Abstract
Effect of bischalcone derivative (2E,5E)-2,5-bis(4-hydroxy-3-methoxybenzylidene) cyclopentanone (CA) on blood glucose level and lipid profile was investigated in normoglycemic and streptozotocin (STZ)-induced diabetic rats. In oral glucose and sucrose tolerance test, treatment with CA (25 mg/kg) and glidenclamide (10 mg/kg) significantly improved the glucose and sucrose tolerance in normal animals. In addition, respective treatment for 7-day resulted in significant percentage reduction in serum glucose (SG). In standardized STZ-induced diabetic rats, a single dose of CA treatment exhibited reduction in SG levels at different time intervals compared to basal levels. The lipid profile levels are restored to near-normal value of all tested parameters. The compound CA was docked into the active site of 5′-AMP (adenosine monophosphate)-activated protein kinase (PDB ID: 2OOX). The binding and docking energy were found to be −6.9 and −45.1 kcal/mol, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a disorder in which homeostasis of carbohydrate and lipid metabolism is improperly regulated by insulin. This results primarily in elevated fasting and postprandial blood glucose levels. If this imbalanced homeostasis does not return to normalcy and continues for a protracted period of time, it leads to hyperglycemia that in due to course turns into a syndrome called diabetes mellitus (Lernmark and Ott, 1998). The global prevalence of diabetes is estimated to be 2.8 % in 2000 and 4.4 % in 2030. The total number of diabetes is projected to rising from 171 million in 2000 to 366 million in 2030. Medication often has an important role to play particularly for the control of blood glucose and lipids profiles (Wild et al., 2004). External insulin preparations can be used to treat type 1 diabetes mellitus as well as in controlled type 2 diabetes mellitus (T2DM) conditions. The oral hypoglycemic agents such as sulfonylureas (tolbutamide and glibenclamide), Biguanides (phenformin and metformin), thiazolidinediones (troglitazone and rosiglitazone), and α-glucosidsase inhibitors (acarbose and miglitol) have been extensively used in T2DM. But none of them are ideal in correcting blood glucose levels round the clock, with single-dose administration. In large doses, these agents cause hypoglycemia and in smaller doses, unchecked hyperglycemia and both these extremes are equally life threatening. Moreover, it has been clearly established that insulin and sulfonylureas, even with excellent glycemic control do not normalize diabetes dyslipidemia or improve hypertension. Parallel to this, recent developments in understanding the pathophysiology of the disease process have opened up several new avenues to identify and develop novel therapies to combat the diabetic plague.
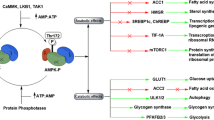
The adenosine monophosphate-activated protein kinase (AMPK) is an important regulatory protein for cellular energy balance and is considered a master switch of glucose and lipid metabolism in various organs, especially in skeletal muscle and liver. In skeletal muscles, AMPK stimulates glucose transport and fatty acid oxidation. In the liver, it augments fatty acid oxidation and decreases glucose output, cholesterol, and triglyceride synthesis. These metabolic effects induced by AMPK are associated with lowering blood glucose levels in hyperglycemic individuals. Two classes of oral antihyperglycemic drugs (biguanidines and thiazolidinediones) have been shown to exert some of their therapeutic effects by directly or indirectly activating AMPK. However, side effects and an acquired resistance to these drugs emphasize the need for the development of novel and efficacious AMPK activators (Arie et al., 2009).
Curcumin chemically is a bis-α,β-unsaturated β-diketone that exhibits keto-enol tautomerism. Curcumin is known for its biological activities. Curcumin and its analog, tetrahydrocurcumin (THC) was found to be a more potent antioxidant and good therapeutic agent for diabetic patients in preventing diabetes vascular abnormalities (Natchaya et al., 2009). In spite of its efficacy and safety, curcumin has not yet been approved as a therapeutic agent. Poor aqueous solubility, relatively low bioavailability and intense staining color of curcumin have been highlighted as major problems; and consequently search for a “super curcumin” without these problems and with efficacy equal to or better than that of curcumin was investigated (Preetha et al., 2008).
This study reports the antidiabetic property of CA [(2E,5E)-2,5-bis(3-methoxy-4-hydroxy-benzylidene) cyclopentanone in rat model system and the compound was docked into the active site of 5′-AMP AMPK (PDB ID: 2OOX).
Materials and methods
Drugs and chemicals
Streptozotocin (STZ) was purchased from HI-Media, Mumbai. Insulin injection biphasic isophane, Ph. Eur. Human Mixtard manufactured by Torrent Pharmaceuticals, Ltd., Standard drug glidenclamide (GLB) 5 mg (Daonil) obtained from Avents Pharma Ltd., Goa. Acarbose (glucobay) was obtained from Bayer Pharma, Pvt Ltd. Standard kits for glucose, triglycerides, cholesterol, and HDL-cholesterol were obtained from ERBA Mannheim, manufactured by Transasia, Biomedical Ltd, Baddi, India.
Synthesis of CA
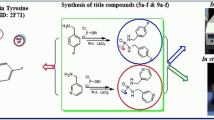
Bischalcone derivative (2E,5E)-2,5-bis(4-hydroxy-3-methoxybenzylidene) cyclopentanone (CA) was synthesized through was carried out according to the reported method (Furniss et al., 1989; Butcher et al., 2007). The compound was characterized by analytical spectral data.
Animals
Young adult male Wistar rats weighing 150–200 g were obtained from inbred animal house Venkateshwara enterprises, Bangalore, Karnataka. The animals were housed in polypropylene cages in standard environmental conditions, 12 h light and 12 h dark cycle at 25 ± 2 °C. Before and during the experiments, the rats were fed with standard laboratory pellet diet and water ad libitum. Animals were acclimatized to the laboratory condition for at least 15 days prior to the experiment and were maintained in a well-ventilated animal house. The experimental protocol was approved by the Institutional animal Ethical Committee (SETCPD/IAEC/09-10/29) animals, and the care of the laboratory was taken as per the CPCSEA regulation.
Effect of CA in normoglycemic rats
Oral glucose tolerance test (OGTT)
The experimental rats were divided into three groups of five rats each. Group 1: NC received 0.5 % tragacanth (10 ml/kg, p.o.); group 2: NC rats treated with CA (25 mg/kg, p.o.); group 3: NC rats treated with GLB (10 mg/kg, p.o.). The CA, GLB, and vehicle were given orally to normoglycemic rats fasted for 18 h. After 30 min, glucose (2 g/kg in distilled water) was administered orally. Blood samples were collected from the retro-orbital plexus at 0 (i.e., immediately after glucose load), 30, 60, and 120 min after glucose administration. Serum glucose (SG) was estimated by the enzymatic glucose oxidase method using diagnostic reagent kit. The results are expressed as integrated area under curve for glucose (AUCglucose) calculated by trapezoid rule
Oral sucrose tolerance test (OSTT)
The experimental rats were divided into three groups of five rats each. Group 1: NC received 0.5 % tragacanth (10 ml/kg, p.o.); group 2: NC rats treated with CA (25 μg/kg, p.o.); group 3: NC rats treated with acarbose (ACR; 7 mg/kg, p.o.). The doses of CA/acarbose and vehicle were given orally to normal rats fasted for 18 h. Thirty minutes later, sucrose (2 g/kg in distilled water) was administered orally. Blood samples were collected from the retro-orbital plexus at 0 (i.e., immediately after sucrose load), 30, 60, and 120 min post sucrose administration (Habbu et al., 2010). SG was estimated by the enzymatic glucose oxidase method using diagnostic reagent kit. The results are expressed as integrated area under curve for glucose (AUCglucose) calculated by trapezoid rule using formula as mentioned above.
Effect of CA in STZ-induced diabetic rats
Induction of diabetes mellitus diabetic condition was induced in male Wistar rats by single intravenous injection of STZ (50 mg/kg) (chosen optimum dose: in house data) after overnight fasting for 12 h (Siddique et al., 1987). Rats showing SG level >200 mg/dl 7 days after STZ administration were considered diabetic and included in the study. Diabetic rats were randomized into different groups based on their SG levels.
Single-dose 1-day study
The experimental rats were divided into four groups of six rats each. Group 1: NC received 0.5 % tragacanth (10 ml/kg, p.o.); group 2: DC received 0.5 % tragacanth (10 ml/kg, p.o.); group 3: DC rats treated with CA (25 μg/kg, p.o.); group 4: DC rats treated with GLB (10 mg/kg, p.o.). Blood samples were collected at 0, 2, and 4 h after CA/GLB administration. SG was estimated by the enzymatic glucose oxidase method. Blood glucose was estimated by the enzymatic glucose oxidase method using a commercial glucometer (Accu-chek® Active, Roche diagnostic, Mannheim, Germany). Percentage reduction in glycemia was calculated with respect to the initial (0 h) level according to: percentage reduction in glycemia = [(G i − G t)/G i] × 100, where G i is the initial glycemia and G t is the glycemia at 2 and 4 h (Yanardag and Colak, 1998).
Multiple-dose 7-day study
The above groups of animals were further treated with CA and GLB for seven consecutive days in order to evaluate the chronic effect of CA/GLB treatment on hyperglycemia. Whereas GLB (0.5 mg/kg, p.o./day) was administered for 7 days. Percent reduction in glycemia was calculated with respect to the initial (0 day) level by above mentioned formula.
Oral glucose tolerance test (OGTT)
On 7th day, glucose tolerance of various groups was estimated by a simple OGTT. Glucose (2 g/kg) was administered to 12 h fasted rats and blood samples were collected from the retro-orbital plexus at 0 (before glucose load), 30, 60, and 120 min after glucose administration. SG was estimated by the enzymatic glucose oxidase method. The results were expressed as integrated area under curve for glucose (AUCglucose), which was calculated by trapezoid rule
Estimation of lipid profile
At the end of the treatment schedule, blood samples were collected from the seven days treated animals. Serum was separated and analyzed spectrophotometrically for triglyceride (STG), total cholesterol (STC), and HDL-cholesterol (HDL-c) using diagnostic reagent kit ERBA diagnostics Mannheim GMBH, Germany.
Docking calculations with ICM™ (Internal Coordinate Mechanics) dock
All the docking calculations of compounds in this article were performed using the ICM™ docking module with the default setup as earlier mentioned (Abagyan et al., 1994; Khan et al., 2009).
Preparations of the inhibitors and target molecules
The 2D structures of the compounds (in mol file formats) have been converted to 3D and energy minimized at the 3D space of ICM environment. The formal charges and 3D topology were assigned using atom types local chemical environment (Merck molecular force field—MMFF). The lowest energy conformers of the compounds were then docked into the 3D space of the active site of 5′-AMP AMPK (PDB code: 2OOX) (Halgren, 1996a, b, c, 1999a, b; Halgren and Nachbar, 1996).
Docking process
All the docking calculations were performed using the “interactive docking” menu at the ICM environment. After docking the stack of docking poses was checked visually. Multiple stack conformations were selected based on their docking energies, rmsd values (compared between the docked model and X-ray conformation), and similarities to closely related X-ray crystal structures from PDB. Then, the best conformations for each of the compounds were finally chosen, and then their binding energies were calculated using ICM script (briefly described in the following “Calculations of free energies of binding” section).
Calculations of free energies of binding
For each of the individual docked complexes, the free energies of binding (Cal. ΔG) between the protein and ligand were calculated using ICM script utilizing the Eqs. 1 and 2
Here ΔG H is the hydrophobic or cavity term, which accounts for the variation of water/non-water interface area. ΔG EL is the electrostatic term composed of coulombic (ΔG COUL) interactions and desolvation (ΔG DESOLV) of partial charges transferred from an aqueous medium to a protein core environment. ΔG S is the entropic term which results from the decrease in the conformational freedom of functional groups buried upon complexation; and finally the C is a constant accounts for the change of entropy of the system due to the decrease of free molecules concentration (cratic factor), and loss of rotational/translational degrees of freedom (Schapira et al., 1999).
Interpretations of intermolecular interactions
To study the intermolecular interactions between the targets and the compounds LigPlot12 were used to plot the interactions from 3D to 2D. Beside LigPlot, ICM (www.molsoft.com) and Discovery Studio Visualizer (www.accelrys.com) also been used to analyze the interactions in 3D space.
Statistical analysis
The data were expressed as mean ± SEM for six rats in each group. Statistical comparisons were performed by one-way ANOVA followed by Tukey’s post test.
Results and discussion
Diabetes mellitus is a metabolic disorder as old as mankind and is characterized by hyperglycemia associated with impairment in insulin secretion/action along with altered carbohydrate, protein, and lipid metabolism. The function of insulin is to maintain normal blood glucose levels either by suppression of glucose output from liver or by the stimulation of glucose uptake and its metabolism. Insufficient release of insulin or loss of insulin action at target tissues causes abnormal glucose and lipid metabolism. This results in elevated glucose levels in blood, the hallmark of diabetes. Type-1 diabetes results from autoimmune destruction of pancreatic β-cells resulting in insulin deficiency. Insulin therapy affords effective glycemic control, yet its drawbacks such as ineffectiveness on oral administration, short shelf life, need for constant refrigeration and hypoglycemia on excess dosage limits its usage (Kasiviswanath et al., 2005). Therefore, efforts continue to find insulin substitutes from synthetic or plant sources. STZ-induced experimental diabetes is a valuable model for induction of type 1 diabetes. Further, it is generally accepted that severe diabetes (SD) is similar to type 1 and mild diabetes (MD) is similar to type II diabetes (Matti et al., 2005; Sharma et al., 2003). This is the first study to investigate the effect of bischalcone derivative (CA) on the glucose levels, lipid profile in STZ-induced diabetic rats.
Effect of CA in normoglycemic rats
Oral glucose tolerance test (OGTT)
Administration of glucose (2 g/kg) produces significant change in SG level of normal rats. Treatment with CA (25 mg/kg) and GLB (10 mg/kg) significantly (P < 0.01; P < 0.001) improved the glucose tolerance and significantly reduced the AUC glucose compared to NC group, and the data are given in Fig. 1.
Animals subjected to sucrose (2 g/kg) load produces significant changes in SG level of normal rats. Treatment CA at 25 mg/kg and acarbose (7 mg/kg) exhibited significant (P < 0.05; P < 0.01) reduction in SG level over the period of 120 min compared to NC group. The data are illustrated in Fig. 2. This result suggested that the CA may inhibit α-glucosidase. The CA caused hypoglycemia and improved glucose tolerance in normal rats in spite of counter regulatory factors avoiding reduction in blood glucose levels. Therefore, hypoglycemic activity of CA could be mediated by stimulation of surviving β-cells to release more insulin and may be through extra-pancreatic mechanisms. Furthermore, treatment of CA significantly improves the sucrose tolerance in normal rats, suggested that the CA may inhibit α-glucosidase, a membrane bound enzyme located on the epithelium of the small intestine, catalyzing the cleavage of disaccharides to form glucose. Therefore, inhibitors can retard the uptake of dietary carbohydrates and suppress postprandial hyperglycemia.
Effect of CA in STZ-induced diabetic rats
Single-dose 1-day study
STZ is well known for its selective pancreatic islet β-cell cytotoxicity and has been extensively used to induce diabetic mellitus in animals. It interferes with cellular metabolic oxidative mechanisms. The present data suggested that CA significantly reduced hyperglycemia in both single-dose 1-day and multiple-dose 7-day diabetic studies. This could be mediated by improving the glycemic control mechanisms (extra-pancreatic) and increasing insulin secretion from remnant pancreatic β-cells in diabetic rats. Single-dose 1-day study, a single dose of CA (25 mg/kg) treatment exhibited reduction in SG levels at different time intervals compared to basal levels (0 h). However, administration of GLB showed significant (P < 0.05; P < 0.001) reduction is SG levels with maximum reduction at 4 h post GLB treatment compared to their basal levels, whereas CA-treated animals showed reduction in SG levels compared to their basal levels, and the data are given in Fig. 3.
Multiple-dose 7-day study
On 7th day, oral administration of glucose (2 g/kg) did not produced significant change in SG level of NC rats and AUC for the 120 min interval was not altered. The diabetic rats exhibited significant elevation in fasting SG (at time zero) and showed significant impairment in glucose tolerance to exogenously administered glucose compared to normal rats. Treatment with CA (25 mg/kg) and GLB (0.5 mg/kg) significantly (P < 0.05; P < 0.01) improve the glucose tolerance, and the data are given in Fig. 4. Further, treatment CA-exhibited significant (P < 0.05; P < 0.01) reduction in SG level over the period of 120 min compared to DC group.
Effect of CA on SG levels in STZ-induced diabetic rats (multiple-dose 7-day study). Bar graph represents the percentage reduction in glycemia with respect to the initial (0 day) level. Each value represents the mean ± SEM n = 5; c P < 0.001 compared to DC. One-way ANOVA followed by Tukey’s post test
Lipid profile studies
Diabetic mellitus is often linked with abnormal lipid metabolism. The impairment of insulin secretion results in enhanced metabolism of lipids from the adipose tissue to the plasma (Habbu et al., 2010). It has been demonstrated that insulin deficiency in diabetes leads to a variety of disruptive changes in metabolic and regulatory processes, which in turn lead to accumulation of lipids. It has also been shown that insulin significantly normalizes lipid levels in diabetic rats (Pathak et al., 1981). The compound CA treatment also results in significant attenuation in triglyceride (STG) and total cholesterol (STC). These effects may be due to low activity of cholesterol biosynthesis enzymes or low levels of lipolysis. CA administration reinstated dyslipidemic markers to near-normal values. It was reported that drug bis-o-hydroxycinnamoylmethane enhances the antioxidant defense against reactive oxygen species produced under hyperglycemic conditions and thus protects the pancreatic β-cell against loss and exhibits antidiabetic property (Anusuya and Venugopal, 2003). Diabetic rats showed significantly (P < 0.001) increased levels of STG, STC, VLDL-c, and LDL-c levels, whereas HDL-c was decreased in diabetic rats compared to normal rats, and the data are given in Table 1. Oral administration of CA for 7-day exhibited significant reduction (P < 0.001) in all tested lipid parameters and restoring them to near-normal values.
The compound CA was docked into the active site of 5′-AMP AMPK (PDB ID: 2OOX). The calculated docking and binding (ΔG) energies (in kcal/mol) of CA is shown in Table 2. The data showed that molecule likely bind with AMPK with similar strength. The molecular interactions between the compound and the active site residues of AMPK at 3D space are shown in Fig. 5.
Conclusion
In these studies, a synthetic bischalcone derivative cyclo [2E,5E-2,5-bis(4-hydroxy-3-methoxybenzylidene) cyclopentanone] (CA) was evaluated for its antidiabetic property. The normoglycemic rats treated with CA showed significant reduction in SG level over the period of 120 min compared to NC group. In addition, estimation of integrated AUCglucose indicated that treatment of normoglycemic rats with CA showed significant improved glucose tolerance over the period of 120 min. Acute antihyperglycemic effect of CA was assessed in STZ-induced diabetic rats. Administration of single dose and chronic administration of CA in diabetic rats showed reduction in SG levels at different time intervals compared to base values, i.e., at 0 h of the same group. Treatment of diabetic rats with CA showed significantly (P < 0.001) improved glucose tolerance and marked fall in SG level over the period of 120 min compared to DC group. Lipid profile studies also supported antidiabetic efficacy of CA. The compound CA has been docked into the active site of 5′-AMP AMPK (PDB ID: 2OOX). The calculated docking and binding (ΔG) energies (in kcal/mol) of the compounds was found to be −45.1 and −6.9 kcal/mol, respectively. These data suggest that the potency of the compound is comparable to standard GLB.
References
Abagyan RA, Totrov MM, Kuznetsov DA (1994) ICM: a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem 15:488–506
Anusuya S, Venugopal PM (2003) Protection of pancreatic β-cell by the potential antioxidant bis-ohydroxycinnamoyl methane, analogue of natural curcuminoid in experimental diabetes. J Pharm Pharm Sci 6:327–333
Arie G, Gali B, Shlomo S (2009) Adenosine monophosphate-activated protein kinase (ampk) as a new target for antidiabetic drugs: a review on metabolic, pharmacological and chemical considerations. Rev Diabetes Stud 6:13–36
Butcher RJ, Jasinski JP, Narayana B, Sarojini BK, Bindya S, Yathirajan HS (2007) 2,5-Bis(3,4-dimethoxybenzylidene)cyclopentanone. Acta Cryst E 63:o3270–o3271
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (1989) Vogel’s text book of practical organic chemistry, 5th edn. Longman Group UK Ltd., London, p 1034
Habbu PV, Mahadevan KM, Kulkarni PV, Daultsingh C, Veerapur VP, Shastry RA (2010) Adaptogenic and in vitro antioxidant activity of argyreia speciosa (burm.f) boj. in acute and chronic stress paradigms in rodents. Indian J Exp Biol 48:53–60
Halgren TA (1996a) Merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. J Comput Chem 17:520–552
Halgren TA (1996b) Merck molecular force field. III. Molecular geometries and vibrational frequencies. J Comput Chem 17:553–586
Halgren TA (1996c) Merck molecular force field. V. Extension of MMFF94 using experimental data, additional computational data and empirical rules. J Comput Chem 17:616–641
Halgren TA (1999a) MMFF VI. MMFF94s option for energy minimization studies. J Comput Chem 20:720–729
Halgren TA (1999b) MMFF VII characterization of MMFF94, MMFF94s, and other widely available force fields for conformational energies and for intermolecular-interaction energies and geometries. J Comput Chem 20:730–748
Halgren TA, Nachbar RB (1996) Merck molecular force field. IV. Conformational energies and geometries for MMFF94. J Comput Chem 17:587–615
Kasiviswanath R, Ramesh A, Kumar KE (2005) Hypoglycemic and antihyperglycemic effect of Gmelina asiatica Linn in normal and alloxan induced diabetic rats. Biol Pharm Bull 28:729–732
Khan MT, Fuskevag OM, Sylte I (2009) Discovery of potent thermolysin inhibitors using structure based virtual screening and binding assays. J Med Chem 52:48–61
Lernmark A, Ott J (1998) Sometimes it’s hot. Sometimes its not. Nat Genet 19:213–218
Matti R, Das UK, Ghosh D (2005) Attenuation of hyperglycemia and hyperlipidemia in streptozotocin induced diabetic rats by aqueous CA of seed of Tamarindus indica. Biol Pharm Bull 28:1172–1176
Natchaya W, Pattarin S, Amporn J, Apichart S, Suthiluk P (2009) Effects of curcumin and tetrahydrocurcumin on diabetes induced endothelial dysfunction. Afr J Biochem Res 3:259–265
Pathak RM, Ansari S, Mahnood A (1981) Changes in chemical composition of intestinal brush border membrane in alloxan induced chronic diabetes. Indian J Exp Biol 9:503–505
Preetha A, Chitra S, Sonia J, Ajaikumar BK, Bharat B (2008) Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett 267:133–164
Schapira M, Totrov M, Abagyan R (1999) Prediction of the binding energy for small molecules, peptides and proteins. J Mol Recognit 12:177–190
Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G (2003) Hypoglycaemic and hypolipidemic effect of ethanolic CA of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol 85:201–206
Siddique O, Sun Y, Lin JC, Chein YW (1987) Facilitated transdermal transport of insulin. J Pharmacol Sci 76:341–345
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes. Diabetes Care 27:1047–1053
Yanardag R, Colak H (1998) Effect of chard (Beta vulgaris L. var. cicla) on blood glucose levels in normal and alloxan-induced diabetic rabbits. Pharm Pharmacol Commun 4:309–311
Conflict of interest
Authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raj, C.G.D., Sarojini, B.K., Khan, M.T.H. et al. In vivo antidiabetic activity and in silico studies on adenosine monophosphate-activated protein kinase (AMPK) of (2E,5E)-2,5-bis(4-hydroxy-3-methoxybenzylidene) cyclopentanone. Med Chem Res 22, 2430–2436 (2013). https://doi.org/10.1007/s00044-012-0237-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0237-1