Abstract
A series of novel Schiff bases; compounds 3a–j were prepared by reacting 1-(2-aminoethyl)-2-methyl-5-nitroimidazole dihydrochloride monohydrate (1) with different aldehydes. The structures of these compounds were confirmed through different spectroscopic methods such as 1H-NMR, 13C-NMR and mass spectrometry and also by elemental analyses. The prepared compounds were evaluated in vitro for their antigiardial, antibacterial and antifungal activities. Compounds 3h, 3b and 3d showed remarkable antigiardial activities and were found to be more active than metronidazole with IC50 of 0.83, 1.36 and 1.83 μM, respectively. Other compounds also exhibited antigiardial activity and were as good as or even more potent than metronidazole. Some of the newly synthesized Schiff bases exhibited more antifungal activities than the parent drug. In addition, a few of the prepared compounds exhibited modest antibacterial activity but were not as active as metronidazole.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schiff bases form an important class of organic compounds with a variety of uses. They have been widely used as ligands in the formation of transition metal complexes (Gulco et al., 2005). In addition, Schiff bases were found to exhibit biological activities including antibacterial, antifungal and anti-inflammatory (Zhou et al., 2007). Moreover, Schiff bases containing heterocycles have attracted much attention due to their diverse biological activity such as anticancer, antiviral, fungicidal, bactericidal and anti-HIV (Patel and Parmar, 2010). Alternatively, development of new chemotherapeutic Schiff bases is now attracting the attention of medicinal chemists (Khan et al., 2009).
Several research groups have been involved in the synthesis and biological screening of Schiff bases (Gulco et al., 2005; Zhou et al., 2007; Patel and Parmar, 2010; Khan et al., 2009; Ronad et al., 2008; Bertinaria et al., 2003). Zhou et al. (2007) synthesized a range of Schiff bases derived from 2-aminothiazoles and substituted benzaldehyde; the in vitro antitumor activity of the prepared compounds with three human tumour cell lines was evaluated. On the other hand, Ronad et al. (2008) have synthesized a series of Schiff bases from 7-amino-4-methylcoumarin and benzaldehydes and studied their anti-inflammatory and analgesic activity. They discovered that the anti-inflammatory and analgesic activities of some of the prepared Schiff bases were either comparable or more potent than the reference drug. In addition, Bertinaria and his colleagues (Bertinaria et al., 2003) have prepared a number of Schiff bases through the condensation of aromatic and heteroaromatic aldehydes with coumarin acetohydrazides under conventional and microwave conditions; the prepared compounds were tested for their antimicrobial activity and were found to display moderate to potent activity against different bacterial strains. Recently, the synthesis and antiglycation activity of bis-Schiff bases of isatins have been reported by Khan and colleagues (2009); a remarkable effect on the antiglycation activity due to the presence of electron withdrawing groups at isatin was observed. Very recently, Patel and Parmar (2010) have reported on the synthesis and antimicrobial activities of novel optically active Schiff bases derived from substituted benzaldehyde with different amines and discovered that the presence of nitro, methoxy and halogen group in the phenyl ring increases the activity of the prepared compounds against bacteria.
Similarly, mertonidazole, 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethanol (1) and its derivatives have a wide range of biological activity (Bertinaria et al., 2003; Martino et al., 2005; Hay et al., 2003; Günay et al., 1999). They are highly effective against trichomoniasis, various forms of amoebiasis and infections with anaerobic bacteria and protozoa (Goldman and Wuest, 1981); it can kill or inhibit the majority of anaerobic bacteria when the metronidazole concentration in serum is in the range from 2 to 8 μg/ml (Salimi et al., 1997). Recently (Abu-Shaireh et al., 2009), a number of novel metronidazole derivatives have been synthesized and their anti-parasitic activity has been evaluated. In view of the wide interest in the activity and profile of Schiff bases and metronidazole, and as part of our ongoing research in the synthesis of new compounds of pharmacological interest (Abu-Shaireh et al., 2009; Al-Zghoul et al., 2005; Saadeh et al., 2009, 2010), we describe herein the synthesis and characterization of a number of new Schiff bases derived from metronidazole which, to the best of our knowledge, have not previously been described in the literature. The antigiardial, antibacterial and antifungal activities of the newly prepared compounds were evaluated.
Results and discussion
Chemistry
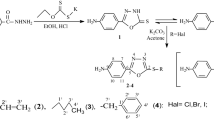
The Schiff bases 3a–j were prepared by reacting 1-(2-aminoethyl)-2-methyl-5-nitroimidazole dihydrochloride monohydrate (1) with different aldehydes in methanol as shown in Scheme 1. The prepared compounds were checked for purity by TLC using glass plates precoated with silica gel 60 GF254, supplied by Fluka as stationary phase and suitable solvent system as mobile phase and was also confirmed by melting point determination. The structures of the prepared compounds were confirmed by NMR, mass spectrometry and elemental analysis. The 1H and 13C-NMR spectra of all prepared compounds are in total agreement with the suggested structures. DEPT experiments were employed to differentiate secondary and quaternary carbons from primary and tertiary ones. Additional supports of the proposed structures come from mass spectral data; mass spectra of the prepared compounds showed the correct molecular ions as suggested by their molecular formulas.
Biological activities
Antigiardial activity
The antigiardial activity of the reported compounds was investigated using in vitro bioassays. Their bioactivity was compared with the standard antigiardial drug, metronidazole. The IC50 values of the compounds against Giardia intestinalis are given in Table 1. As shown in the table, all the tested compounds exhibited biological activity against Giardia. Compounds 3a–e, 3i and 3h have IC50 values ranging from 0.83 to 2.8 μM compared to 3.74 μM for metronidazole. Compound 3h showed the highest antigiardial activity with IC50 of 0.83 μM and was around five times more potent than metronidazole. The molecular modifications on our derivatives did not render any of the compounds inactive, but 3f, 3g and 3j exhibited less antigiardial activity than metronidazole. The giardicidal activity exhibited by the derivatives, especially compounds 3h, 3b and 3d suggest that the derivatives may be used as new lead compounds in the development of new antiparasitic drugs. This cidal activity of the prepared Schiff bases could extend to cover other related anaerobic protozaol parasites such as Entamoeba histolytica and Trichomonas vaginalis; this, however, needs further investigations. Although, drug resistance of Giardia and other target pathogens such as E. histolytica does not, so far, appear to be a serious problem, occasional reports of failure with metronidazole (Knight, 1980) and the reported variations in drug sensitivities of isolates may be alarming (Majewska et al., 1991; Aley et al., 1994). Therefore, the importance of such biologically active metronidazole derivatives lies in their potential contribution to overcome the problem of resistance of pathogens to the standard drugs (Elizondo et al., 1996; Hager and Rapp, 1992). In addition, because of the limited number of drugs available in the market against anaerobic protozoan parasites and bacteria there is a serious need for new active compounds. The molecular modification on the original drugs, therefore, offers alternatives that may bypass the already developed mechanisms adopted by the anaerobic pathogens against the standard drugs. Our new compounds, especially 3h, 3b and 3d are good drug candidates to be tested against metronidazole-resistant parasites and bacteria.
Antimicrobial activity
The antibacterial and antifungal activities of the prepared compounds are presented in Table 2. Results revealed that the Schiff bases derived from metronidazole were significantly less active in their antimicrobial activity towards anaerobic culture of Clostridium sporogenes relative to the parent drug, metronidazole. In addition, as shown in Table 2, the relatively weak activity displayed by metronidazole against the tested gram positive and gram negative bacteria is weak, however, Schiff base formation led to the increased activity of 3f towards both gram positive and gram negative species tested. Interestingly, Schiff base formation of metronidazole increased the antifungal activity, where the parent drug was devoid of any anti-candida effect under the experimental conditions. The tested compounds exhibited MIC values ranging from 0.17 to 2.0 mM.
Conclusions
A series of new metronidazole-derived Schiff bases were prepared by reacting 1-(2-aminoethyl)-2-methyl-5-nitroimidazole dihydrochloride monohydrate with different aldehydes. Structures of the newly synthesized compounds were confirmed by means of 1H-NMR, 13C-NMR, mass spectrometry and elemental analyses. The prepared compounds were tested in vitro for their antibacterial, antigiardial and antifungal activity. Some of them displayed remarkable antigiardial activity and were more potent than metronidazole itself. Similarly, the newly synthesized Schiff bases exhibited more antifungal activities than the parent drug, metronidazole. In contrast, a modest antibacterial activity was exhibited by some of the prepared compounds.
Experimental section
Chemistry
All reagents were used as received from commercial sources without further purification. Progress of reactions was monitored by thin layer chromatography (TLC) using glass plates pre-coated with silica gel (E. Merck Kiesegel 60 F254 layer thickness 0.25 mm). Melting points were measured with a Fischer-Johns melting point apparatus and were uncorrected. 1H- and 13C-NMR spectra were acquired with the aid of a Bruker DPX 300 MHz spectrometer (Germany) with TMS as the internal standard. Chemical shifts are expressed in δ units; J values for 1H–1H, 1H–F and 13C–F coupling constants are given in Hertz. High resolution mass spectra (HRMS) were obtained (in positive/or negative mode) using electrospray ion trap (ESI) technique by collision-induced dissociation on a Bruker APEX-4 (7-Tesla) instrument (Bremen, Germany). The samples were dissolved in acetonitrile, diluted in spray solution (methanol/water 1:1 v/v + 0.1% formic acid) and infused using a syringe pump with a flow rate of 2 μl/min. External calibration was conducted using arginine cluster in a mass range m/z 175–871. Elemental analyses were obtained using a Eurovector Euro EA3000, C, H, N and S elemental analyzer and the obtained results agreed with the calculated percentages to within ±0.4%. Compounds were checked for their purity by TLC using glass plates, precoated with silica gel 60 GF254, supplied by Fluka.
Synthesis of imines 3a–j
Compounds 3a–j were synthesized and purified according to the following general procedure: A mixture of 1-(2-aminoethyl)-2-methyl-5-nitroimidazole dihydrochloride monohydrate 1 (2.50 mmol), triethylamine (5.50 mmol) and the corresponding aldehyde (2.60 mmol) in methanol (15 ml) was stirred at room temperature for 24 h. The desired products were collected by filtration, washed with water and then recrystallized form methanol. Using the aforementioned general procedure, the following compounds were synthesized.
(4-Fluoro-benzylidene)-[2-(2-methyl-5-nitro-imidazol-1-yl)-ethyl]-amine (3a)
Yield 77%; Mp 134–135°C. 1H-NMR (CDCl3): δ = 2.45 (s, 3H), 3.95 (t, J = 5.5 Hz, 2H), 4.65 (t, J = 5.5 Hz, 2H), 7.05 (m, J = 7.9 Hz, 2H), 7.60 (m, J = 7.9 Hz, 2H), 7.92 (s, 1H), 8.05 (s, 1H). 13C-NMR (CDCl3): δ = 14.9 (CH3), 47.0 (CH2), 60.6 (CH2), 116.0 (d, 2 J C–F = 22 Hz, C), 130.1 (d, 4 J C–F = 3.6 Hz, CH), 133.4 (CH), 138.5 (C), 151.6 (C), 162.2 (CH), 163.7 (d, 1 J C–F = 250 Hz, C). HRMS (EIMS) m/z: [M + Na]+ calcd. for C13H13FN4 NaO2 299.09202; found 299.09257. Anal. Calcd. for C13H13FN4O2: C, 56.52; H, 4.74; N, 20.28. Found: C, 56.41; H, 4.69; N, 20.13.
(4-Methyl-benzylidene)-[2-(2-methyl-5-nitro-imidazol-1-yl)-ethyl]-amine (3b)
Yield 86%; Mp 163–164°C. 1H-NMR (CDCl3): δ = 2.35 (s, 3H), 2.45 (s, 3H), 3.95 (t, J = 5.5 Hz, 2H), 4.65 (t, J = 5.5 Hz, 2H), 7.15 (d, J = 7.9 Hz, 2H), 7.50 (d, J = 7.9 Hz, 2H), 7.90 (s, 1H), 8.00 (s, 1H). 13C-NMR (CDCl3): δ = 14.6 (CH3), 21.6 (CH3), 47.1 (CH2), 60.8 (CH2), 128.2 (CH), 129.5 (CH), 132.8 (C), 133.4 (CH), 141.8 (C), 151.6 (C), 163.5 (CH). HRMS (EIMS) m/z: [M + Na]+ calcd. for C14H16N4NaO2 295.11709; found 295.12575. Anal. Calcd. for C14H16N4O2: C, 61.75; H, 5.92; N, 20.58. Found: C, 61.68; H, 5.87; N, 20.44.
(4-Methoxy-benzylidene)-[2-(2-methyl-5-nitro-imidazol-1-yl)-ethyl]-amine (3c)
Yield 80%; Mp 69–71°C. 1H-NMR (CDCl3): δ = 2.38 (s, 3H), 3.85 (s, 3H), 3.95 (t, J = 5.5 Hz, 2H), 4.65 (t, J = 5.5 Hz, 2H), 6.90 (d, J = 8.0 Hz, 2H), 7.55 (d, J = 8.0 Hz, 2H), 7.93 (s, 1H), 8.00 (s, 1H). 13C-NMR (CDCl3): δ = 14.8 (CH3), 47.2 (CH2), 60.7 (CH3), 60.7 (CH2), 114.2 (CH), 128.3 (C), 129.8 (CH), 133.3 (CH), 138.4 (C), 151.5 (C), 162.1 (C), 162.9 (CH). HRMS (EIMS) m/z: [M − H]+ calcd. for C14H15N4O3 287.11442, found 287.11496. Anal. Calcd. for C14H16N4O3: C, 58.32; H, 5.59; N, 19.43 found C, 58.25; H, 5.56; N, 19.33.
[2-(2-Methyl-5-nitro-imidazol-1-yl)-ethyl]-(4-nitro-benzylidene)-amine (3d)
Yield 81%; Mp 190–191°C. 1H-NMR (CDCl3): δ = 2.45 (s, 3H), 4.00 (t, J = 5.6 Hz, 2H), 4.65 (t, J = 5.6 Hz, 2H), 7.80 (d, J = 8.0 Hz, 2H), 7.93 (s, 1H), 8.15 (s, 1H), 8.20 (d, J = 8.0 Hz, 2H). 13C-NMR (CDCl3): δ = 14.8 (CH3), 46.8 (CH2), 60.7 (CH2), 124.1 (CH), 128.9 (CH), 133.4 (CH), 138.4 (C), 140.6 (C), 151.3 (C), 161.4 (CH). HRMS (EIMS) m/z: [M]+ calcd. for C13H13N5O4 303.09676; found 303.09730. Anal. Calcd. for C13H13N5O4: C, 51.48; H, 4.32; N, 23.09 found C, 51.39; H, 4.29; N, 23.98.
2-{[2-(2-Methyl-5-nitro-imidazol-1-yl)-ethylimino]-methyl}-phenol (3e)
Yield 91%; Mp 105–106°C. 1H-NMR (CDCl3): δ = 2.45 (s, 3H), 4.00 (t, J = 5.6 Hz, 2H), 4.70 (t, J = 5.6 Hz, 2H), 6.85 (t, J = 7.1 Hz, 1H), 6.95 (d, J = 7.7 Hz, 1H), 7.15 (dd, J = 7.6, 1.5 Hz, 1H), 7.35 (dt, J = 7.7, 1.7 Hz, 1H), 7.95 (s, 1H), 8.20 (s, 1H). 13C-NMR (CDCl3): δ = 14.7 (CH3), 46.8 (CH2), 59.4 (CH2), 117.1 (CH), 118.1(C), 119.2 (CH), 131.9 (CH), 133.2 (CH), 133.6 (CH), 138.5 (C), 151.2 (C), 160.7 (C), 167.9 (CH). HRMS (EIMS) m/z: [M + H]+ calcd. for C13H15N4O3 275.11442; found 275.11387. Anal. Calcd. for C13H14N4O3: C, 56.93; H, 5.14; N, 20.43. Found: C, 56.85; H, 5.11; N, 20.34.
4-Chloro-2-{[2-(2-methyl-5-nitro-imidazol-1-yl)-ethylimino]-methyl}-phenol (3f)
Yield 86%; Mp 160–161°C. 1H-NMR (CDCl3): δ = 2.45 (s, 3H), 4.00 (t, J = 5.6 Hz, 2H), 4.70 (t, J = 5.6 Hz, 2H), 6.90 (t, J = 8.6 Hz, 1H), 7.20 (s, 1H), 7.30 (d, J = 6.2, 1H), 7.95 (s, 1H), 8.10 (s, 1H), 12.6 (s, 1H). 13C-NMR (CDCl3): δ = 14.6 (CH3), 46.7 (CH2), 59.3 (CH2), 118.8 (CH), 118.9 (C), 123.8 (C), 130.9 (CH), 133.1 (CH), 133.6 (CH), 138.5 (C), 151.0 (C), 159.3 (C), 166.8 (CH). HRMS (EIMS) m/z: [M − H]+calcd. for C13H12ClN4O3 307.05979; found 307.06034. Anal. Calcd. for C13H13ClN4O3: C, 50.58; H, 4.24; N, 18.15. Found: C, 50.52; H, 4.19; N, 18.06.
(2-Chloro-benzylidene)-[2-(2-methyl-5-nitro-imidazol-1-yl)-ethyl]-amine (3g)
Yield 80%; Mp 142–143°C. 1H-NMR (CDCl3): δ = 2.50 (s, 3H), 3.85 (t, J = 5.5 Hz, 2H), 4.65 (t, J = 5.5 Hz, 2H), 7.38 (m, 3H), 7.58 (d, J = 7.7 Hz, 1H), 7.15 (dd, J = 7.6, 1.5 Hz, 1H), 7.55 (t, J = 7.7, 1H), 7.90 (s, 1H), 8.00 (s, 1H). 13C-NMR (CDCl3): δ = 14.8 (CH3), 46.9 (CH2), 60.6 (CH2), 126.5 (CH), 127.8 (CH), 130.1 (CH), 131.3 (CH), 133.4 (CH), 135.0 (C), 137.0 (C), 138.6 (C), 151.5 (C), 162.2 (C). HRMS (EIMS) m/z: [M + H]+ calcd. for C13H14ClN4O2 293.08053; found 293.07998. Anal. Calcd. for C13H13ClN4O2: C, 53.34; H, 4.48; Cl, 12.11; N, 19.14. Found: C, 53.25; H, 4.46; N, 19.07.
[2-(2-Methyl-5-nitro-imidazol-1-yl)-ethyl]-thiophen-2-ylmethylene-amine (3h)
Yield 89%; Mp 88–90°C. 1H-NMR (CDCl3): δ = 2.40 (s, 3H), 3.85 (t, J = 5.4 Hz, 2H), 4.55 (t, J = 5.4 Hz, 2H), 6.95 (t, 1H), 7.15 (d, J = 5.0 Hz, 1H), 7.30 (d, J = 5.0 Hz, 1H), 7.80 (s, 1H), 8.05 (s, 1H). 13C-NMR (CDCl3): δ = 14.8 (CH3), 47.0 (CH2), 60.3 (CH2), 127.7 (CH), 129.7 (CH), 131.6 (CH), 133.3 (CH), 138.5 (C), 141.4 (C), 151.8 (C), 156.7 (C). HRMS (EIMS) m/z: [M + Na]+ calcd. for C11H12N4NaO2S 287.05786; found 287.05732. Anal. Calcd. for C11H12N4O2S: C, 49.99; H, 4.58; N, 21.20; S, 12.13 Found: C, 49.91; H, 4.55; N, 21.13; S, 12.04.
Furan-2-ylmethylene-[2-(2-methyl-5-nitro-imidazol-1-yl)-ethyl]-amine (3i)
Yield 67%; Mp 74–76°C. 1H-NMR (CDCl3): δ = 2.50 (s, 3H), 3.90 (t, J = 5.6 Hz, 2H), 4.65 (t, J = 5.6 Hz, 2H), 6.45 (dd, J = 3.5, 1.8 Hz 1H), 6.70 (d, J = 3.5 Hz, 1H), 7.50 (d, J = 3.5 Hz, 1H), 7.85 (s, 1H), 7.95 (s, 1H). 13C-NMR (CDCl3): δ = 14.9 (CH3), 47.0 (CH2), 61.0 (CH2), 111.9 (CH), 115.4 (CH), 133.4 (CH), 138.5 (C), 145.5 (CH), 150.9 (C), 151.6 (C), 152.0 (CH). HRMS (EIMS) m/z: [M − H]+ calcd. for C11H11N4O3 247.08312; found 247.08366. Anal. Calcd. for C11H12N4O3: C, 53.22; H, 4.87; N, 22.57. Found: C, 53.17; H, 4.83; N, 22.46.
[2-(2-Methyl-5-nitro-imidazol-1-yl)-ethyl]-pyridin-4-ylmethylene-amine (3j)
Yield 90%; Mp 109–110°C. 1H-NMR (CDCl3): δ = 2.45 (s, 3H), 4.00 (t, J = 5.5 Hz, 2H), 4.70 (t, J = 5.5 Hz, 2H), 7.50 (d, J = 5.8 Hz, 2H), 7.90 (s, 1H), 8.10 (s, 1H), 8.65 (d, J = 5.8 Hz, 2H). 13C-NMR (CDCl3): δ = 14.8 (CH3), 46.7 (CH2), 60.7 (CH2), 121.2 (CH), 133.4 (CH), 138.4 (C), 141.9 (CH), 150.7 (C), 151.3 (C), 161.9 (CH). HRMS (EIMS) m/z: [M − H]+ calcd. for C12H12N5O2 258.09910; found 258.09972. Anal. Calcd. for C12H13N5O2: C, 55.59; H, 5.05; N, 27.01. Found: C, 55.35; H, 5.01; N, 26.74.
Antigiardial activity
Test organism
Giardia intestinalis WB strain (ATCC number 30957) was grown in a modified YI-S medium with antibiotics. The parasite was cultivated in 15 ml screw-capped borosilicate glass tubes containing 13 ml medium. The tubes were incubated on a 15° horizontal slant at 36–37°C. Culture maintenance and sub-culturing was performed as described in a previous publication (Saadeh et al., 2009). Giardia was harvested from confluent cultures by chilling of the tubes on ice for 5–10 min to detach cells, followed by centrifugation at 800×g for 5 min.
Antigiardial assay
The antigiardial activity of the prepared molecules and metronidazole as the standard antigiardial drug were tested as described (Saadeh et al., 2009). In brief, the tested compounds and metronidazole were dissolved in dimethyl sulfoxide (DMSO) then in medium and filter-sterilized. Twofold dilutions starting at 15 μg/ml were prepared in a final volume of 15 ml to exclude air from the tube. Each tube was inoculated with 20,000 cells of Giardia. Each compound was assayed in duplicate in each of three independent experiments. In each assay, the appropriate controls were performed, including the one without any compound and another with metronidazole as the positive control. The biological activity of the compounds was evaluated by counting the parasites in each tube using the standard hemacytometer. For counting, the parasites were harvested as described in the above section. In each count, trypan blue was employed to distinguish live from dead parasites (Aley et al., 1994).
Antibacterial and antifungal activity
Broth microdilution method in 96 microtitre plates (Cellstar®, Greinerbio-one, Germany) was employed to assess the antimicrobial activity of the prepared Schiff bases. In brief, stock solution (10 mg/ml) of each substrate was prepared in DMSO under aseptic conditions. The first experimental well was filled with the sabauroud dextrose broth (190 μl) and the other wells were filled with 100 μl of the sabauroud dextrose broth. A volume of 10 μl of each substance stoke solutions was added to the first well and a twofold serial dilution was then carried out across the plate. The antimicrobial activity of the reference substances of ampicillin and miconazole (both obtained as a gift from Dar Al Dawa Pharmaceutical Company, Naour-Jordan) and metronidazole was determined in the same manner and their results were used as quality control. Overnight batch cultures (10 μl) of Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6538P, C. sporogenes ATCC 19404 and Candida albicans ATCC 10231 were used to inoculate the wells to achieve a final inoculum size of 1 × 106 cfu/ml and the plate was incubated for 24 h at 37°C. Growth media, conditions and positive and negative controls were performed according to published procedures (Saadeh et al., 2010). MIC was expressed as the mean concentration between the well showing growth and the well showing no growth. Growth was detected as turbidity (630 nm), relative to an un-inoculated well using a microtitre plate reader (Biotek, USA). Each MIC determination was carried out in triplicate.
References
Abu-Shaireh EAM, Saadeh HAM, Mosleh IMI, Al-Arif MTA, Mubarak MSHM (2009) Synthesis of new metronidazole derivatives as antiparasitic agents. Eur Pat Appl EP 2985394 A2, p 19 (European Patent No: 09250251.7-2117)
Aley SB, Zimmerman M, Hetsko M, Selsted ME, Gillin FD (1994) Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect Immun 62:5397
Al-Zghoul KHA, Salih KSM, Ayoub MT, Mubarak MS (2005) A convenient procedure for the synthesis of substituted 4-methylaminocoumarins. Heterocycles 65(12):2937
Bertinaria M, Galli U, Sorba G, Fruttero R, Gasco A, Brenciaglia MI, Scaltrito MM, Dubini F (2003) Synthesis and anti-Helicobacter pylori properties of donor/metronidazole hybrids and related compounds. Drug Dev Res 60:225
Elizondo G, Gonsebatt ME, Salazar AM, Lares I, Santiago P, Herrera J, Hong E, Ostrosky-Weigman P (1996) Genotoxic effects of metronidazole. Mutat Res 370:75–80
Goldman P, Wuest JD (1981) Reactions of nitroimidazoles. Nucleophilic substitution of the nitro group. J Am Chem Soc 103:6224
Gulco A, Tumer A, Demirelli H, Wheatley RA (2005) Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: synthesis, characterization, properties and biological activity. Inorg Chim Acta 358:1785
Günay NS, Çapan G, Ulusoy N, Ergenç N, Ötük G, Kaya D (1999) 5-Nitroimidazole derivatives as possible antibacterial and antifungal agents. Farmaco 54:826
Hager WD, Rapp RP (1992) Metronidazole. Obstet Gynecol Clin North Am 19:497
Hay MP, Anderson RF, Ferry DM, Wilson WR, Denny WA (2003) Synthesis and evaluation of nitroheterocyclic carbamate prodrugs for use with nitroreductase-mediated gene-directed enzyme prodrug therapy. J Med Chem 46:5533
Khan KM, Khan M, Ali M, Taha M, Rasheed S, Perveen S, Choudhary MI (2009) Synthesis of bis-Schiff bases of isatins and their antiglycation activity. Bioorg Med Chem 17(22):7795
Knight R (1980) The chemotherapy of amebiasis. J Antimicrob Chemother 6:577–593
Majewska AC, Kasprzak W, De Jonckheere JF, Kaczmarek E (1991) Heterogeneity in the sensitivity of stocks and clones of Giardia to metronidazole and ornidazole. Trans R Soc Trop Med Hyg 85:67
Martino GD, Regina GL, Pasquali AD, Ragno R, Bergamini A, Ciaprini C, Sinistro A, Maga G, Crespan E, Artic M, Silvestri R (2005) Novel 1-[2-(diarylmethoxy)ethyl]-2-methyl-5-nitroimidazoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. A structure–activity relationship investigation. J Med Chem 48:4378
Patel IJ, Parmar SJ (2010) Synthesis and studies of novel optically active Schiff’s base derivatives and their antimicrobial activities. E J Chem 7(2):617
Ronad P, Dharbamalla S, Hunshal R, Maddi V (2008) Synthesis of novel substituted 7-(benzylideneamino)-4-methyl-2H-chromen-2-one derivatives as anti-inflammatory and analgesic agents. Arch Pharm 341(11):696
Saadeh HA, Mosleh MI, Mubarak MS (2009) Synthesis of novel hybrid molecules from precursors with known antiparasitic activity. Molecules 14:1483
Saadeh HA, Mosleh IM, Al-Bakri AG, Mubarak MS (2010) Synthesis and antimicrobial activities of new 1,2,4-triazole-3-thiole metronidazole derivatives. Mon fur Chem 141:471–478
Salimi A, Izadi M, Hallaj R, Rashidi M (1997) Simultaneous determination of ranitidine and metronidazole at glassy carbon electrode modified with single wall carbon nanotubes. Electroanalysis 19:1668
Zhou X, Shao L, Jin Z, Liu J-B, Dai H, Fang J-X (2007) Synthesis and antitumor activity evaluation of some Schiff bases derived from 2-aminothiazole derivatives. Hetroatom Chem 18(1):55
Acknowledgments
I.M. Mosleh was supported by a joint grant from the Deanship of Scientific Research, The University of Jordan, and Hamdi Manko Center for Scientific Research (HMCSR), The University of Jordan. We also wish to thank Miss Lina Al-Natsheh for the technical assistance and the maintenance of cultures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saadeh, H.A., Shaireh, E.A.A., Mosleh, I.M. et al. Synthesis, characterization and biological activity of Schiff bases derived from metronidazole. Med Chem Res 21, 2969–2974 (2012). https://doi.org/10.1007/s00044-011-9830-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9830-y