2002; Refsgaard et al., 2005). Molecular properties such as membrane permeability and bioavailability are always associated with some basic molecular descriptions such as log P (partition coefficient), molecular weight (MW), or hydrogen bond acceptor and donor count in a molecule (Muegge, 2003). Lipinski et al. (1997) used these molecular properties in formulating his ‘Rule of Five’. The rule states that most molecules with good membrane permeability have log P ≤ 5, MW ≤ 500, the number of hydrogen bond acceptors ≤ 10, and the number of hydrogen bond donors ≤ 5. The rule is widely used as a filter for drug-like properties. A compound that fulfils at least three out of the four criteria is said to adhere to ‘Lipinski’s Rule of Five’. A poor permeation or absorption is more likely when there are more than five H-bond donors and ten H-bond acceptors. The series (4a–i) under investigation has not only the most of the compounds possessing less number of hydrogen bond donors (<5) but also does possess considerable number of acceptors (<10). Table 1 lists the values of these properties for the new scaffolds and suggest that the active compounds can be used as templates for a drug discovery effort.
Disc diffusion assay
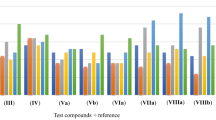
Table 2 summarizes the results obtained from antimicrobial activity of pyrazoline derivatives with test compounds, Kanamycin and Fluconazole. All the isolates Bacillus subtilis, Staphylococcus epidermidis, Proteus vulgaris, Pseudomonas aeruginosa, Aspergillus niger and Penicillium chrysogenum showed high degrees of sensitivity towards all the test compounds (sharing a common structure) with slight variation because of the presence of different substituents on the phenyl ring. Compound ‘4d’ with a ‘chloro’ group at para position of the phenyl ring showed higher antimicrobial activity followed closely by compound 4i having a bromo group at the same position. Compound ‘4f’ with a ‘fluoro’ group at meta position showed the least antimicrobial activity. From the results, it can be inferred that the presence and position of different groups on phenyl ring of the compounds have marked effect on the antimicrobial efficiency of the test compounds. Besides, an optimum electron density is must for a compound to attain maximum activity. The most important thing noticed is that the test compounds were slightly more effective against bacteria as compared with fungi. 1 % DMSO is used as a solvent (control), having no effect on the tested organism. Hence, we can effectively conclude here that whole of the antimicrobial effect is because of the test compounds.
Antioxidant activity
The results summarized in Table 3 give the antioxidant data in terms of mean % inhibition by DPPH method. All the synthesized pyrazolines showed good antioxidant activity with slight variation as expected because of the presence of different substituents on phenyl ring. Compound 4h showed maximum antioxidant activity as compared with other derivatives, perhaps because of the availability of more electron cloud on the core molecule. Inhibition due to control (ethanol) was not seen during the test as shown in the table. Ascorbic acid was used as a positive control.
Conclusion
The present study has achieved the efficient synthesis of pyrazoline bearing a pyridyl moiety and examined their preliminary in vitro antimicrobial activity by disc diffusion method and antioxidant activity by DPPH method. From these activities, it was found that the compounds 4a, 4d and 4i exhibited maximum antimicrobial activity, while compounds 4e, 4f and 4h showed maximum antioxidant activity. The molecular properties were predicted, which showed that these active compounds can be used as templates for development of new drugs. Also, it was found that all the compounds followed Lipinski’s Rule of Five. Thus, the idea of appending the pyridyl moiety to the pyrazoline nucleus so as to combine the beneficial effects in a single structure proved to be successful. In conclusion, the present study showed that synthesized compounds can be used as template for future development through modification and derivatization.
Experimental section
General methods
Melting points were recorded on Buchi melting point apparatus D-545; IR spectra (KBr discs) were recorded on Bruker Vector 22 instrument. NMR spectra were recorded on Bruker DPX200 instrument in CDCl3 with TMS as internal standard for protons and solvent signals as internal standard for carbon spectra. Chemical shift values are mentioned in δ (ppm). Mass spectra were recorded on EIMS (Shimadzu) and ESI-esquire 3000 Bruker Daltonics instrument. The progress of all reactions was monitored by TLC on 2 × 5 cm2 pre-coated silica gel 60 F254 plates of thickness of 0.25 mm (Merck). The chromatograms were visualized under UV 254–366 nm and iodine vapours.
Chemical synthesis
General procedure for the preparation of chalcones (3a–i)
To the stirring solution of 2-acetyl pyridine 1 (0.01 mol) and aryl aldehyde 2 (0.01 mol) in ethanol (30 ml) was added an aqueous solution of KOH (40 %, 15 ml). The mixture was kept overnight at room temperature. It was poured into crushed ice and acidified with concentrated HCl. The product obtained was filtered, washed with water and crystallized from ethanol.
General procedure for the preparation of pyrazolines (4a–i)
The appropriate chalcones (0.001 mol) and hydrazine hydrate (0.001) were immediately dissolved in acetic acid (5 ml) and refluxed for 24 h. After completion of reaction, the reflux condenser was removed, and the reaction mixture was washed, first with cold water and then with excess sodium bicarbonate. It was filtered and crystallized to give pure compound. However, impure compounds were readily purified by silica gel column chromatography using a methanol/chloroform eluent.
2-(5-Phenyl-4,5-dihydro-1H-pyrazol-3-yl)pyridine (4a)
The compound 4a was prepared by reacting (2E)-3-phenyl-1-(pyridin-3-yl)prop-2-en-1-one (3a) with hydrazine hydrate in acetic acid. The product was obtained as coloured powder. The yield was 45 %. IR (KBr) cm−1: 3,104 (N–H Str.), 3,050 (C–H Str.), 1,637 (C=N Str.), 1,403 (C=C Str.); 1H NMR (δ ppm) (CDCl3): δ 2.4 (d, 2H, CH2,), δ 5.4 (s, 1H, N–H), δ 7.12–7.9 (m, Ar–H), δ 8.02 (d, 1H, N–C–H,); 13C NMR (500 MHz, CDCl3): δ 44.6 (C-4), δ 55.6 (C-5), δ 124.69–150.6 (Ar–C), δ 147.6 (C-3); ESI–MS: 222 (M+H); Anal. Calcd. for C17H21N3O3: C, 76.01; H, 4.91; Found C, 76.37; H, 4.83.
2-[5-(3,4,5-Trimethoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl]pyridine (4b)
The compound 4b was prepared by reacting (2E)-3-(3,4,5-trimethoxyphenyl)-1-(pyridin-3-yl)prop-2-en-1-one (3b) with hydrazine hydrate in acetic acid. The product was obtained as coloured powder. The yield was 40 %. IR (KBr) cm−1: 3,114 (N–H Str.), 3,012 (C–H Str.), 1,627 (C=N Str.), 1,403 (C=C Str.); 1H NMR (CDCl3): δ 2.5 (d, 2H, CH2), δ 2.67 (s, 9H, OCH3), δ 6.0 (s, 1H, N–H), δ 7.3–7.9 (m, Ar–H), δ 8.2 (d, 1H, N–C–H); 13C NMR (500 MHz, CDCl3): δ 47.3 (C-4), δ 56.3 (C-5), δ 120–139 (C–Ar), δ 149 (C-3); ESI-MS: 312 (M+H); Anal. Calcd. for C17H17N3O3: C, 65.30; H, 5.41; N, 13.5; O, 15.4; Found C, 64.87; H, 5.63; N, 13.46; O, 15.00.
2-[5-(4-Nitrophenyl)-4,5-dihydro-1H-pyrazol-3-yl]pyridine (4c)
The compound 4c was prepared by reacting (2E)-3-(4-nitrophenyl)-1-(pyridin-3-yl)prop-2-en-1-one (3c) with hydrazine hydrate in acetic acid. The product was obtained as coloured powder. The yield was 45 %. IR (KBr) cm−1: 3,125 (N–H Str.), 3,100 (C–H Str.), 1,630 (C=N Str.), 1,500 (C=C Str.),1H NMR (δ ppm) (CDCl3): δ 3.1 (d, 2H, CH2), δ 6.0 (s, 1H, N–H), δ 7.38–7.99 (m, Ar–H), δ 8.2 (d, 1H, N–C–H); 13C NMR (500 MHz, CDCl3): δ 44.3 (C-4), δ 53.1 (C-5,), δ 121.3–137.2 (C–Ar), δ 147.1 (C-3); ESI-MS: 267 (M+H); Anal. Calcd. for C14H10N4O2: C, 63.15; H, 3.7; N, 21.06; O, 12.03; Found C, 63.27; H, 3.23.; N, 21.46; O, 12.20.
2-[5-(4-Chlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl]pyridine (4d)
The compound 4d was prepared by reacting (2E)-3-(4-chlorophenyl)-1-(pyridin-3-yl)prop-2-en-1-one (3d) with hydrazine hydrate in acetic acid. The product was obtained as coloured powder. The yield was 41 %. IR (KBr) cm−1: 3,105 (N–H Str.), 3,090 (C–H Str.), 1,617 (C=H Str.), 1,513 (C=C Str.); 1H NMR (δ ppm) (CDCl3): δ 2.9 (d, 2H, CH2), δ 6.12 (s, 1H, N–H), δ 7.18–7.8 (m, Ar–H), δ 8.52 (d, 1H, N–C–H); 13C NMR (500 MHz, CDCl3): δ 46.13 (C-4,), δ 56.13 (C-5,), δ 118.3–137.32 (C–Ar), δ 151.1 (C-3); ESI-MS: 256 (M+H); Anal. Calcd. for C14H10ClN3: C, 65.7; H, 3.9; N, 16.42; Found C, 65.63; H, 3.83.; N, 16.56;
2-[5-(4-Fluorophenyl)-4,5-dihydro-1H-pyrazol-3-yl]pyridine (4e)
The compound 4e was prepared by reacting (2E)-3-(4-fluorophenyl)-1-(pyridin-3-yl)prop-2-en-1-one (3e) with hydrazine hydrate in acetic acid. The product was obtained as coloured powder. The yield was 45 %. IR (KBr) cm−1: 3,115 (N–H Str.), 3,080 (C–H Str.), 1,605 (C=H Str.), 1,505 (C=C Str.); 1H NMR (δ ppm) (CDCl3): δ 2.6 (d, 2H, CH2), δ 6.42 (s, 1H, N–H), δ 7.2–7.8 (m, Ar–H), δ 8.49 (d, 1H, N–C–H); 13C NMR (500 MHz, CDCl3): δ 41.23 (C-4), δ 46.53 (C-5,), δ 116.2–137.3 (C–Ar), δ 150.4 (C-3); ESI-MS: 240 (M+H); Anal. Calcd. for C14H10FN3: C, 70.22; H, 4.17; N, 17.55; Found C, 70.16; H, 4.0; N, 17.46;
2-[5-(3-Fluorophenyl)-4,5-dihydro-1H-pyrazol-3-yl]pyridine (4f)
The compound 4f was prepared by reacting (2E)-3-(3-fluorophenyl)-1-(pyridin-3-yl)prop-2-en-1-one (3f) with hydrazine hydrate in acetic acid. The product was obtained as coloured powder. The yield was 40 %. IR (KBr) cm−1: 3,125 (N–H Str.), 3,085 (C–H Str.), 1,600 (C=N Str.), 1,495 (C=C Str.); 1H NMR (δ ppm) (CDCl3): δ 3.1 (d, 2H, CH2), δ 6.0 (s, 1H, N–H), δ 6.9–7.8 (m, Ar–H), δ 8.6 (d, 1H, N–C–H); 13C NMR (500 MHz, CDCl3): δ 45.1 (C-4), δ 52.7 (C-5), δ 120.19–147.8 (Ar–C), δ 150.6 (C-3); ESI-MS: 240 (M+H); Anal. Calcd. for C14H10FN3: C, 70.22; H, 4.17; N, 17.55; Found C, 70.26; H, 4.1; N, 17.51;
2-[5-(4-Methoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl]pyridine (4g)
The compound 4g was prepared by reacting (2E)-3-(4-methoxyphenyl)-1-(pyridin-3-yl)prop-2-en-1-one (3g) with hydrazine hydrate in acetic acid. The product was obtained as coloured powder. The yield was 40 %. IR (KBr) cm−1: 3,110 (N–H Str.), 3,085 (C–H Str.), 1,665 (C=N Str.), 1,625 (C=C Str.); 1H NMR (δ ppm) (CDCl3): δ 3.12 (s, 3H, OCH3), δ 2.9 (d, 2H, CH2), δ 5.1 (s, 1H, N–H), δ 6.1–7.6 (m, Ar–H), δ 8.6 (d, 1H, N–C–H); 13C NMR (500 MHz, CDCl3): δ 43.1 (C-4), δ 53.97 (C-5), δ 105.19–137.8 (Ar–C), δ 148.1 (C-3); ESI-MS: 252 (M+H); Anal. Calcd. for C15H13N3O: C, 71.70; H, 5.17; N, 16.13; Found C, 71.79; H, 5.13; N,16.23.
2-[5-(4-N,N-dimethylphenyl)-4,5-dihydro-1H-pyrazol-3-yl]pyridine (4h)
The compound 4h was prepared by reacting (2E)-3-(4-N,N-dimethylphenyl)-1-(pyridin-3-yl)prop-2-en-1-one (3h) with hydrazine hydrate in acetic acid. The product was obtained as coloured powder. The yield was 43 %. IR (KBr) cm−1: 3,105 (N–H Str.), 2,900 (C–H Str.), 1,680 (C=N Str.), 1,490 (C=C Str.); 1H NMR (δ ppm) (CDCl3): δ 2.52 (s, 6H, N(CH3)2), δ 2.7 (d, 2H, CH2), δ 5.91 (s, 1H, N–H), δ 6.2–7.9 (m, Ar–H), δ 8.5 (d, 1H, N–C–H); 13C NMR (500 MHz, CDCl3): δ 44.1 (C-4), δ 52.27 (C-5), δ 114.6–137 (Ar–C), δ 147.21 (C-3); ESI-MS: 265 (M+H); Anal. Calcd. for C16H16N4: C, 64.36; H, 6.0; N; 21.2; Found C, 64.17; H, 5.83; N, 20.89.
2-[5-(4-Bromophenyl)-4,5-dihydro-1H-pyrazol-3-yl]pyridine (4i)
The compound 4i was prepared by reacting (2E)-3-(4-bromophenyl)-1-(pyridin-3-yl)prop-2-en-1-one (3i) with hydrazine hydrate in acetic acid. The product was obtained as coloured powder. The yield was 40 %. IR (KBr) cm−1: IR (KBr) cm−1: 3,125 (N–H Str.), 3,038 (C–H Str.), 1,650 (C=N Str.), 1,412 (C=C Str.); 1H NMR (δ ppm) (CDCl3): δ 2.4 (d, 2H, CH2), δ 5.86 (s, 1H, N–H), δ 7.2–7.8 (m, Ar–H), δ 8.3 (d, 1H, N–C–H); 13C NMR (500 MHz, CDCl3): δ 42.8 (C-4), δ 50.97 (C-5), δ 120.5–147.5 (Ar–C), δ 149.21 (C-3); ESI-MS: 301 (M+H); Anal. Calcd. for C14H10BrN3: C,56; H,3.3; N,14.2; Br, 26.2; Found C, 56.37; H, 3.13; N, 14.6; Br, 26.5;
Antimicrobial activity
Strain and media
The bacterial and fungal strains used in the study were B. subtilis (MTCC 619), S. epidermidis (MTCC 435), P. vulgaris (MTCC 426), P. aeruginosa (MTCC 424), A. niger (MTCC 1344), and P. chrysogenum (MTCC 947). All the bacterial and fungal strains were maintained on Mueller Hinton agar (MHA) and potato dextrose agar (PDA), respectively.
Growth conditions
Stock cultures of all the bacterial and fungal strains were maintained on agar slants at 4 °C to initiate growth; one loopful of cells from the agar culture was inoculated into their respective broth media and incubated at 35 ± 2 °C for 24–28 h up to the stationary phase (primary culture). The cells from the primary culture (108 cells ml) were re-inoculated into 100-ml fresh media and grown for 8 ± 2 h, i.e. up to mid-log phase (106 cells/ml).
Disc diffusion assay
The target compounds (4a–i) were evaluated for antimicrobial activity against the test strains by agar disc diffusion method (Stefan et al., 2010). Strains were inoculated into liquid media and kept overnight for growth at 35 ± 2 °C. The cells were then pelleted, and washed three times with distilled water. Approximately, 105 cells/ml were inoculated in molten agar media at 40 °C and poured into 100-mm-diameter petriplates. 4-mm filter discs were impregnated on solid agar, and test compounds (1,000 μg/ml dissolved in 1 % DMSO) were spotted on the filter discs. Solvent control (1 % DMSO) was pipetted onto filter disc to serve as positive control. The diameter of zone of inhibition was recorded in millimetres after 48 h and was compared to that of control. This experiment was independently repeated thrice, and values are shown in terms of mean ± standard error of mean (SEM).
Antioxidant activity
General free radical scavenging-DPPH assay
The antioxidant potential of any compound can be determined on the basis of its capacity to trap the stable 1,1-diphenyl-2-picryl hydrazyl (DPPH) free radical (Sherwin, 1978; Brand-Willams et al., 1995; Espin et al., 2000). The antioxidant activity by this method is measured as the decrease in the absorbance of DPPH at 517 nm resulting from the colour change from purple to yellow. The decrease in absorbance is because of formation of stable molecule of DPPH on reaction with an antioxidant through donation of hydrogen or electron by an antioxidant. The free electron on DPPH radical is responsible for giving absorbance peak at 517 nm and appears purple in colour. The antioxidant agents pair up through donation of electron or release of hydrogen with the free electron on DPPH radical and form stable molecule of DPPH-H. The change of colour from purple to yellow is attributed to the decrease of molar absorptivity of DPPH radical when the odd electron of DPPH pairs up with the antioxidant agent. The resulting decrease in colour is also stoichiometric with the number of electrons captured.
The formula used for % inhibition is as follows:
References
Avdeef A (2001) Physicochemical profiling (solubility, permeability and charge state). Curr Top Med Chem 1:277–351
Balogh GT, Gyarmati B, Nagy B, Molnar L, Keseru GM (2009) Comparative evaluation of in silico pKa prediction tool on the gold standard dataset. QSAR Comb Sci 28:1148–1155
Brand-Willams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensmittel Wissenschaft und Technologie 28:25–30
Espin JC, Solar Rivas C, Wichers HG (2000) J Agric Food Chem 48:648–656
Hou TJ, Xu XJ (2004) Recent development and application of virtual screening in drug discovery an over view. Curr Pharm Des 10:1011–1033
Hou T, Wang J, Zhang W, Xu X (2007) ADME evaluation in drug discovery: prediction of oral absorption by correlation and classification. J Chem Inf Model 47:460–463
Ji-Tai L, Xiao-Hui Z, Zhi-Ping L (2007) An improved synthesis of 1,3,5-triaryl-2-pyrazolines in acetic acid aqueous solution. Beilstein J Org Chem 3:13
Jyh-Haur C, Chung-Chi L, Chih-Shiang C, Yen-Chun L, Chia-Liang T, Ying-Ting L, Kak-Shan S, Ching-Yin L, Shin-Ru S (2004) Synthesis and antienteroviral activity of series of novel oxime ether-containing pyridyl imidazolidinones. Bioorg Med Chem Lett 14:5051–5056
Kumar S, Bawa S, Drabu S, Kumar R, Gupta H (2009) Biological activity of pyrazoline derivatives—a recent development. Recent Pat Antiinfect Drug Discov 4:154–163
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development setting. Adv Drug Deliv Rev 23:23–25
Mohamad Y, Payal J (2011) Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arab J Chem. doi:10.1016/09.013
Muegge I (2003) Selection criteria for drug-like compounds. Med Res Rev 23:302–321
Norinder U, Bergstorm AS (2006) Prediction of ADMET properties. Chem Med Chem 1:920–934
Oprea TI (2002) Current trends in lead discovery: are we looking for the appropriate properties. Comput J Aided Mol Des 16:325–334
Ozdemir Z, Kandillei HB, Gumusel B, Calis U, Bilgin AA (2007) Synthesis on antidepression and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur J Med Chem 42:373–379
Prasad YR, Kumar PP, Kumar PR, Rao AS (2008) Synthesis and antimicrobial activity of some new chalcones of 2-acetyl pyridine. E J Chem 5:144–148
Refsgaard HHF, Jensen BF, Brockhoff PB, Padkjaer SB, Guldbrandt M, Chistensen MS (2005) In silico prediction of membrane permeability from calculated molecular parameters. J Med Chem 48:805–811
Sherwin FR (1978) J Am Oil Chem Soc 55:809–841
Sivakumar PM, Ganesan S, Veluchamy P, Doble M (2010) Novel chalcones and 1,3,5-triphenyl-2-pyrazoline derivatives as antimicrobial agents. Chem Biol Drug 76:407–411
Stefan S, Jeroen SD, Brigitte K, Irene W, Randi D, Florenz S (2010) Biological activity of volatiles from marine and terrestrial bacteria. Mar Drugs 8:2976–2987
Turan ZG, Chevallet P, Kilic FS, Erol K (2000) Synthesis of some thiazolyl pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur J Med Chem 35:635–641
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kapple KD (2002) Molecular properties that influences the oral bioavailability of drug candidates. J Med Chem 45:2615–2623
Waterbeemed HD, Gifford E (2003) ADMET in silico modeling: towards prediction paradise. Nat Rev Drug Discov 2:192–204
Acknowledgments
The authors would like to thank UGC, India for their financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lone, I.H., Khan, K.Z. & Fozdar, B.I. Synthesis, physicochemical properties, antimicrobial and antioxidant studies of pyrazoline derivatives bearing a pyridyl moiety. Med Chem Res 23, 363–369 (2014). https://doi.org/10.1007/s00044-013-0643-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0643-z